Biocontrol Potential of Selected Phyllospheric Yeasts Against Botrytis cinerea and Fusarium fujikuroi
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Preparation of Yeast Cells and Fungal Spores
2.3. Determination of Antagonistic Activity of Yeasts Against Spoilage Mould
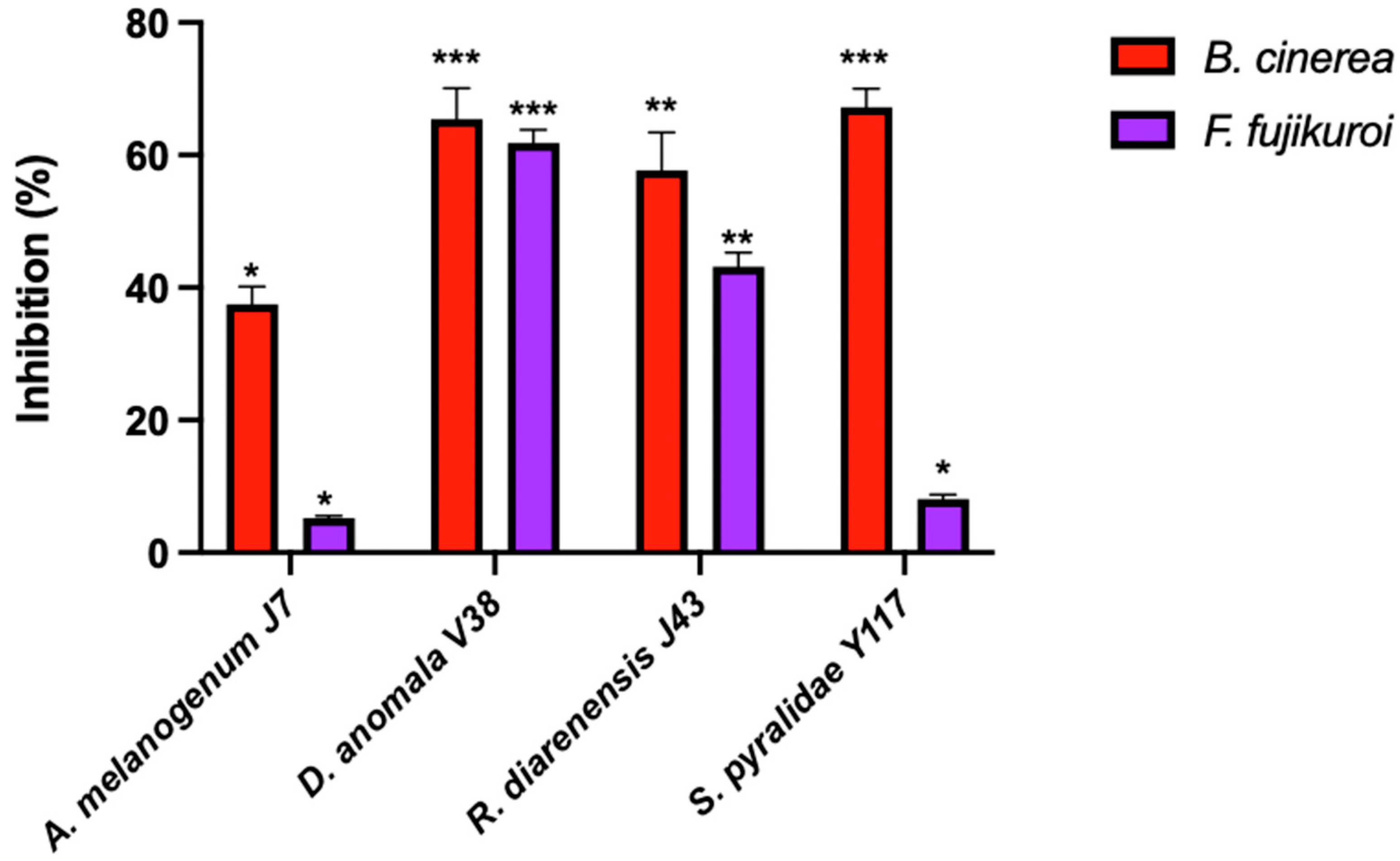
2.3.1. Radial Growth Inhibition
2.3.2. Mouth-to-Mouth Assay
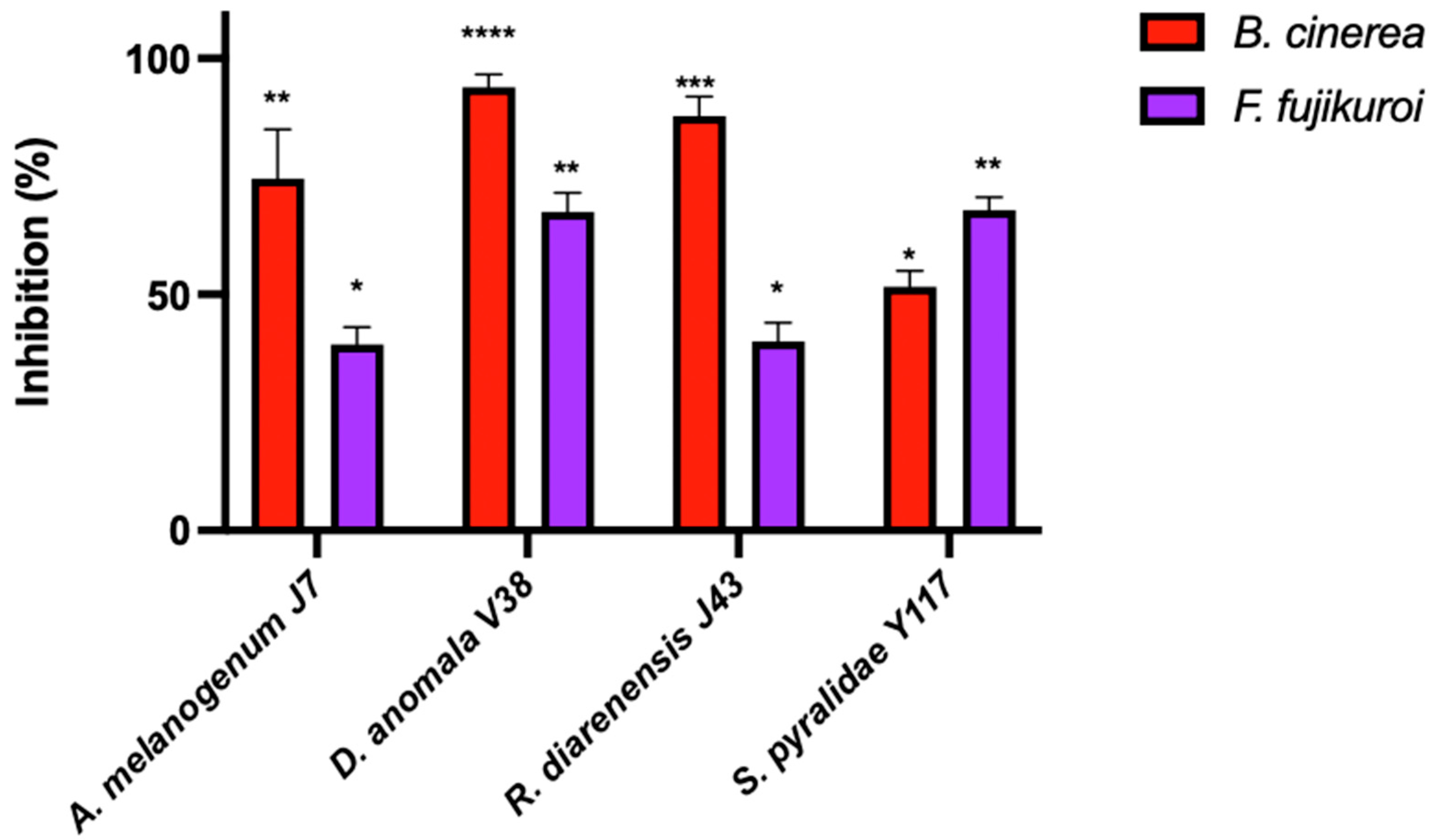
2.3.3. Dual Culture Assays
2.4. Screening of Yeasts for Cell Wall Degrading Enzyme Production
2.5. Production, Extraction and Analysis of VOC by Gas Chromatography
2.6. Biocontrol Activities of Yeast Cultures on Infected Pears and Tomatoes Post-Harvest
2.7. Statistical Analyses
3. Results and Discussion
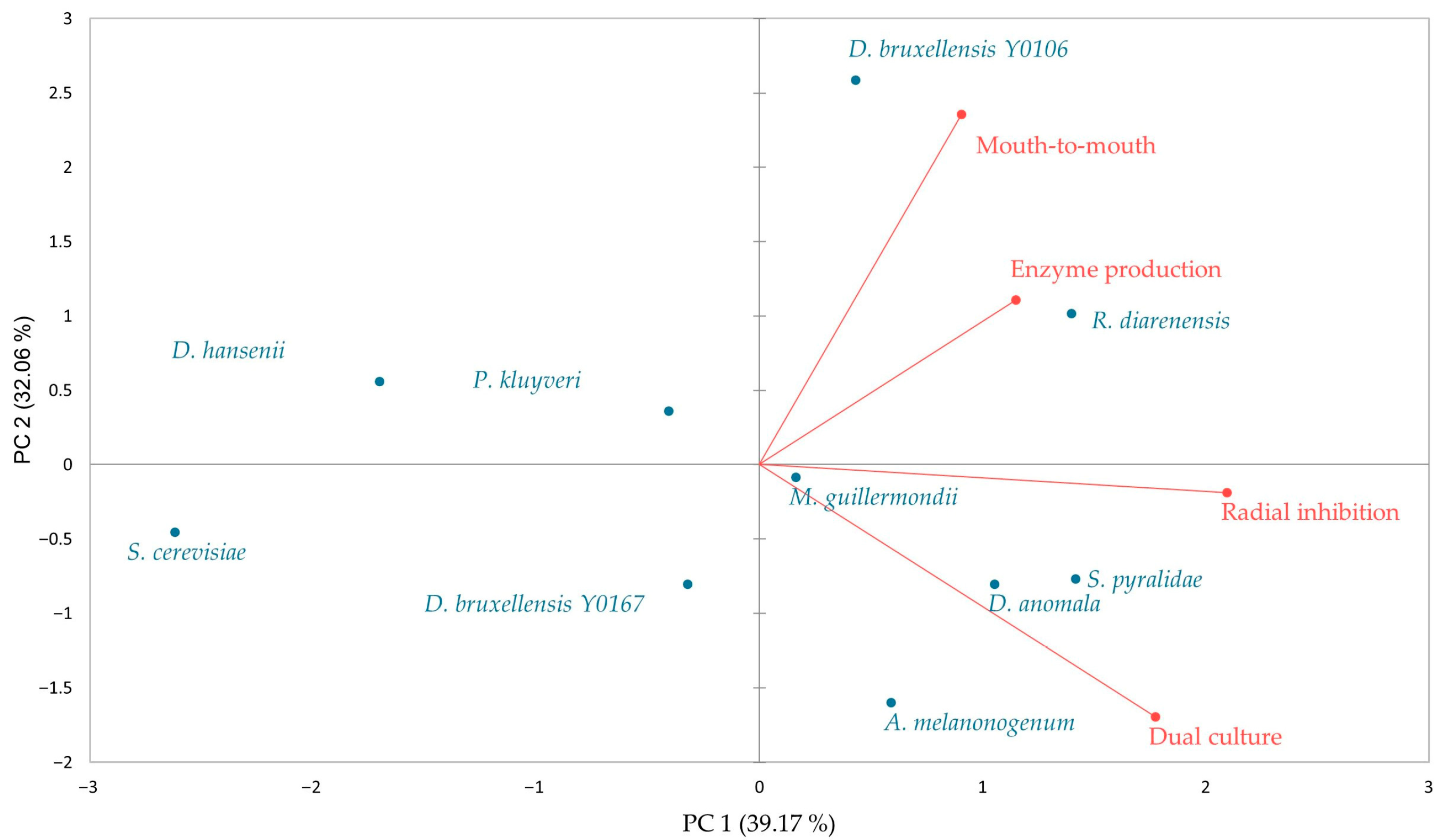
3.1. Antagonistic Activity of Yeasts Against Spoilage Mould
3.2. Cell Wall-Degrading Enzyme Production
3.3. Identification of Volatile Organic Compounds (VOCs) Produced by Yeasts
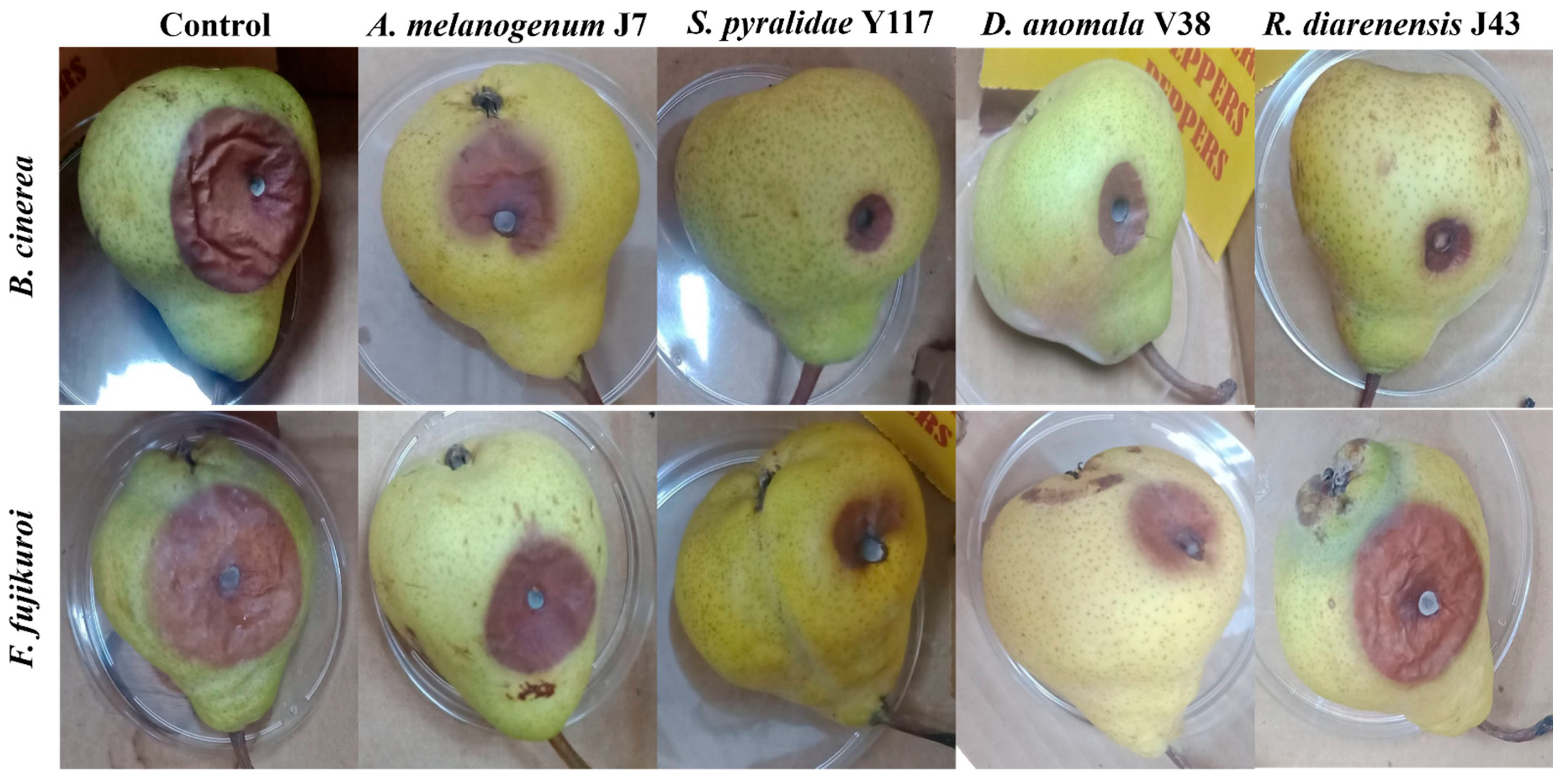
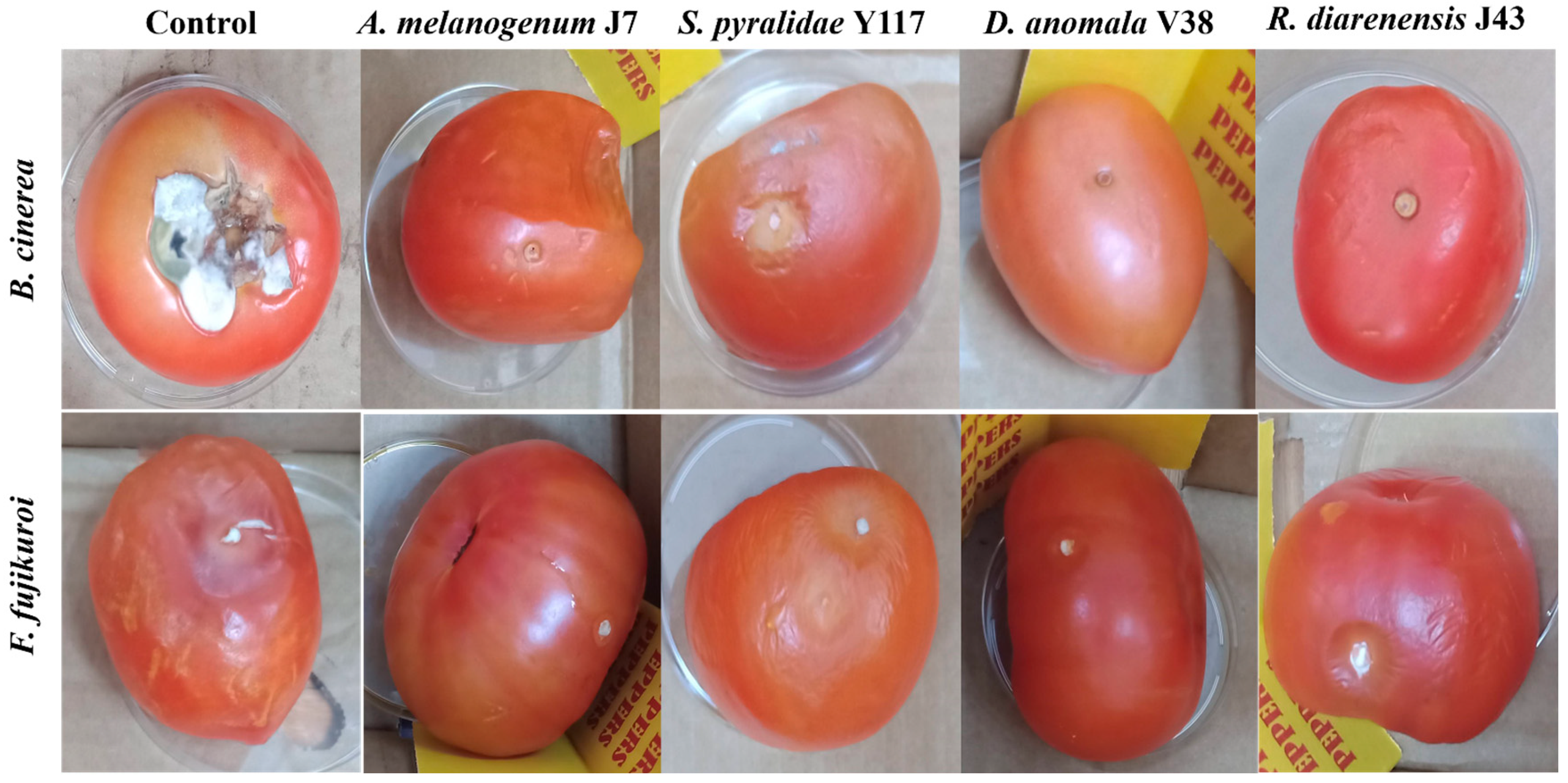
3.4. Application of Biocontrol Yeasts in Preventing Fruit Spoilage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef]
- Stathers, T.; Holcroft, D.; Kitinoja, L.; Mvumi, B.M.; English, A.; Omotilewa, O.; Kocher, M.; Ault, J.; Torero, M. A scoping review of interventions for crop postharvest loss reduction in sub-Saharan Africa and South Asia. Nat. Sustain. 2020, 3, 821–835. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and postharvest factors affecting bruise damage of fresh fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Pretscher, J.; Fischkal, T.; Branscheidt, S.; Jäger, L.; Kahl, S.; Schlander, M.; Thines, E.; Claus, H. Yeasts from different habitats and their potential as biocontrol agents. Fermentation 2018, 4, 31. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, S.; Deng, L.; Ming, J.; Zeng, K. Different mechanisms of action of isolated epiphytic yeasts against Penicillium digitatum and Penicillium italicum on citrus fruit. Postharvest Biol. Technol. 2019, 152, 100–110. [Google Scholar] [CrossRef]
- Bano, A.; Gupta, A.; Prusty, M.R.; Kumar, M. Elicitation of fruit fungi infection and its protective response to improve the post-harvest quality of fruits. Stresses 2023, 3, 231–255. [Google Scholar] [CrossRef]
- Yilmaz, N.; Sandoval-Denis, M.; Lombard, L.; Visagie, C.M.; Wingfield, B.D.; Crous, P.W. Redefining species limits in the Fusarium fujikuroi species complex. Persoonia-Mol. Phylogeny Evol. Fungi 2021, 46, 129–162. [Google Scholar] [CrossRef]
- Zakaria, L. Fusarium species associated with diseases of major tropical fruit crops. Horticulturae 2023, 9, 322. [Google Scholar] [CrossRef]
- Richards, J.K.; Xiao, C.L.; Jurick, W.M. Botrytis spp.: A contemporary perspective and synthesis of recent scientific developments of a widespread genus that threatens global food security. Phytopathology 2021, 111, 432–436. [Google Scholar] [PubMed]
- Huang, R.; Che, H.J.; Zhang, J.; Yang, L.; Jiang, D.H.; Li, G.Q. Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits. Biol. Control 2012, 62, 53–63. [Google Scholar]
- Elad, Y.; Vivier, M.; Fillinger, S. Botrytis, the good, the bad and the ugly. In Botrytis–The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Bahadur, A. Their Management Strategies. Fusarium: An Overview of the Genus; IntechOpen: London, UK, 2022. [Google Scholar]
- Dukare, A.S.; Singh, R.K.; Jangra, R.K.; Bhushan, B. Non-fungicides-based promising technologies for managing post-production penicillium induced spoilage in horticultural commodities: A comprehensive review. Food Rev. Int. 2022, 38, 227–267. [Google Scholar] [CrossRef]
- Liu, X.; Wei, L.; Miao, C.; Zhang, Q.; Yan, J.; Li, S.; Chen, H.; Qin, W. Application of exogenous phenolic compounds in improving postharvest fruits quality: Classification, potential biochemical mechanisms and synergistic treatment. Food Rev. Int. 2024, 40, 1776–1795. [Google Scholar]
- Khadiri, M.; Boubaker, H.; Lahmamsi, H.; Taoussi, M.; Ezzouggari, R.; Askarne, L.; Farhaoui, A.; Ait Barka, E.; Lahlali, R. Challenges in apple preservation: Fungicide resistance and emerging biocontrols. Physiol. Mol. Plant Pathol. 2024, 129, 102205. [Google Scholar]
- Marciano, L.P.; Costa, L.F.; Cardoso, N.S.; Freire, J.; Feltrim, F.; Oliveira, G.S.; Paula, F.B.; Silvério, A.C.; Martins, I. Biomonitoring and risk assessment of human exposure to triazole fungicides. Regul. Toxicol. Pharmacol. 2024, 147, 105565. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A.; Silva, C.L.; Brandao, T.R. A review on ozone-based treatments for fruit and vegetables preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Chen, O.; Yi, L.; Deng, L.; Ruan, C.; Zeng, K. Screening antagonistic yeasts against citrus green mold and the possible biocontrol mechanisms of Pichia galeiformis (BAF03). J. Sci. Food Agr. 2020, 100, 3812–3821. [Google Scholar]
- González-Gutiérrez, K.N.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Narváez-Zapata, J.A.; Calderón-Santoyo, M. Yeasts with potential biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass) and characterization of Yamadazyma mexicana mechanisms. Eur. J. Plant Pathol. 2023, 165, 525–543. [Google Scholar]
- Sipiczki, M. Identification of antagonistic yeasts as potential biocontrol agents: Diverse criteria and strategies. Int. J. Food Microbiol. 2023, 406, 110360. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for post-harvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Zhu, J.; Sun, J.; Shao, X. Volatile organic compounds of Scheffersomyces spartinae W9 have antifungal effect against Botrytis cinerea on strawberry fruit. Foods 2023, 12, 3619. [Google Scholar] [CrossRef]
- Oufensou, S.; Ul Hassan, Z.; Balmas, V.; Jaoua, S.; Migheli, Q. Perfume guns: Potential of yeast volatile organic compounds in the biological control of mycotoxin-producing fungi. Toxins 2023, 15, 45. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of post-harvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Hoff, J.; Van der Rijst, M.; Okudoh, V.; Kriel, J.; du Plessis, H.W. Control of mould spoilage on apples using yeasts as biological control agents. Pol. J. Food Nutr. Sci. 2022, 72, 119–128. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Ruzengwe, F.M.; Singh, S.; Pillai, S. Pear juice clarification using polygalacturonase from Beauveria bassiana: Effects on rheological, antioxidant and quality properties. Pol. J. Food Nutr. Sci. 2022, 72, 57–67. [Google Scholar] [CrossRef]
- Tenorio-Salgado, S.; Tinoco, R.; Vazquez-Duhalt, R.; Caballero-Mellado, J.; Perez-Rueda, E. Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered 2013, 4, 236–243. [Google Scholar]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, R.Y.; Chou, J.Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 2018, 46, 33–46. [Google Scholar]
- Mangunwardoyo, W.; Aprilismulan, A.; Oetari, A.; Sjamsuridzal, W. Screening cellulose activity of yeast isolated from soil, sediment and water river from Taman Nasional Gunung Halimun, West Java, Indonesia. Malays. J. Microbiol. 2011, 7, 210–216. [Google Scholar]
- Charoenchai, C.; Fleet, G.H.; Henschke, P.A.; Todd, B.E.N.T. Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Öztekin, S.; Karbancioglu-Guler, F. Biological control of green mould on mandarin fruit through the combined use of antagonistic yeasts. Biol. Control 2023, 180, 105186. [Google Scholar]
- Di Francesco, A.; Di Foggia, M.; Zajc, J.; Gunde-Cimerman, N.; Baraldi, E. Study of the efficacy of Aureobasidium strains belonging to three different species: A. pullulans, A. subglaciale and A. melanogenum against Botrytis cinerea of tomato. Ann. Appl. Biol. 2020, 177, 266–275. [Google Scholar] [CrossRef]
- Khunnamwong, P.; Lertwattanasakul, N.; Jindamorakot, S.; Suwannarach, N.; Matsui, K.; Limtong, S. Evaluation of antagonistic activity and mechanisms of endophytic yeasts against pathogenic fungi causing economic crop diseases. Folia Microbiol. 2020, 65, 573–590. [Google Scholar] [CrossRef]
- Chacón-Navarrete, H.; Ruiz-Pérez, F.; Ruiz-Castilla, F.J.; Ramos, J. Exploring biocontrol of unwanted fungi by autochthonous Debaryomyces hansenii strains isolated from dry meat products. J. Fungi 2022, 8, 873. [Google Scholar] [CrossRef] [PubMed]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Chidi, B.S.; Hoff, J.W.; Rijst, M.V.D.; Mokwena, L.; Setati, M.E.; du Plessis, H.W. The Use of Specific Non-Saccharomyces Yeasts as Sustainable Biocontrol Solutions Against Botrytis Cinerea on Apples and Strawberries. J. Fungi 2025, 11, 26. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major biological control strategies for plant pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef]
- Ferreira, J.M.; de Freitas Soares, F.E. Entomopathogenic fungi hydrolytic enzymes: A new approach to biocontrol? J. Nat. Pestic. Res. 2023, 3, 100020. [Google Scholar] [CrossRef]
- Thakur, D.; Bairwa, A.; Dipta, B.; Jhilta, P.; Chauhan, A. An overview of fungal chitinases and their potential applications. Protoplasma 2023, 260, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Elkhairy, B.M.; Salama, N.M.; Desouki, A.M.; Abdelrazek, A.B.; Soliman, K.A.; Ibrahim, S.A.; Khalil, H.B. Towards unlocking the biocontrol potential of Pichia kudriavzevii for plant fungal diseases: In vitro and in vivo assessments with candidate secreted protein prediction. BMC Microbiol. 2023, 23, 356. [Google Scholar] [CrossRef]
- Masih, E.I.; Paul, B. Secretion of β-1, 3-glucanases by the yeast Pichia membranifaciens and its possible role in the biocontrol of Botrytis cinerea causing grey mold disease of the grapevine. Curr. Microbiol. 2002, 44, 391–395. [Google Scholar] [CrossRef]
- Lin, L.; Zhuo, Y.; Dong, Q.; Yang, C.; Cheng, C.; Liu, T. Plasma activated Ezhangfeng Cuji as innovative antifungal agent and its inactivation mechanism. AMB Express 2023, 13, 65. [Google Scholar] [CrossRef]
- Oztekin, S.; Karbancioglu-Guler, F. Recruiting grape-isolated antagonistic yeasts for the sustainable bio-management of Botrytis cinerea on grapes. Food Energy Secur. 2024, 13, e528. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Mu, S.; Han, Y.; Shi, Y.; Li, X.; Li, D. Assessment of the contributions of Saccharomyces cerevisiae, Hansenula sp. and Pichia kudriavzevii to volatile organic compounds and sensory characteristics of waxy rice wine. Eur. Food Res. Technol. 2023, 249, 685–697. [Google Scholar] [CrossRef]
- Paszkot, J.; Gasiński, A.; Kawa-Rygielska, J. Evaluation of volatile compound profiles and sensory properties of dark and pale beers fermented by different strains of brewing yeast. Sci. Rep. 2023, 13, 6725. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ordorica, A.; Patiño-Medina, J.A.; Meza-Carmen, V.; Macías-Rodríguez, L. Volatile fingerprint mediates yeast-to-mycelial conversion in two strains of Beauveria bassiana exhibiting varied virulence. J. Fungi 2023, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Hatanaka, K.; Ohata, I.; Yamashita-Kitaguchi, Y.; Kurata, A.; Kishimoto, N. Antifungal activities of volatile substances generated by yeast isolated from Iranian commercial cheese. Food Control 2012, 26, 472–478. [Google Scholar] [CrossRef]
- Piechulla, B.; Lemfack, M.C. Microbial volatiles and their biotechnological applications. In Plant Specialized Metabolism; CRC Press: Boca Raton, FL, USA, 2016; pp. 251–268. [Google Scholar]
- Kovanda, L. Antimicrobial Effects of Organic Acids and Their Derivatives. Master’s Thesis, University of California, Davis, CA, USA, 2020. [Google Scholar]

| Yeast strain | Radial Growth Inhibition | Mouth-to-Mouth | Dual Culture | |||
|---|---|---|---|---|---|---|
| B. cinerea | F. fujikuroi | B. cinerea | F. fujikuroi | B. cinerea | F. fujikuroi | |
| A. melanogenum J7 | 100.0 ± 0.00 a | 90.7 ± 2.31 b | 8.0 ± 6.93 c | 13.3 ± 10.84 cdef | 61.11 ± 0.96 a | 47.5 ± 2.50 a |
| S. pyralidae Y1117 | 86.4 ± 1.68 c | 92.0 + 0.00 b | 4.8 ± 1.38 cde | 9.64 ± 3.61 def | 62.78 ± 0.96 a | 45.8 ± 1.44 ab |
| D. hansenii MY1 | 69.9 ± 1.68 e | 50.7 ± 2.31 e | 27.2 ± 3.67 b | 19.3 ± 5.11 cde | 41.67 ± 1.67 c | 25.8 ± 1.44 e |
| D. anomala V38 | 89.3 ± 1.68 b | 100.0 ± 0.00 a | 6.4 ± 2.40 cd | 27.7 ± 3.61 bc | 63.33 ± 1.67 a | 45.0 ± 0.00 b |
| D. bruxellensis Y0106 | 100.0 ± 0.00 a | 86.7 ± 2.31 c | 46.4 ± 3.67 a | 63.9 ± 3.61 a | 46.67 ± 1.67 bc | 25.0 ± 0.00 e |
| D. bruxellensis Y0167 | 72.8 ± 1.68 d | 81.3 ± 2.31 d | 4.0 ± 2.40 cde | 16.9 ± 7.23 cde | 58.89 ± 0.96 ab | 37.5 ± 0.00 d |
| M. guilliermondii J26 | 100.0 ± 0.00 a | 82.7 ± 2.31 d | 7.2 ± 2.77 c | 20.5 ± 9.56 | 53.89 ± 0.96 abc | 35.8 ± 1.44 d |
| P. kluyveri Y1125 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 4.8 ± 1.38 cde | 24.1 ± 3.61 cd | 41.11 ± 0.96 c | 22.5 ± 2.50 f |
| R. diarenensis J43 | 98.1 ± 3.36 a | 86.7 ± 2.31 c | 3.2 ± 1.39 de | 39.8 ± 2.09 b | 57.78 ± 0.96 ab | 40.0 ± 0.00 c |
| S. cerevisiae Y0936 | 90.3 ± 1.68 b | 46.7 ± 2.31 f | 1.6 ± 1.41 e | 6.0 ± 3.61 ef | 41.67 ± 0.00 c | 25.0 ± 0.00 e |
| Yeast Species | Enzyme | ||
|---|---|---|---|
| Cellulase | Chitinase | Protease | |
| Aureobasidium melanogenum J7 | + | - | + |
| Suhomyces pyralidae Y1117 | + | + | + |
| D. hansenii MY1 | + | + | - |
| D. anomala V38 | + | - | + |
| D. bruxellensis Y0106 | + | - | + |
| D. bruxellensis Y0167 | - | - | + |
| M. guilliermondii J26 | + | - | + |
| P. kluyveri Y1125 | - | - | + |
| R. diarenensis J43 | + | + | + |
| S. cerevisiae Y0936 | + | - | - |
| Aureobasidium melanogenum J7 | Suhomyces pyralidae Y1117 | Dekkera anomala V38 | Rhodotorula diarenensis J43 | |||
|---|---|---|---|---|---|---|
| VOC | Chemical Formula | Molar Mass (g/mol) | Area Ratio | |||
| 1-Butanol | C4H9OH | 74.12 | 0.011 | 0.009 | 0.042 | 0.017 |
| 1-Propanol | C3H7OH | 60.1 | 0.039 | 0.072 | 0.081 | 0.051 |
| 2-Phenylethanol | C8H9OH | 122.16 | 0.133 | 0.013 | 0.022 | 0.029 |
| 2,3-Butandiol | (CH3CHOH)2 | 90.121 | 0.054 | 0.059 | 0.082 | 0.143 |
| 3-Methylbutanol | C5H11OH | 88.15 | 0.138 | 0.149 | 0.189 | 0.459 |
| Acetic acid | CH3COOH | 60.05 | 0.154 | 0.080 | 0.156 | 0.164 |
| Butyric acid | C3H7COOH | 88.11 | 0.160 | 0.089 | 0.117 | 0.069 |
| Ethyl lactate | C5H10O3 | 118.13 | 0.000 | 0.000 | 0.014 | 0.000 |
| Isobutanol | C4H9OH | 74.12 | 0.007 | 0.007 | 0.007 | 0.007 |
| Isobutyric acid | (CH3)2CHCOOH | 88.11 | 0.028 | 0.020 | 0.132 | 0.057 |
| Isovaleric acid | (CH3)2CHCH2COOH | 102.13 | 0.144 | 0.053 | 0.171 | 0.168 |
| Propionic acid | CH 3CH 2CO 2H | 74.08 | 0.014 | 0.008 | 0.014 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkomonde, S.N.; du Plessis, H.W.; Bhagwat, P.; Amobonye, A.; Gomomo, Z.; Mewa-Ngongang, M.; Hoff, J.W.; Pillai, S. Biocontrol Potential of Selected Phyllospheric Yeasts Against Botrytis cinerea and Fusarium fujikuroi. Fermentation 2025, 11, 606. https://doi.org/10.3390/fermentation11110606
Nkomonde SN, du Plessis HW, Bhagwat P, Amobonye A, Gomomo Z, Mewa-Ngongang M, Hoff JW, Pillai S. Biocontrol Potential of Selected Phyllospheric Yeasts Against Botrytis cinerea and Fusarium fujikuroi. Fermentation. 2025; 11(11):606. https://doi.org/10.3390/fermentation11110606
Chicago/Turabian StyleNkomonde, Sibusisiwe Nobuhle, Heinrich Wilbur du Plessis, Prashant Bhagwat, Ayodeji Amobonye, Zukisani Gomomo, Maxwell Mewa-Ngongang, Justin Wallace Hoff, and Santhosh Pillai. 2025. "Biocontrol Potential of Selected Phyllospheric Yeasts Against Botrytis cinerea and Fusarium fujikuroi" Fermentation 11, no. 11: 606. https://doi.org/10.3390/fermentation11110606
APA StyleNkomonde, S. N., du Plessis, H. W., Bhagwat, P., Amobonye, A., Gomomo, Z., Mewa-Ngongang, M., Hoff, J. W., & Pillai, S. (2025). Biocontrol Potential of Selected Phyllospheric Yeasts Against Botrytis cinerea and Fusarium fujikuroi. Fermentation, 11(11), 606. https://doi.org/10.3390/fermentation11110606








