Isolation and Purification of Extracellular Inhibitory Products from Bacillus velezensis YJ0-1 and Optimization of Fermentation Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation, Identification and Antimicrobial Activity of Active Compounds
2.1.1. Microbial Strains
2.1.2. Purification and Identification of Active Substances
2.1.3. Antimicrobial Activity and Thermal Stability Assays
2.2. Medium Optimization
2.2.1. Single-Factor Medium Optimization
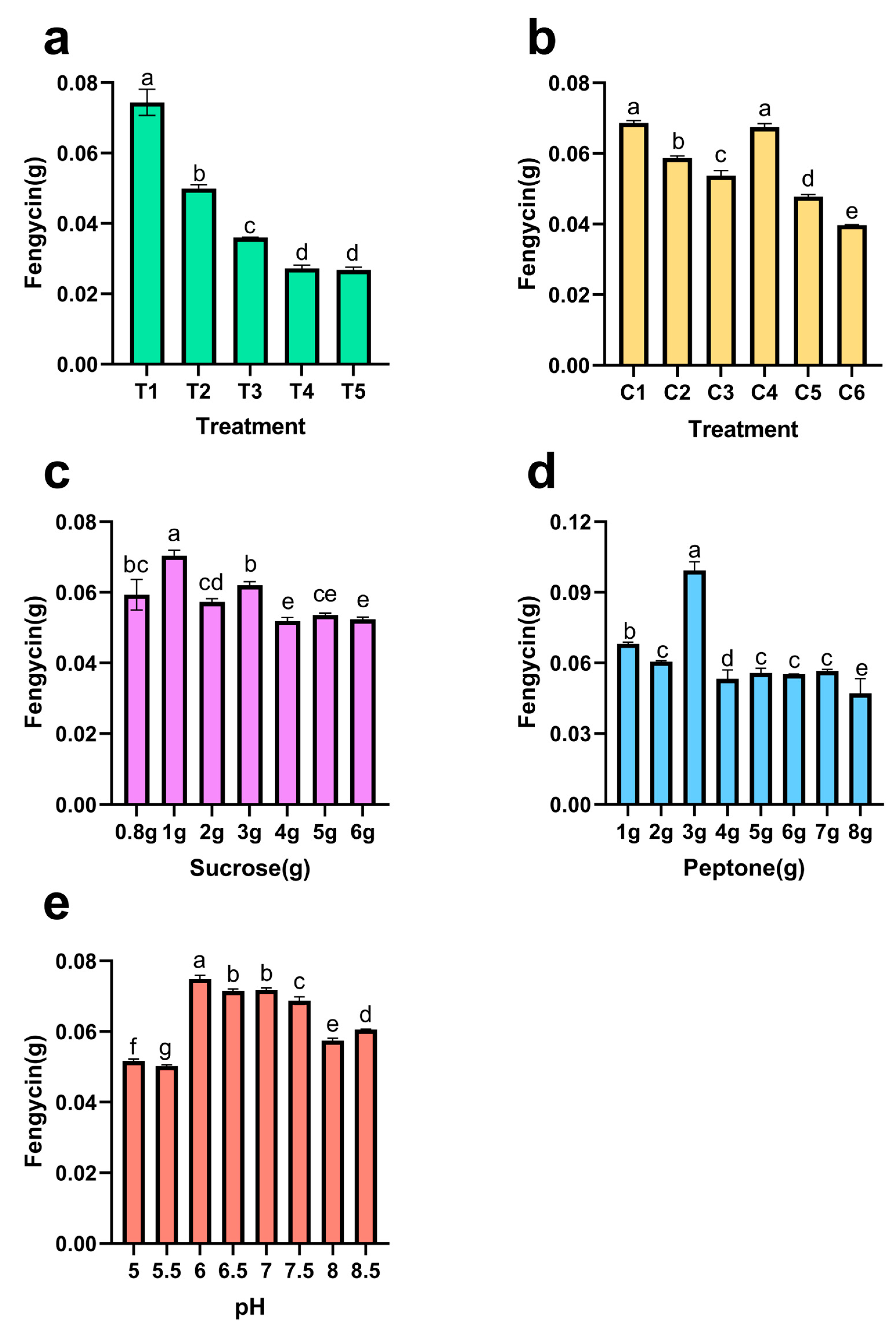
Screening of Supplemental Nitrogen Sources
Screening of Supplemental Carbon Sources
Optimization of Supplemental Sucrose Concentration
Optimization of Supplemental Peptone Concentration
Optimization of Initial pH
Experimental Design
2.2.2. Culture and Product Extraction
2.2.3. Standard Preparation and HPLC Analysis
2.2.4. Determination of the Bacterial Growth Curve of Bacillus velezensis YJ0-1
2.2.5. Optimization Using Response Surface Methodology
2.2.6. Validation Experiments
3. Results
3.1. Purification, Identification, and Antimicrobial Activity Analysis of Active Compounds
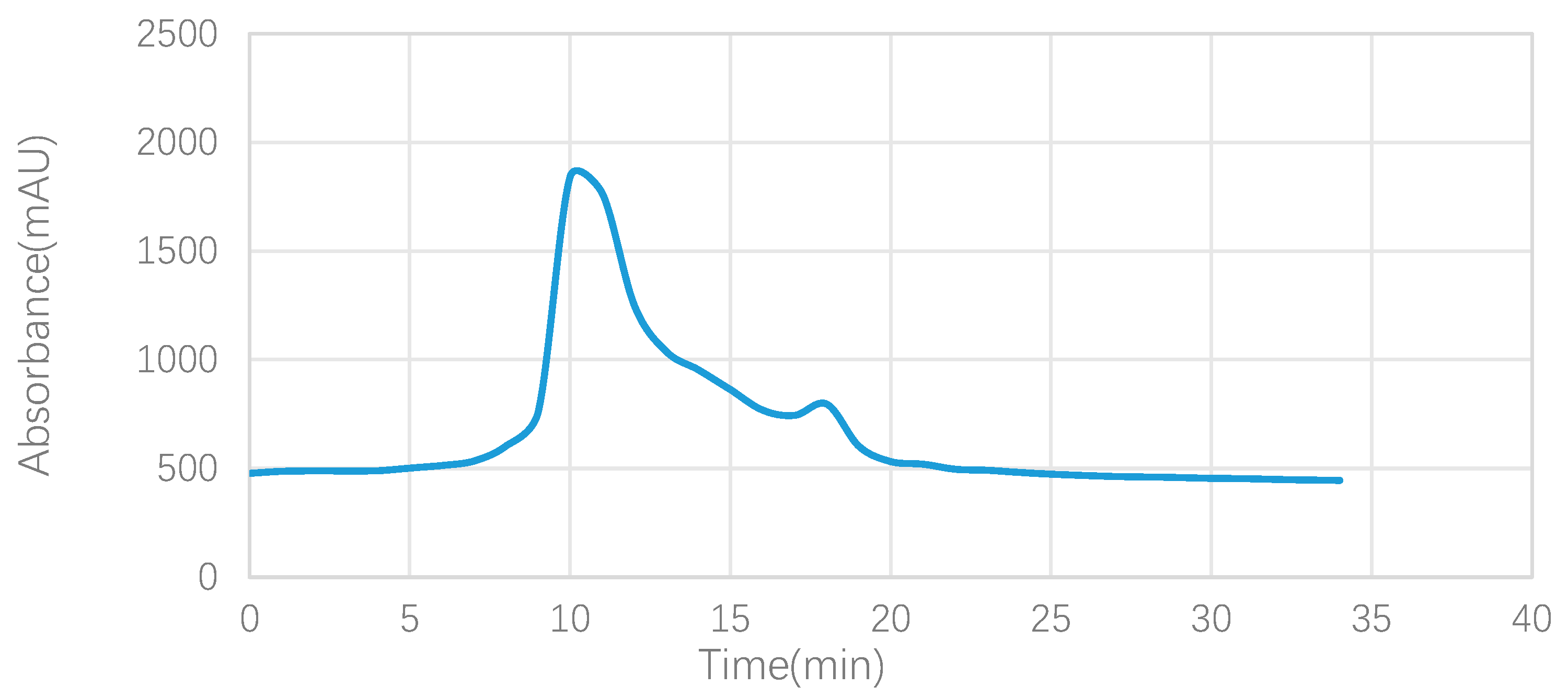
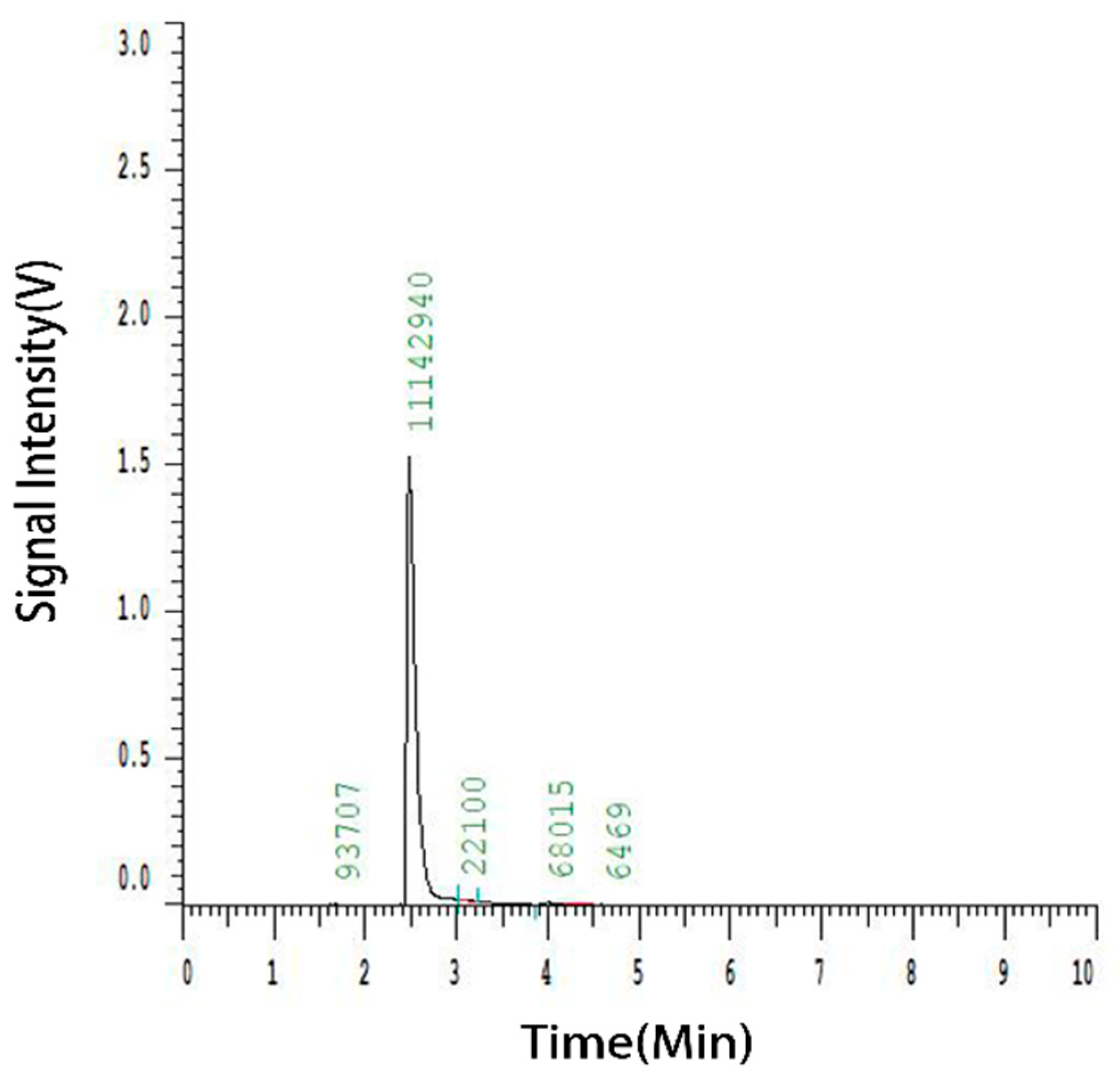
3.1.1. Analysis of the Elution Profile of the Crude Extract
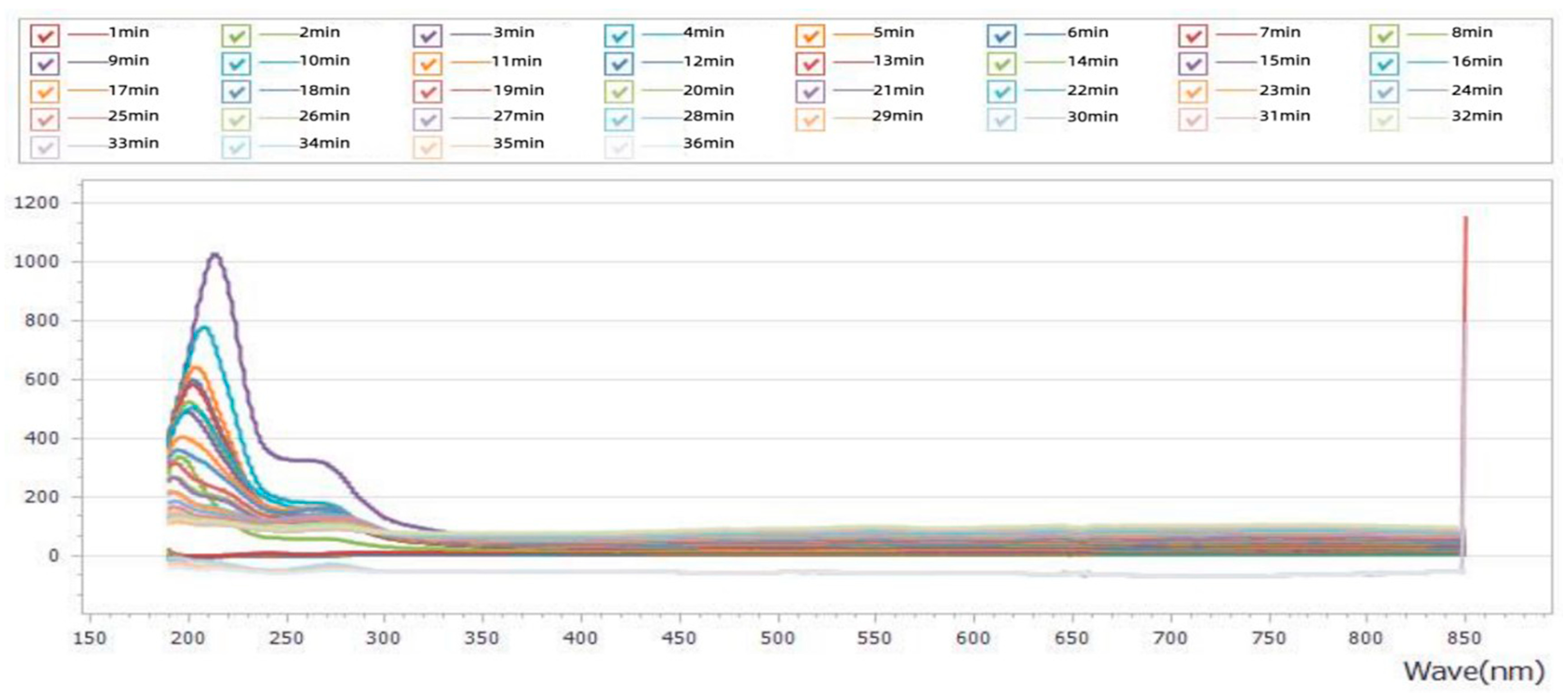
3.1.2. Full-Spectrum Analysis of Crude Extracts
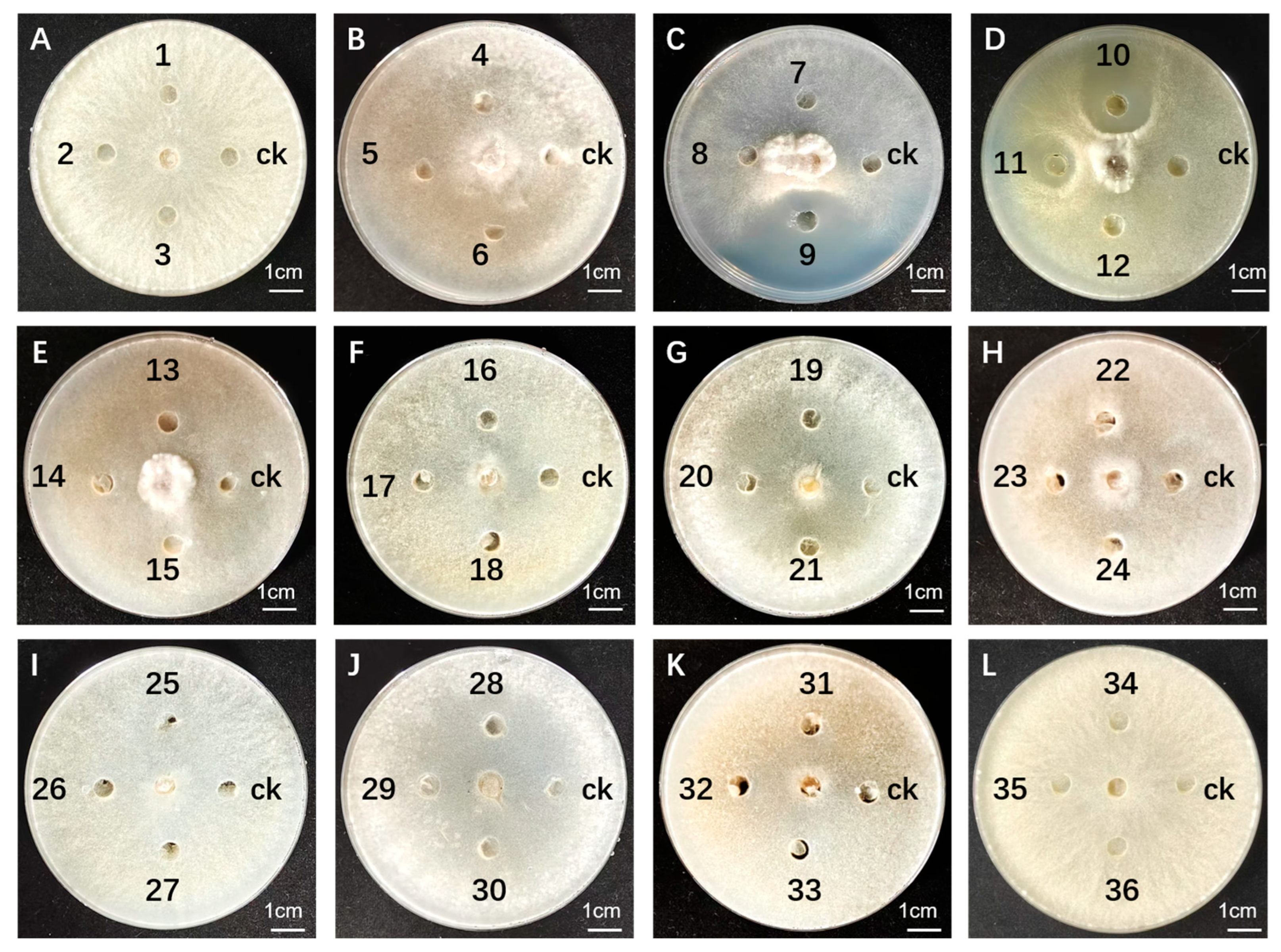
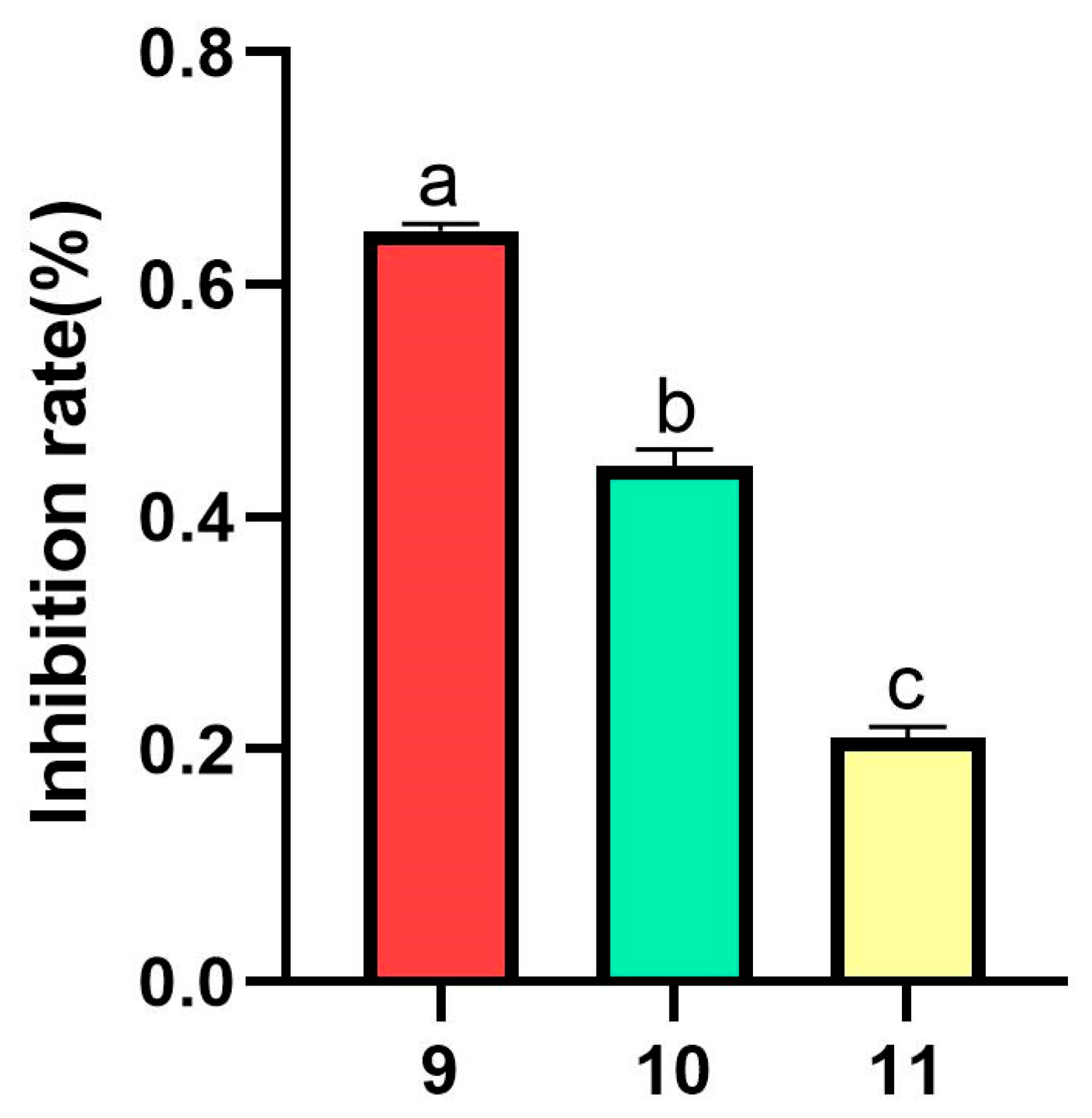
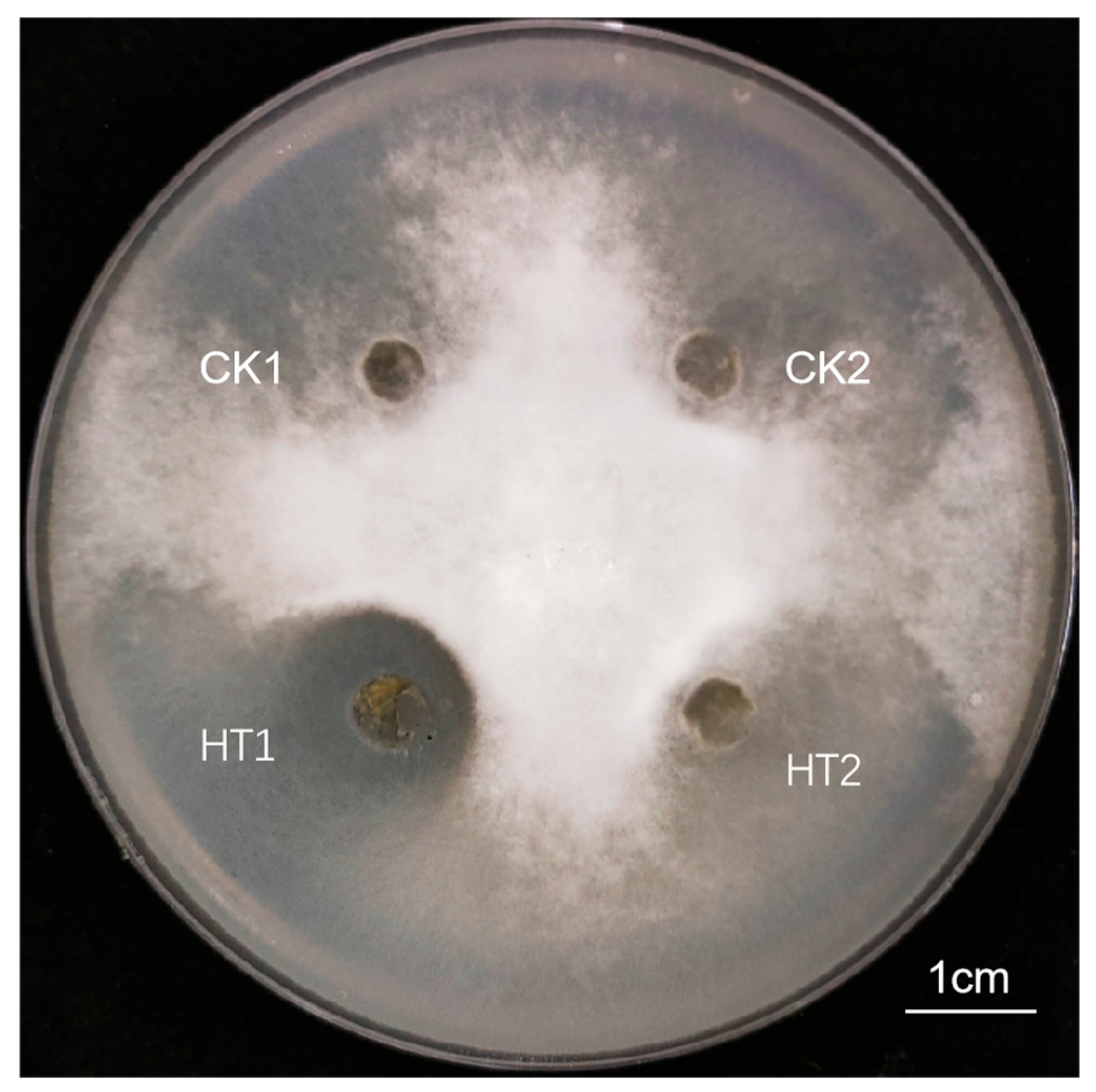
3.1.3. Antimicrobial Activity Assay
3.1.4. Optimization of Separation Conditions
3.1.5. Thermal Stability Analysis
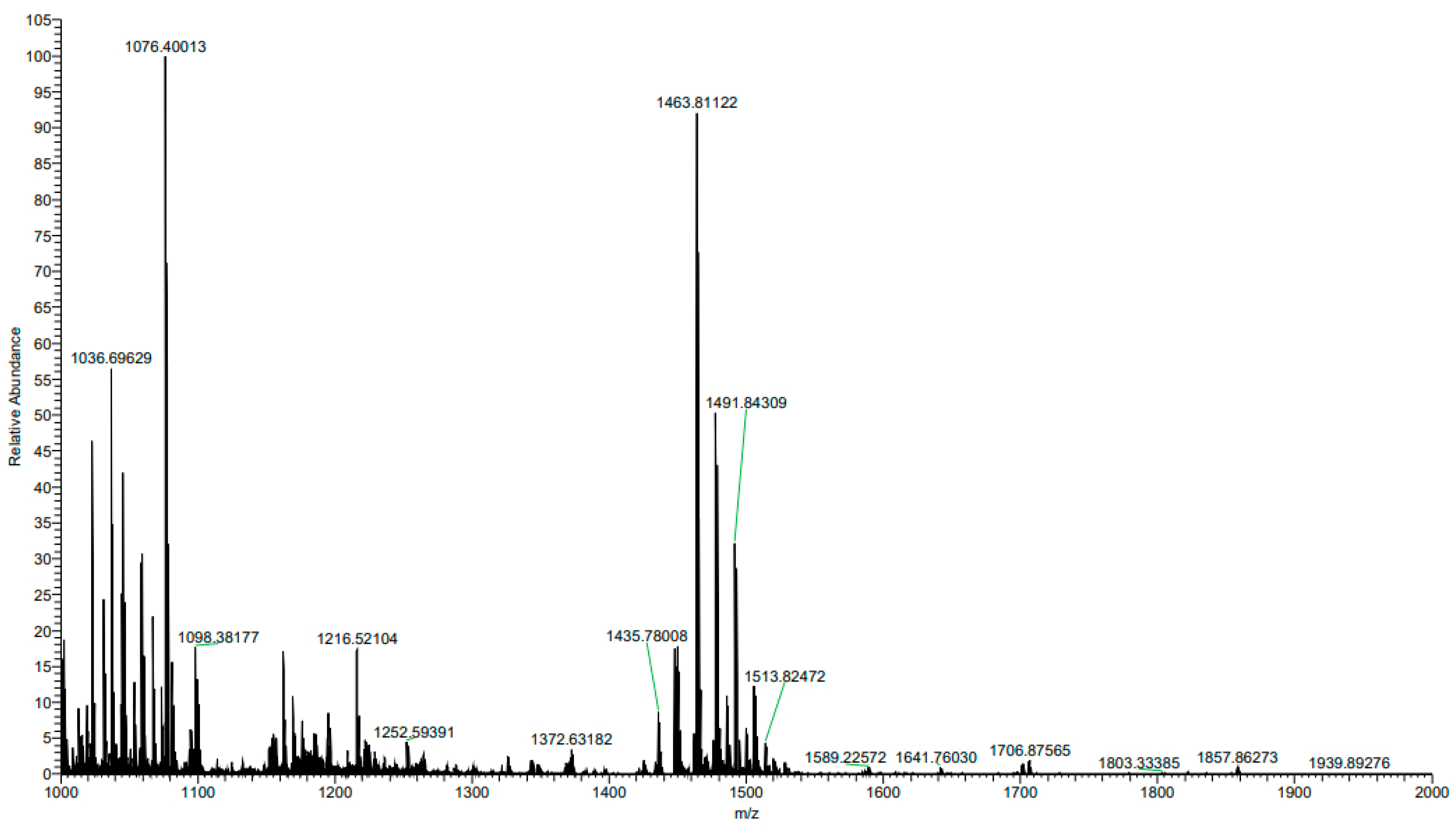
3.1.6. Mass Spectrometric Analysis of Purified Fraction
3.2. Medium Optimization
3.2.1. Standard Calibration Curve
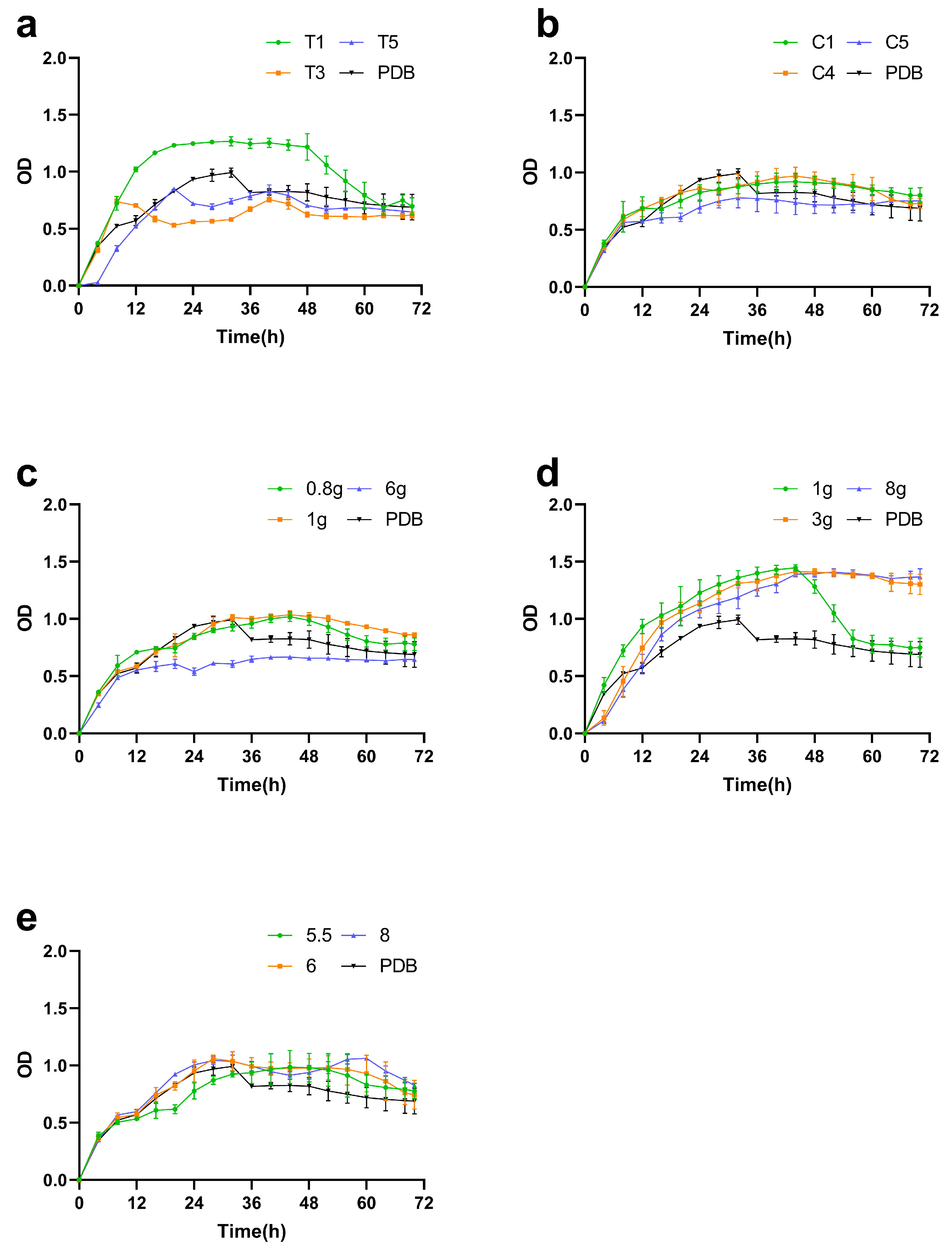
3.2.2. Single-Factor Analyses
Screening of Supplemental Nitrogen Sources
Screening of Supplemental Carbon Sources
Optimization of Supplemental Sucrose Concentration
Optimization of Supplemental Peptone Concentration
Optimization of Initial pH
3.2.3. Response Surface Methodology (RSM)
+ 0.022917 × AC + 0.006083 × BC − 0.010783 × A2 − 0.009021 × B2 − 0.025544 × C2
pH-Peptone Interaction
pH-Sucrose Interaction
Peptone-Sucrose Interaction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y. Progress in the Study of Pathogenic Factors of Sclerotinia sclerotiorum. Theor. Nat. Sci. 2024, 59, 138–145. [Google Scholar] [CrossRef]
- Willbur, J.; McCaghey, M.; Kabbage, M.; Smith, D.L. An Overview of the Sclerotinia sclerotiorum Pathosystem in Soybean: Impact, Fungal Biology, and Current Management Strategies. Trop. Plant Pathol. 2018, 44, 3–11. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Jedryczka, M. Inoculum and Inoculation Techniques: Key Steps in Studying Pathogenicity and Resistance to Sclerotinia Stem Rot in Oilseed Rape. Front. Plant Sci. 2025, 16, 1610049. [Google Scholar] [CrossRef] [PubMed]
- Sarenqimuge, S.; Koopmann, B.; von Tiedemann, A. Long-Term Survival of Sclerotinia sclerotiorum Sclerotia and Verticillium longisporum Microsclerotia in Soil and the Effects of Soil Depth, Soil Temperature, and a Biocontrol Agent. Appl. Soil Ecol. 2025, 210, 106101. [Google Scholar] [CrossRef]
- Ding, L.-N.; Li, T.; Guo, X.-J.; Li, M.; Liu, X.-Y.; Cao, J.; Tan, X.-L. Sclerotinia Stem Rot Resistance in Rapeseed: Recent Progress and Future Prospects. J. Agric. Food Chem. 2021, 69, 2965–2978. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Yang, Q.; Wei, D.; Yang, L.; Zhang, X.-L.; Chen, X.-L. Research on Destructive of Sclerotinia sclerotiorum to Host Soybean in Black Soil Area of Northeast China. Soybean Sci. 2008, 27, 633–636. [Google Scholar]
- Antwi-Boasiako, A.; Zheng, L.; Begum, N.; Amoah, S.; Zhao, T. Progress towards Germplasm Evaluation and Genetic Improvement for Resistance to Sclerotinia White Mold in Soybean. Euphytica 2021, 217, 178. [Google Scholar] [CrossRef]
- Zhu, G.-Y.; Shi, X.-C.; Wang, S.-Y.; Wang, B.; Laborda, P. Antifungal Mechanism and Efficacy of Kojic Acid for the Control of Sclerotinia sclerotiorum in Soybean. Front. Plant Sci. 2022, 13, 845698. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-F.; Wang, C.-L.; Hu, Y.-F.; Wen, Y.-C.; Sun, Z.-B. Biocontrol of Three Severe Diseases in Soybean. Agriculture 2022, 12, 1391. [Google Scholar] [CrossRef]
- Morais, E.M.; Silva, A.A.R.; de Sousa, F.W.A.; de Azevedo, I.M.B.; Silva, H.F.; Santos, A.M.G.; Beserra Júnior, J.E.A.; de Carvalho, C.P.; Eberlin, M.N.; Porcari, A.M.; et al. Endophytic Trichoderma Strains Isolated from Forest Species of the Cerrado-Caatinga Ecotone Are Potential Biocontrol Agents against Crop Pathogenic Fungi. PLoS ONE 2022, 17, e0265824. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Sautua, F.; Scandiani, M.; Carmona, M.; Asurmendi, S. Current Recommendations and Novel Strategies for Sustainable Management of Soybean Sudden Death Syndrome. Pest Manag. Sci. 2021, 77, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Sumida, C.H.; Canteri, M.G.; Peitl, D.C.; Tibolla, F.; Orsini, I.P.; Araújo, F.A.; Chagas, D.F.; Calvos, N.S. Chemical and Biological Control of Sclerotinia Stem Rot in the Soybean Crop. Cienc. Rural. 2015, 45, 760–766. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Belt, K.; Thatcher, L.F. Tackling Control of a Cosmopolitan Phytopathogen: Sclerotinia. Front. Plant Sci. 2021, 12, 707509. [Google Scholar] [CrossRef]
- Dickman, M. Approaches for Improving Crop Resistance to Soilborne Fungal Diseases through Biotechnology Using Sclerotinia sclerotiorum as a Case Study. Australas. Plant Pathol. 2007, 36, 116–123. [Google Scholar] [CrossRef]
- Saharan, G.S.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural Functions of Lipopeptides from Bacillus and Pseudomonas: More than Surfactants and Antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Valenzuela Ruiz, V.; Gándara-Ledezma, A.; Villarreal-Delgado, M.F.; Villa-Rodríguez, E.D.; Parra-Cota, F.I.; Santoyo, G.; Gómez-Godínez, L.J.; Cira Chávez, L.A.; de los Santos-Villalobos, S. Regulation, Biosynthesis, and Extraction of Bacillus-Derived Lipopeptides and Its Implications in Biological Control of Phytopathogens. Stresses 2024, 4, 107–132. [Google Scholar] [CrossRef]
- Yuan, Q.-S.; Yang, P.; Liu, Y.-K.; Tabl, K.M.; Guo, M.-W.; Zhang, J.-B.; Wu, A.-B.; Liao, Y.-C.; Huang, T.; He, W.-J. Iturin and Fengycin Lipopeptides Inhibit Pathogenic Fusarium by Targeting Multiple Components of the Cell Membrane and Their Regulative Effects in Wheat. J. Integr. Plant Biol. 2025, 67, 2184–2197. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, T.; Li, S. β-1,3-1,4-Glucanase Gene from Bacillus velezensis ZJ20 Exerts Antifungal Effect on Plant Pathogenic Fungi. World J. Microbiol. Biotechnol. 2016, 32, 26. [Google Scholar] [CrossRef]
- Tyagi, A.; Lama Tamang, T.; Kashtoh, H.; Mir, R.A.; Mir, Z.A.; Manzoor, S.; Manzar, N.; Gani, G.; Vishwakarma, S.K.; Almalki, M.A.; et al. A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae 2024, 10, 805. [Google Scholar] [CrossRef]
- Tsvetkov, V.; Yarullina, L.; Sorokan, A.; Khabibullina, V.; Mardanshin, I. Activity of Hydrolases and Their Inhibitors in Potato Plants Treated with Bacillus subtilis, Salicylic, and Jasmonic Acids and Affected by the Combined Effect of the Late Blight and the Lack of Moisture. Int. J. Plant Biol. 2023, 14, 329–338. [Google Scholar] [CrossRef]
- Gao, X. Mechanism Analysis of Bacillus Velezensis YJ0-1 Inhibiting Sclerotinia sclerotiorum. Master’s Thesis, Jilin Normal University, Siping, China, 2023. [Google Scholar]
- Wei, Y.-H.; Wang, L.-C.; Chen, W.-C.; Chen, S.-Y. Production and Characterization of Fengycin by Indigenous Bacillus Subtilis F29-3 Originating from a Potato Farm. Int. J. Mol. Sci. 2010, 11, 4526–4538. [Google Scholar] [CrossRef]
- Roy, A.; Mahata, D.; Paul, D.; Korpole, S.; Franco, O.L.; Mandal, S.M. Purification, Biochemical Characterization and Self-Assembled Structure of a Fengycin-like Antifungal Peptide from Bacillus thuringiensis Strain SM1. Front. Microbiol. 2013, 4, 66994. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, H.; Zhang, C.; Zhang, Y.; Sun, H.; Yu, L.; Zhang, Q.; Chen, X.; Xiao, H. Recent Advances in Antimicrobial Lipopeptide Fengycin Secreted by Bacillus: Structure, Biosynthesis, Antifungal Mechanisms, and Potential Application in Food Preservation. Food Chem. 2025, 489, 144937. [Google Scholar] [CrossRef]
- Medeot, D.; Sannazzaro, A.; Estrella, M.J.; Torres Tejerizo, G.; Contreras-Moreira, B.; Pistorio, M.; Jofré, E. Unraveling the Genome of Bacillus velezensis MEP218, a Strain Producing Fengycin Homologs with Broad Antibacterial Activity: Comprehensive Comparative Genome Analysis. Sci. Rep. 2023, 13, 22168. [Google Scholar] [CrossRef] [PubMed]
- Ait Kaki, A.; Smargiasso, N.; Ongena, M.; Kara Ali, M.; Moula, N.; De Pauw, E.; Kacem Chaouche, N. Characterization of New Fengycin Cyclic Lipopeptide Variants Produced by Bacillus Amyloliquefaciens (ET) Originating from a Salt Lake of Eastern Algeria. Curr. Microbiol. 2020, 77, 443–451. [Google Scholar] [CrossRef]
- Fan, H.; Ru, J.; Zhang, Y.; Wang, Q.; Li, Y. Fengycin Produced by Bacillus subtilis 9407 Plays a Major Role in the Biocontrol of Apple Ring Rot Disease. Microbiol. Res. 2017, 199, 89–97. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, Z.; Chen, Y.; Wang, J.; Xiao, R.; Wang, X.; Liu, B.; Chen, M.; He, J. Lipopeptide C17 Fengycin B Exhibits a Novel Antifungal Mechanism by Triggering Metacaspase-Dependent Apoptosis in Fusarium oxysporum. J. Agric. Food Chem. 2024, 72, 7943–7953. [Google Scholar] [CrossRef]
- da Silva Junior, A.L.; Borges, Á.V.; da Silva, H.A.O.; Leite, I.C.H.L.; Alves, K.S.; de Medeiros, L.S.; Abreu, L.M. de Lipopeptide-Enriched Extracts of Bacillus Velezensis B157 for Controlling Tomato Early Blight. Crop Prot. 2023, 172, 106317. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Zhao, X.; Ma, G.; Liu, N.; Zheng, Y.; Tan, J.; Qi, G. Systemically Engineering Bacillus amyloliquefaciens for Increasing Its Antifungal Activity and Green Antifungal Lipopeptides Production. Front. Bioeng. Biotechnol. 2022, 10, 961535. [Google Scholar] [CrossRef]
- Tan, W.; Yin, Y.; Wen, J. Increasing Fengycin Production by Strengthening the Fatty Acid Synthesis Pathway and Optimizing Fermentation Conditions. Biochem. Eng. J. 2022, 177, 108235. [Google Scholar] [CrossRef]
- Wang, D.-J.; Ding, M.-Z.; Hou, Z.-J.; Zhang, Y.; Shang, W.; Duan, T.-X.; Xu, Q.-M.; Cheng, J.-S. Carbon Metabolism Modification in Bacillus subtilis for Improving Fengycin Production and Investigating Antifungal Activity of Its Homologous Components. ACS Synth. Biol. 2025, 14, 2644–2656. [Google Scholar] [CrossRef]
- Lu, H.; Xu, H.; Yang, P.; Bilal, M.; Zhu, S.; Zhong, M.; Zhao, L.; Gu, C.; Liu, S.; Zhao, Y.; et al. Transcriptome Analysis of Bacillus amyloliquefaciens Reveals Fructose Addition Effects on Fengycin Synthesis. Genes 2022, 13, 984. [Google Scholar] [CrossRef]
- Pererva, P.; Kobielieva, T.; Keršys, R.; Nagy, S.; Kosenko, O.; Tkachova, N.; Marchuk, L.; Kosenko, A.; Tkachov, M.; Pohorielov, S. Development of Methods for Forming the Cost of Production and Assessment of Its Impact on the Efficiency of an Industrial Enterprise. Technol. Audit. Prod. Reserves 2025, 3, 26–33. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, H.; Wei, H.; Zhang, D.; Yao, S.; Hao, H.; Bie, X. Current State in Fermentation, Isolation, and Purification of Lipopeptide Antibiotics from Bacillus sp. Chin. J. Biotechnol. 2011, 31, 114–122. [Google Scholar]
- Qiu, C.; Bao, Y.; Lü, D.; Yan, M.; Li, G.; Liu, K.; Wei, S.; Wu, M.; Li, Z. The Synergistic Effects of Humic Acid, Chitosan and Bacillus Subtilis on Tomato Growth and against Plant Diseases. Front. Microbiol. 2025, 16, 1574765. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Hu, J.; Cai, K.; Jing, W.; Liu, M.; Liang, W. The Intelligent Infectious Disease Active Surveillance and Early Warning System in China: An Application of Dengue Prevention and Control. Glob. Transit. 2024, 6, 249–255. [Google Scholar] [CrossRef]
- Tiwari, A.K. IPM Essentials: Combining Biology, Ecology, and Agriculture for Sustainable Pest Control. J. Adv. Biol. Biotechnol. 2024, 27, 39–47. [Google Scholar] [CrossRef]


| Factors | Variable and Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| pH | 5.5 | 6 | 6.5 |
| Peptone | 2 | 3 | 4 |
| Sucrose | 0.8 | 1.4 | 2 |
| Std | Run | A: pH | B: Peptone (g) | C: Sucrose (g) | Response (g) |
|---|---|---|---|---|---|
| 1 | 15 | 5.5 | 2 | 1.4 | 0.0919 |
| 2 | 9 | 6.5 | 2 | 1.4 | 0.0866 |
| 3 | 13 | 5.5 | 4 | 1.4 | 0.093 |
| 4 | 2 | 6.5 | 4 | 1.4 | 0.0939 |
| 5 | 10 | 5.5 | 3 | 0.8 | 0.0977 |
| 6 | 7 | 6.5 | 3 | 0.8 | 0.0856 |
| 7 | 5 | 5.5 | 3 | 2 | 0.083 |
| 8 | 4 | 6.5 | 3 | 2 | 0.0984 |
| 9 | 6 | 6 | 2 | 0.8 | 0.0859 |
| 10 | 11 | 6 | 4 | 0.8 | 0.0865 |
| 11 | 8 | 6 | 2 | 2 | 0.0759 |
| 12 | 14 | 6 | 4 | 2 | 0.0911 |
| 13 | 12 | 6 | 3 | 1.4 | 0.1057 |
| 14 | 1 | 6 | 3 | 1.4 | 0.1064 |
| 15 | 3 | 6 | 3 | 1.4 | 0.0971 |
| Std. Dev. | Mean | R2 | Adjusted R2 | Predicted R2 | C.V % | Adeq Precision |
|---|---|---|---|---|---|---|
| 0.0037 | 0.0919 | 0.929 | 0.8011 | 0.6181 | 4.05 | 8.4807 |
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 0.1031 | 1 | 0.0022 | 0.0975 | 0.1086 | |
| A-pH | −0.0001 | 1 | 0.0013 | −0.0035 | 0.0032 | 1.0000 |
| B-Peptone | 0.0030 | 1 | 0.0013 | −0.0004 | 0.0064 | 1.0000 |
| C-Sucrose | −0.0009 | 1 | 0.0013 | −0.0043 | 0.0025 | 1.0000 |
| AB | 0.0016 | 1 | 0.0019 | −0.0032 | 0.0063 | 1.0000 |
| AC | 0.0069 | 1 | 0.0019 | 0.0021 | 0.0117 | 1.0000 |
| BC | 0.0037 | 1 | 0.0019 | −0.0011 | 0.0084 | 1.0000 |
| A2 | −0.0027 | 1 | 0.0019 | −0.0077 | 0.0023 | 1.01 |
| B2 | −0.0090 | 1 | 0.0019 | −0.0140 | −0.0040 | 1.01 |
| C2 | −0.0092 | 1 | 0.0019 | −0.0142 | −0.0042 | 1.01 |
| Source | Sun of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.0009 | 9 | 0.0001 | 7.27 | 0.0209 | significant |
| A-pH | 1.512 × 10−7 | 1 | 1.512 × 10−7 | 0.0109 | 0.9209 | |
| B-Peptone | 0.0001 | 1 | 0.0001 | 5.27 | 0.0701 | |
| C-Sucrose | 6.661 × 10−6 | 1 | 6.661 × 10−6 | 0.4797 | 0.5194 | |
| AB | 9.610 × 10−6 | 1 | 9.610 × 10−6 | 0.6920 | 0.4434 | |
| AC | 0.0002 | 1 | 0.0002 | 13.61 | 0.0141 | |
| BC | 0.0001 | 1 | 0.0001 | 3.84 | 0.1074 | |
| A2 | 0.0000 | 1 | 0.0000 | 1.93 | 0.2232 | |
| B2 | 0.0003 | 1 | 0.0003 | 21.64 | 0.0056 | |
| C2 | 0.0003 | 1 | 0.0003 | 22.48 | 0.0051 | |
| Residual | 0.0001 | 5 | 0.0000 | |||
| Lack of Fit | 0.0000 | 3 | 5.263 × 10−6 | 0.1962 | 0.8916 | not significant |
| Pure Error | 0.0001 | 2 | 0.0000 | |||
| Cor Total | 0.0010 | 14 |
| Number | pH | Peptone (g) | Sucrose (g) | Extract (g) | Desirability | |
|---|---|---|---|---|---|---|
| 1 | 6.500 | 3.331 | 1.634 | 0.102 | 0.928 | Selected |
| 2 | 6.500 | 3.340 | 1.635 | 0.102 | 0.928 | |
| 3 | 6.500 | 3.339 | 1.631 | 0.102 | 0.928 | |
| 4 | 6.500 | 3.325 | 1.638 | 0.102 | 0.928 | |
| 5 | 6.500 | 3.332 | 1.641 | 0.102 | 0.928 | |
| 6 | 6.500 | 3.316 | 1.626 | 0.102 | 0.928 | |
| 7 | 6.500 | 3.345 | 1.645 | 0.102 | 0.928 | |
| 8 | 6.500 | 3.354 | 1.643 | 0.102 | 0.928 | |
| 9 | 6.500 | 3.310 | 1.637 | 0.102 | 0.928 | |
| 10 | 6.500 | 3.353 | 1.619 | 0.102 | 0.928 | |
| 11 | 6.500 | 3.293 | 1.625 | 0.102 | 0.928 | |
| 12 | 6.500 | 3.344 | 1.668 | 0.102 | 0.927 | |
| 13 | 6.500 | 3.390 | 1.641 | 0.102 | 0.927 | |
| 14 | 6.500 | 3.392 | 1.617 | 0.102 | 0.927 | |
| 15 | 6.500 | 3.276 | 1.663 | 0.102 | 0.927 | |
| 16 | 6.500 | 3.288 | 1.670 | 0.102 | 0.927 | |
| 17 | 6.500 | 3.319 | 1.681 | 0.102 | 0.927 | |
| 18 | 6.500 | 3.231 | 1.614 | 0.102 | 0.926 | |
| 19 | 6.500 | 3.273 | 1.562 | 0.102 | 0.925 | |
| 20 | 6.500 | 3.479 | 1.787 | 0.102 | 0.916 | |
| 21 | 6.500 | 3.552 | 1.797 | 0.101 | 0.912 | |
| 22 | 6.500 | 2.912 | 1.510 | 0.100 | 0.898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Yang, S.; Li, Y.; Deng, Y. Isolation and Purification of Extracellular Inhibitory Products from Bacillus velezensis YJ0-1 and Optimization of Fermentation Medium. Fermentation 2025, 11, 595. https://doi.org/10.3390/fermentation11100595
Zou X, Yang S, Li Y, Deng Y. Isolation and Purification of Extracellular Inhibitory Products from Bacillus velezensis YJ0-1 and Optimization of Fermentation Medium. Fermentation. 2025; 11(10):595. https://doi.org/10.3390/fermentation11100595
Chicago/Turabian StyleZou, Xinqi, Siqi Yang, Yuqing Li, and Yijie Deng. 2025. "Isolation and Purification of Extracellular Inhibitory Products from Bacillus velezensis YJ0-1 and Optimization of Fermentation Medium" Fermentation 11, no. 10: 595. https://doi.org/10.3390/fermentation11100595
APA StyleZou, X., Yang, S., Li, Y., & Deng, Y. (2025). Isolation and Purification of Extracellular Inhibitory Products from Bacillus velezensis YJ0-1 and Optimization of Fermentation Medium. Fermentation, 11(10), 595. https://doi.org/10.3390/fermentation11100595




