4. Discussion
Effects of protein concentration in rumen fluid on rumen tympanism. Under high-concentrate feeding conditions, goats’ rumens produce substantial amounts of stable foam [
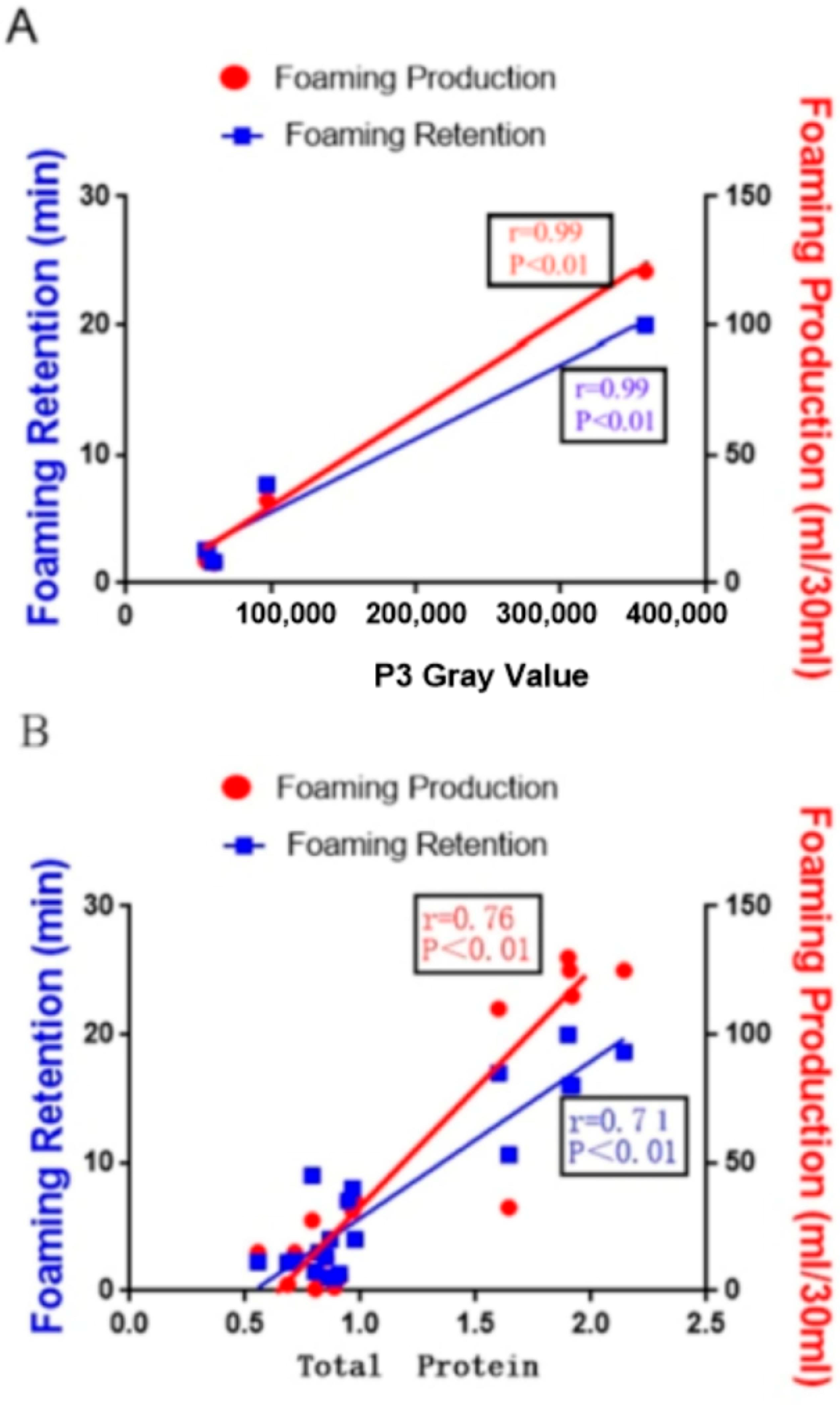
19]. Trial 1 demonstrated a significant positive correlation between the total protein concentration in rumen fluid and its foaming properties (
Table 5). As the protein content in rumen fluid increased, foam production per unit volume also rose, but foam stability may not always improve. Protein acts as a surfactant, significantly influencing the liquid’s foaming properties when dissolved [
20]. Rumen microorganisms ferment feed, producing gas that can become trapped in rumen contents, forming bubbles that may accumulate into foam [
6]. During the ascent, hydrolyzed protein peptides unfold at the liquid surface, facilitating intermolecular forces that create a viscoelastic film adhering to bubble edges. The hydrophobic side stabilizes the gas, while the hydrophilic side stabilizes the aqueous phase [
21,
22], reducing surface tension and enhancing foam stability by preventing bubble collapse and rupture [
23].
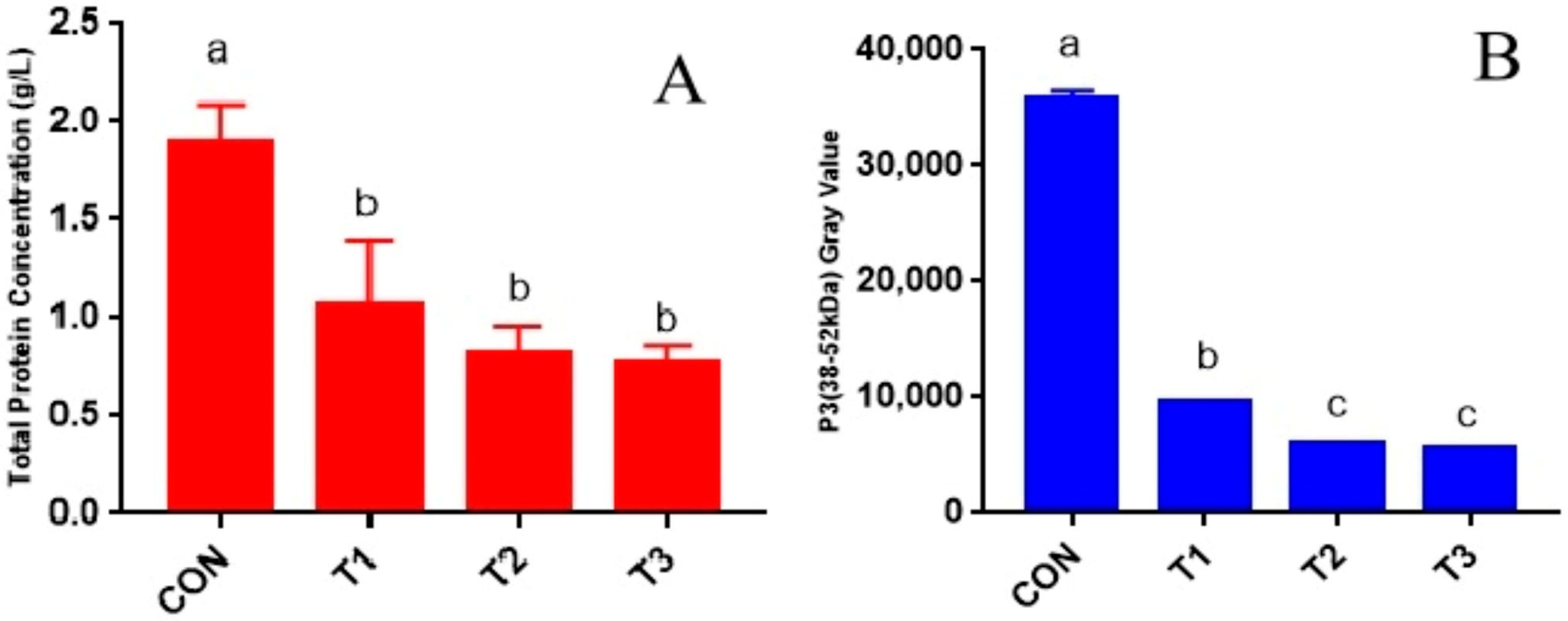
Furthermore, protein hydrolysis may induce peptide chain cross-linking and increase viscosity, further contributing to foam stabilization. This phenomenon elucidates the observed positive correlation between protein concentration in rumen fluid and foam stability identified in this study. To our knowledge, this is the first report on the impact of rumen fluid’s protein concentration on its foaming performance. Research on beer foam has shown that barley hordein, poorly soluble in water, forms soluble proteins after microbial hydrolysis, significantly improving beer foam’s performance [
24]. However, not all proteins enhance liquid foaming [
25]. This study found that only a protein component with a molecular weight of 38–52 kDa positively correlates with rumen fluid’s foaming power and bubble retention (
Table 5). Previous beer foam studies identified a 40 kDa protein, Protein Z, as a key factor affecting beer foam volume and stability, termed “beer foam protein” [
26,
27]. It binds with sugar groups during wort boiling, significantly enhancing beer foam stability [
28,
29]. Since the rumen of ruminants lacks a secretory function, it is postulated that the proteins forming foam in the rumen primarily originate from dietary and microbial sources. Most proteins in ruminant feed ingredients, including gluten and prolamins in corn, wheat, and rice, as well as globulins in soybeans and cottonseeds, are largely insoluble in water and possess ordered structures [
30]. Consequently, proteins in natural grain feeds exhibit poor foaming properties. However, microbial fermentation in the rumen alters feed proteins, increasing their hydrophobicity and potentially enhancing foaming ability. Additionally, hydrolysis may increase peptide chain cross-linking and lamellar viscosity, thereby improving foam stability. Rumen microorganisms are capable of synthesizing substantial amounts of secretory proteins. If ruminal foam formation is associated with microbial secretory proteins and high-concentrate diets are the primary inducer of ruminal foam, it can be inferred that such diets may modify the structure and composition of rumen microorganisms, affecting the type or quantity of secretory proteins produced and ultimately inducing ruminal foam formation [
31]. Therefore, the amino acid composition and spatial structure of the 38–52 kDa protein component in rumen fluid remain unclear, and its origin—whether from feed or de novo synthesis by rumen microorganisms—requires further investigation. On the other hand, studies [
32] have demonstrated that polysaccharides in rumen fluid can bind to proteins via electrostatic interactions, forming protein–polysaccharide complexes. These complexes, stabilized by hydrophobic forces as well as electrostatic interactions, contribute to maintaining foam stability.
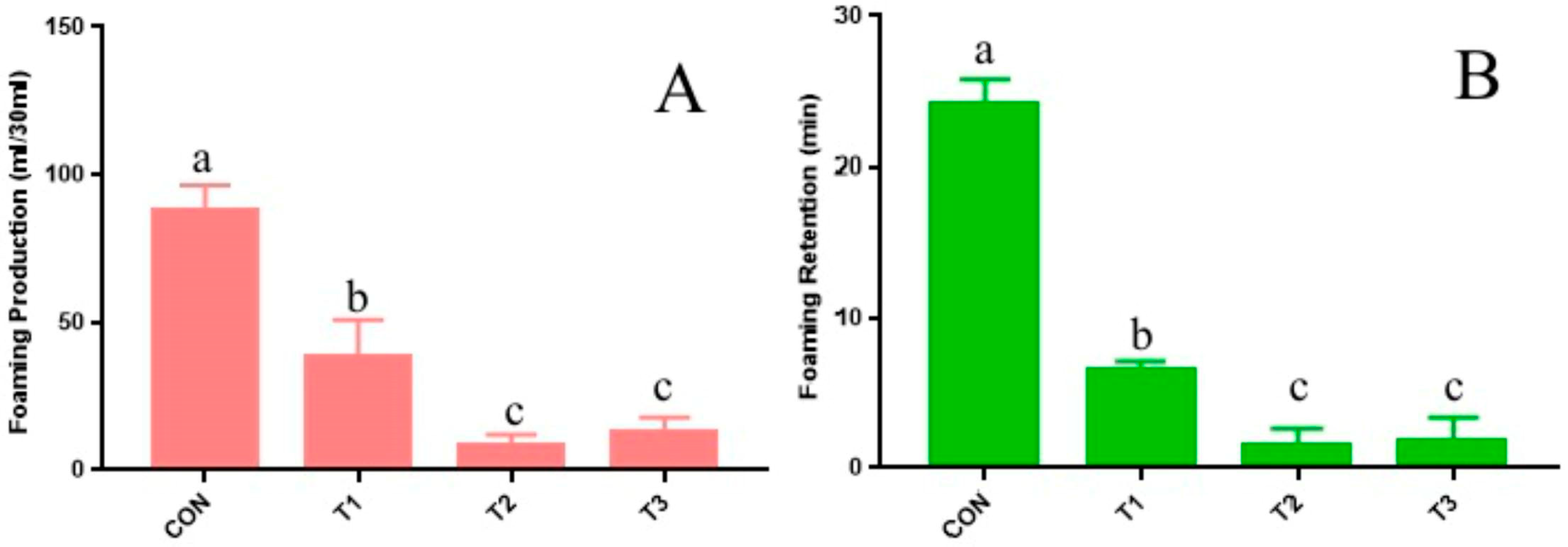
Effects of CT on rumen tympanism. The results of Trial 1 reveal that adding CT significantly diminishes the foaming power and bubble retention of rumen fluid (
Figure 2). It is important to note that CT itself does not function as an anti-foaming agent; rather, the reduction in foaming performance can be attributed to its interaction with proteins, leading to the formation of insoluble substances that lower protein concentrations in rumen fluid (
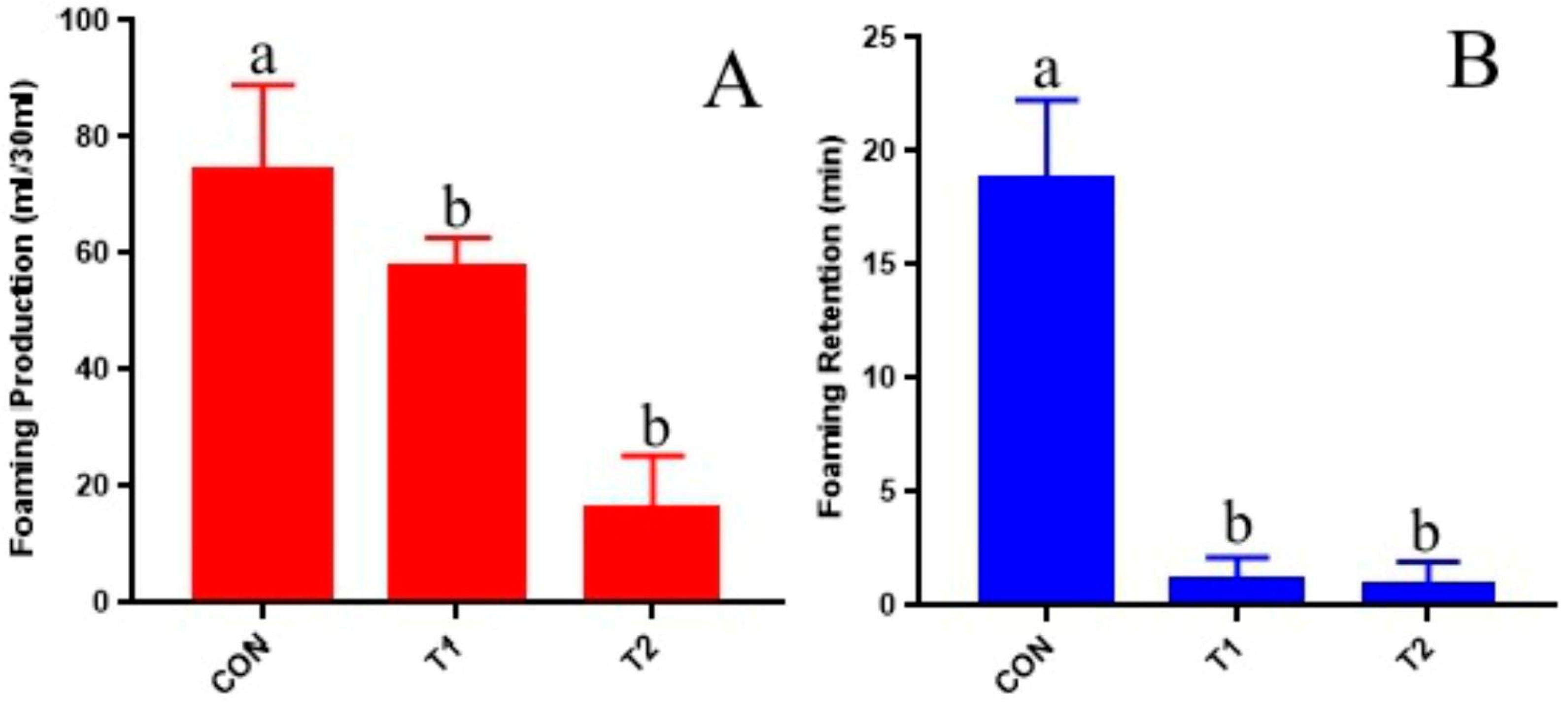
Figure 1). These in vitro findings suggest that CT may alleviate the risk of ruminal tympanism by binding to soluble proteins. Subsequent animal trials confirmed this hypothesis. Specifically, Trial 2 demonstrated that, compared to the CON group, goats in the 1% CT and 2% CT groups exhibited a significant reduction in the incidence and severity of ruminal tympanism (
Table 6). Furthermore, the foaming power and bubble retention of rumen fluid in the 2% CT group were significantly decreased (
Figure 4), indicating that an appropriate dosage of CT incorporated into a high-concentrate diet effectively prevents ruminal tympanism. The phenolic hydroxyl groups and aromatic ring structures present in CT exhibit a strong binding affinity for protein carbonyl groups [
33], facilitating the formation of stable tannin–protein complexes through surface adsorption [
34]. These complexes are insoluble and resistant to microbial degradation within the typical rumen pH range (5.0–7.0) [
11,
35], maintaining stability in the ruminal environment. Previous studies have shown that CT efficacy in ruminants is influenced by various factors, including dosage, source, animal species, microorganism, and basal diet composition [
34,
36]. In Trial 1, it was observed that 3% CT did not further decrease the foaming properties of rumen fluid. This may be due to the fact that proteins with a molecular weight of 38–52 kDa are crucial in determining these properties, and CT reduces them by binding to these proteins. However, at higher CT concentrations, the available proteins within this molecular weight range in the rumen fluid may already be fully saturated, thereby preventing any further reduction in foam characteristics from additional CT. The current study preliminarily explores the feasibility of adding CT to HCDs to prevent ruminal tympanism and finds that 2% CT effectively prevents ruminal tympanism in goats. Further research is needed to investigate the impact of other additives and nutrient levels on CT efficacy.
Effects of CT on rumen fermentation and nutrient digestibility in goats. The results of Trial 2 indicate that adding 1% CT and 2% CT to HCDs had no significant impact on the digestibility of nutrients such as DM, CP, NDF, and ADF in goat diets. This finding is consistent with previous research by Xiaofeng [
37], where the addition of 3% poplar CT did not alter the digestibility of DM, NDF, and ADF in dairy cattle diets. However, contrasting studies have shown that the inclusion of argan tannins in Holstein cow diets significantly reduced the apparent digestibility of fiber [
38]. This inconsistency may stem from the varying doses of CT used in different trials. High doses of CT in the diet can disrupt rumen fermentation, thereby affecting the digestibility of fiber and other nutrients. The CT utilized in this study originated from bayberry extract, suggesting that bayberry CT at concentrations of 1% and 2% has no effect on nutrient digestibility in goats.
The stability of the rumen environment can be visually evaluated by measuring the pH value of rumen fluid. A fermentation environment is deemed more stable when the pH ranges between 6.2 and 7.0 [
39]. In our animal experiment, all groups exhibited a normal pH range for rumen fluid, yet the CON group displayed a significantly lower pH compared to both the 1% CT and 2% CT groups. Research conducted by Chengyun [
40] has indicated that enhancing the diet of Yanbian yellow cattle with higher concentrations of hazelnut-leaf condensed tannins (CT) results in an increase in rumen pH, which aligns with our experimental results.
Volatile fatty acids (VFAs), crucial products stemming from ruminal fermentation, primarily consist of acetic, propionic, and butyric acids [
41], serve as pivotal energy sources for the host organism. Post-feeding with HCDs, VFAs concentrations in the rumen progressively rise until peaking, fueled by accelerated fermentation processes. Of note, in the treatment groups that received CT supplementation during our animal trial, concentrations of acetic, propionic, and butyric acids were notably lower than those recorded in the CON group. This implies that CT supplementation may effectively mitigate VFA concentrations, aligning with previous findings [
42,
43] which observed a reduction in acetic and propionic acid concentrations within VFAs upon CT addition.
On the other hand, during foamy tympanism, the significant accumulation of foam in the rumen can impede the interaction between VFAs and the rumen wall. This results in reduced VFA absorption efficiency and subsequent accumulation within the rumen. The inclusion of CT acts to bind with proteins and curb foam formation in the rumen, thereby enhancing VFA absorption by minimizing obstacles. Consequently, this minimized the accumulation of VFAs in the rumen, resulting in a significant reduction in their concentration in rumen fluid, which effectively prevents ruminal acidosis. Additionally, the decrease in foam enhances the absorption surface area of VFAs on the rumen wall, potentially boosting their contribution to energy supply in goats. Additionally, this explains why pH values in CT-supplemented groups were markedly higher compared to the control group. In Trial 2, although the 1% CT group exhibited a significantly higher relative abundance of the rumen-dominant bacterial phylum Fibrobacterota compared to the other groups, its overall abundance remained relatively low, potentially having a minor impact on VFA production. Consequently, the more pronounced reduction in VFA concentration observed in the CT groups was more likely due to the dissipation of rumen foam.
Effects of CT on goat health. Interleukins play a crucial role in information transmission, as well as the activation and regulation of immune cells and inflammatory responses. IL-1β, IL-6, and TNF-α are categorized as pro-inflammatory factors that stimulate or amplify inflammatory responses. Conversely, IL-10 serves as an anti-inflammatory factor, mitigating or even halting such responses. In Trial 2, we observed an elevated concentration of IL-10 in the 2% CT group compared to the CON group, while levels of pro-inflammatory factors such as IL-1β, IL-6, and TNF-α were notably reduced relative to the CON group. These findings imply that the addition of 2% CT may enhance the immune status of goats. Furthermore, previous research has demonstrated that plant-derived CTs possess anti-inflammatory properties [
44], supporting our experimental observations.
Blood routine analysis serves as a vital indicator for assessing animal health. White blood cells (WBCs), functioning as defense cells within the organism, reflect changes in immune status and directly signify immunity levels, particularly cellular immunity and overall immune health [
45]. As evident from
Table 10, WBC counts in all three groups remained within normal ranges [
46]. Notably, however, the 2% CT group demonstrated significantly elevated WBC levels compared to the other two groups. This finding indicates that the addition of 2% CT may enhance goats’ immune status.
Effects of CT on dominant rumen flora in goats. In the present study,
Bacteroidetes,
Proteobacteria, and
Firmicutes were identified as the dominant microflora present in goats’ rumens. Analysis of microbial relative abundance (
Table 12) failed to uncover any significant differences among treatment groups, either at the phylum or genus levels, in terms of the composition of the dominant flora. Consequently, it can be inferred that supplementing with 1–2% CT does not alter the structural composition of the dominant flora within goats’ rumens.
Some studies [
47] have shown that long-term consumption of high levels of condensed tannins may impair animals’ absorption of certain minerals, such as iron and zinc. However, the extent of these antinutritional effects varies greatly depending on the source and dose of the condensed tannins, as well as the animal species and their physiological state. This trial, conducted over a period of one month, aimed to investigate whether tannins can eliminate rumen foam induced by high-concentrate diets during intensive fattening periods. Current research indicates that supplementing with 2% bayberry condensed tannins does not adversely affect goats’ metabolic health. Nevertheless, the long-term impacts of condensed tannin supplementation on feed efficiency and animal growth remain to be fully explored.