Release and Purification of Poly(3-hydroxybutyrate) P(3HB) via the Combined Use of an Autolytic Strain of Azotobacter vinelandii OP-PhbP3+ and Non-Halogenated Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strain, Culture Medium and Inoculum Generation
2.2. Scaling from Shake Flask to Bioreactor
2.3. Bioreactor Cultures
2.4. Analytical Methods
2.4.1. Microbial Growth
2.4.2. P(3HB) Quantification
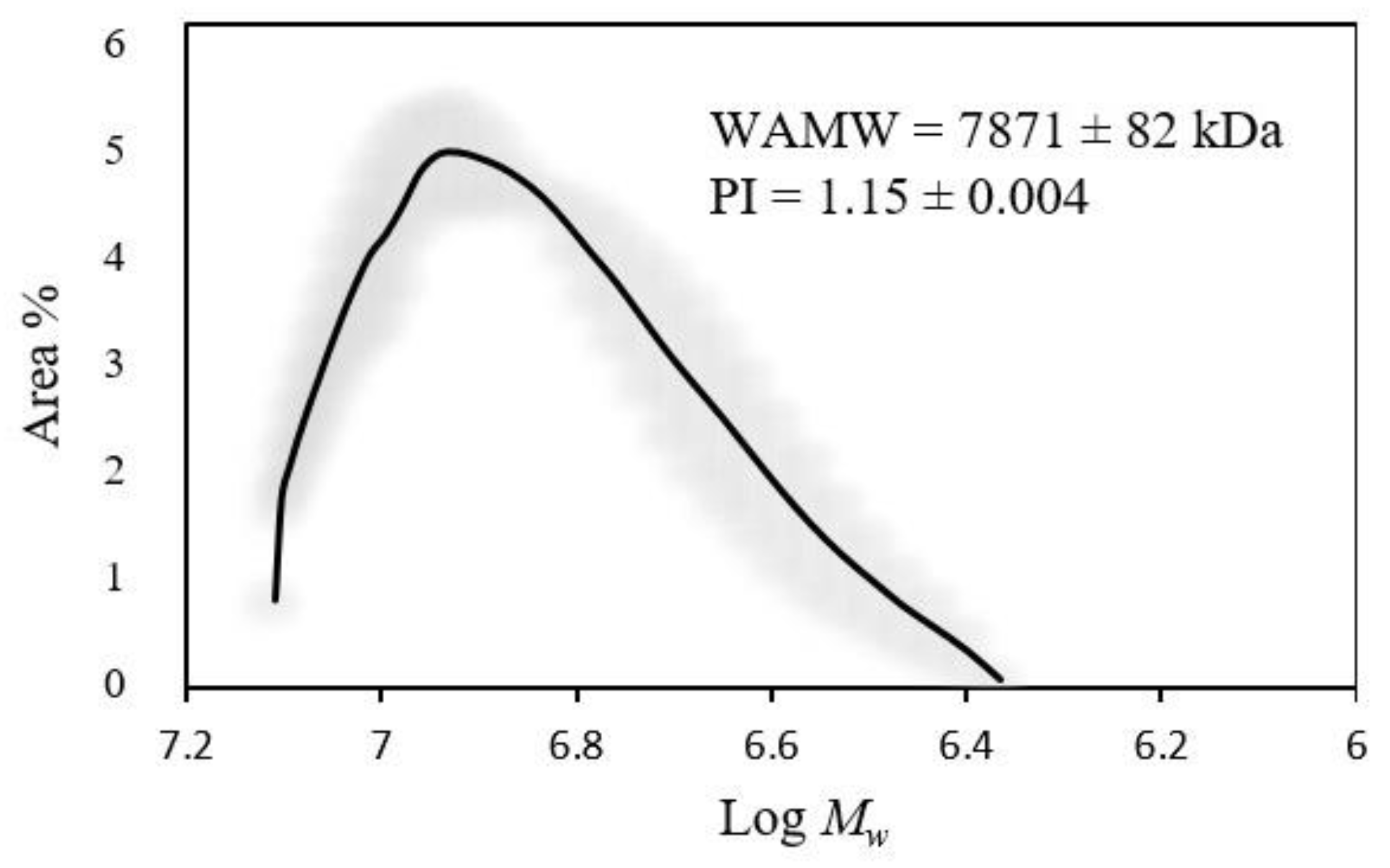
2.4.3. Weight Average Molecular Weight (WAMW) Determination
2.5. Electron Microscopy Assay
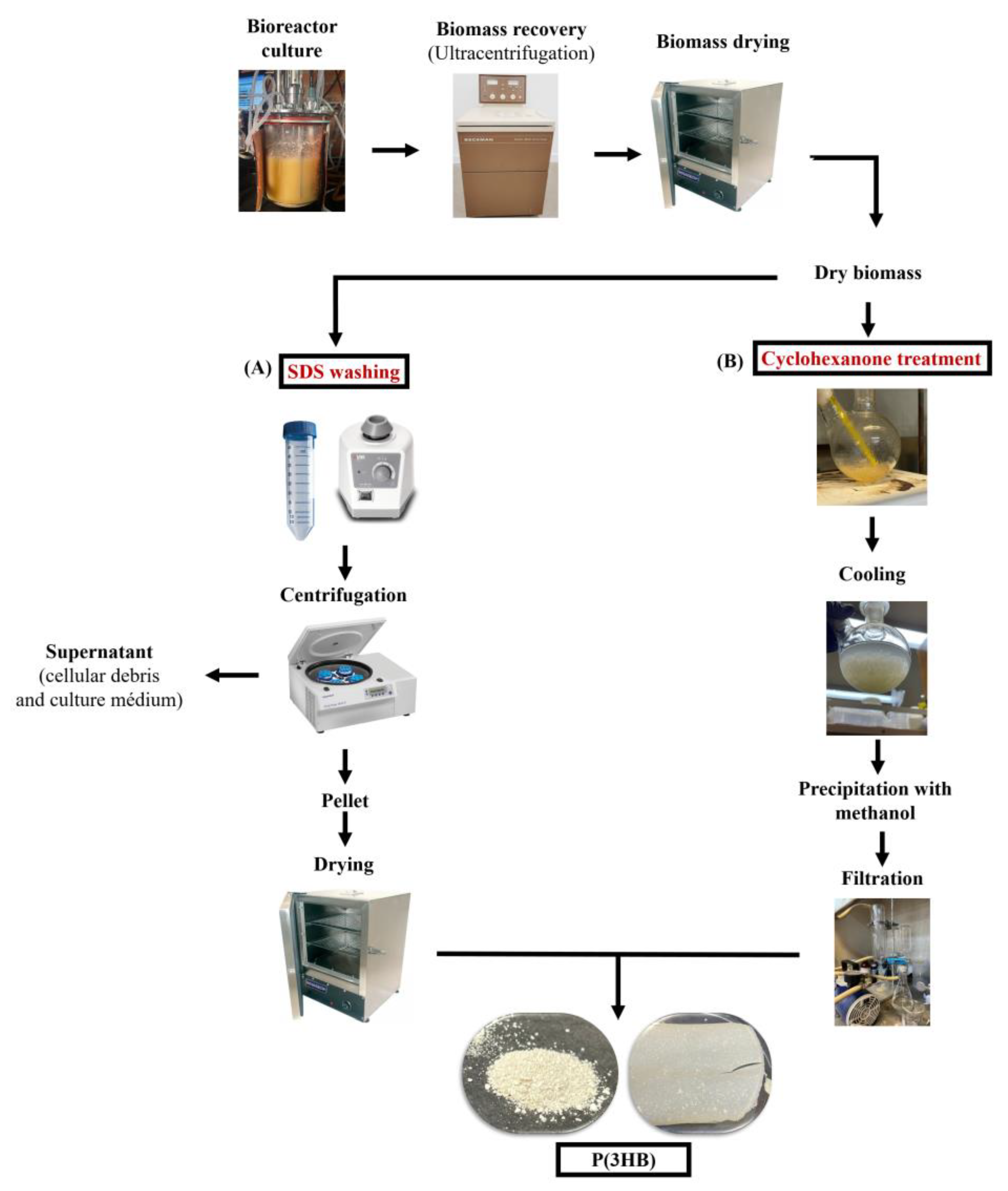
2.6. P(3HB) Recovery and Washing Using SDS
2.7. P(3HB) Recovery and Purification Using Cyclohexanone (CYC)
2.8. Yield and Purity Determination of P(3HB) Extracted from Cultivations
2.9. Preparation of Staining for Flow Cytometry Analysis
3. Results and Discussion
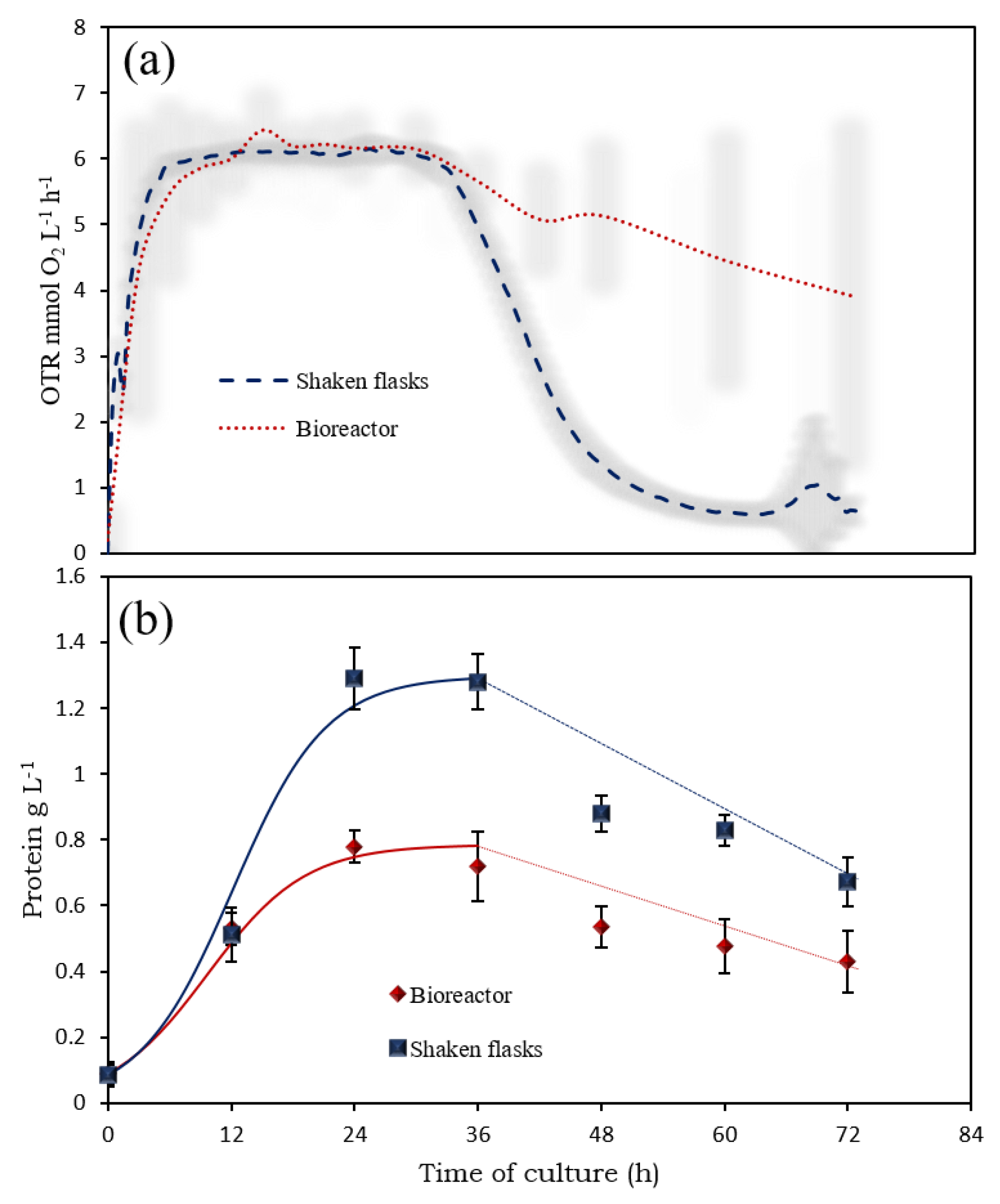
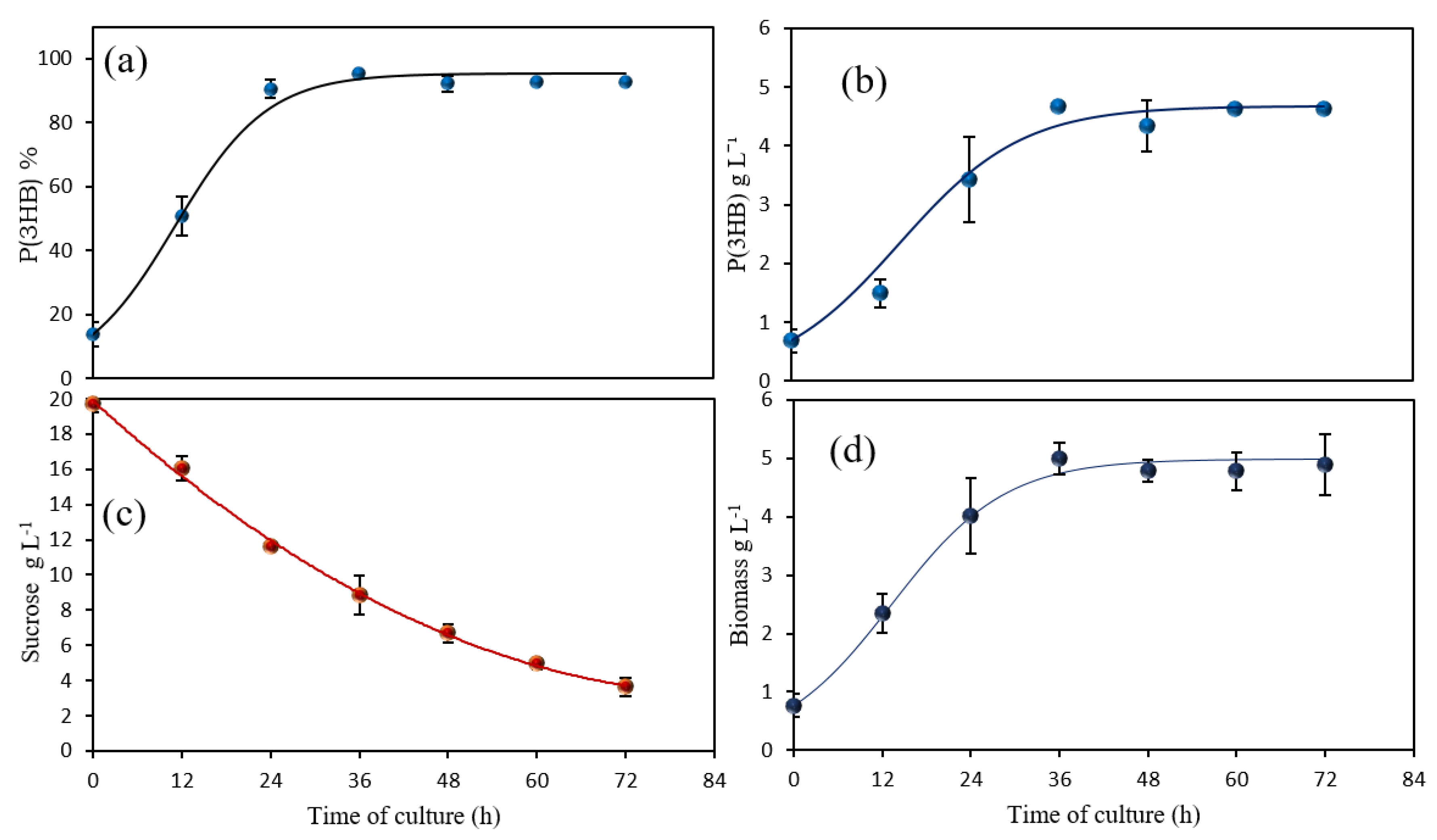
3.1. Scaling-Up from Shake Flasks to Bioreactors
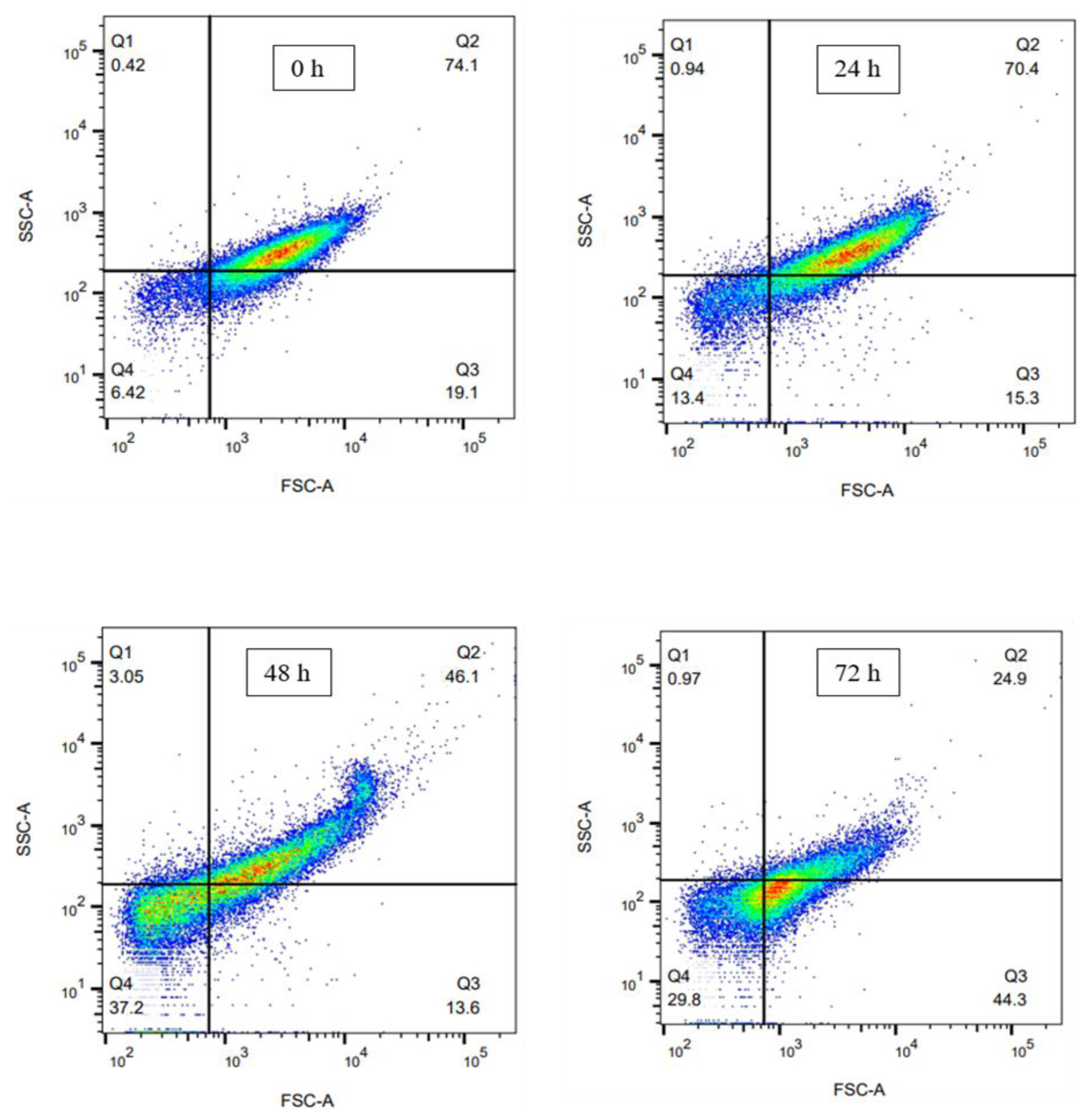
3.2. Cell Population Analysis by Flow Cytometry
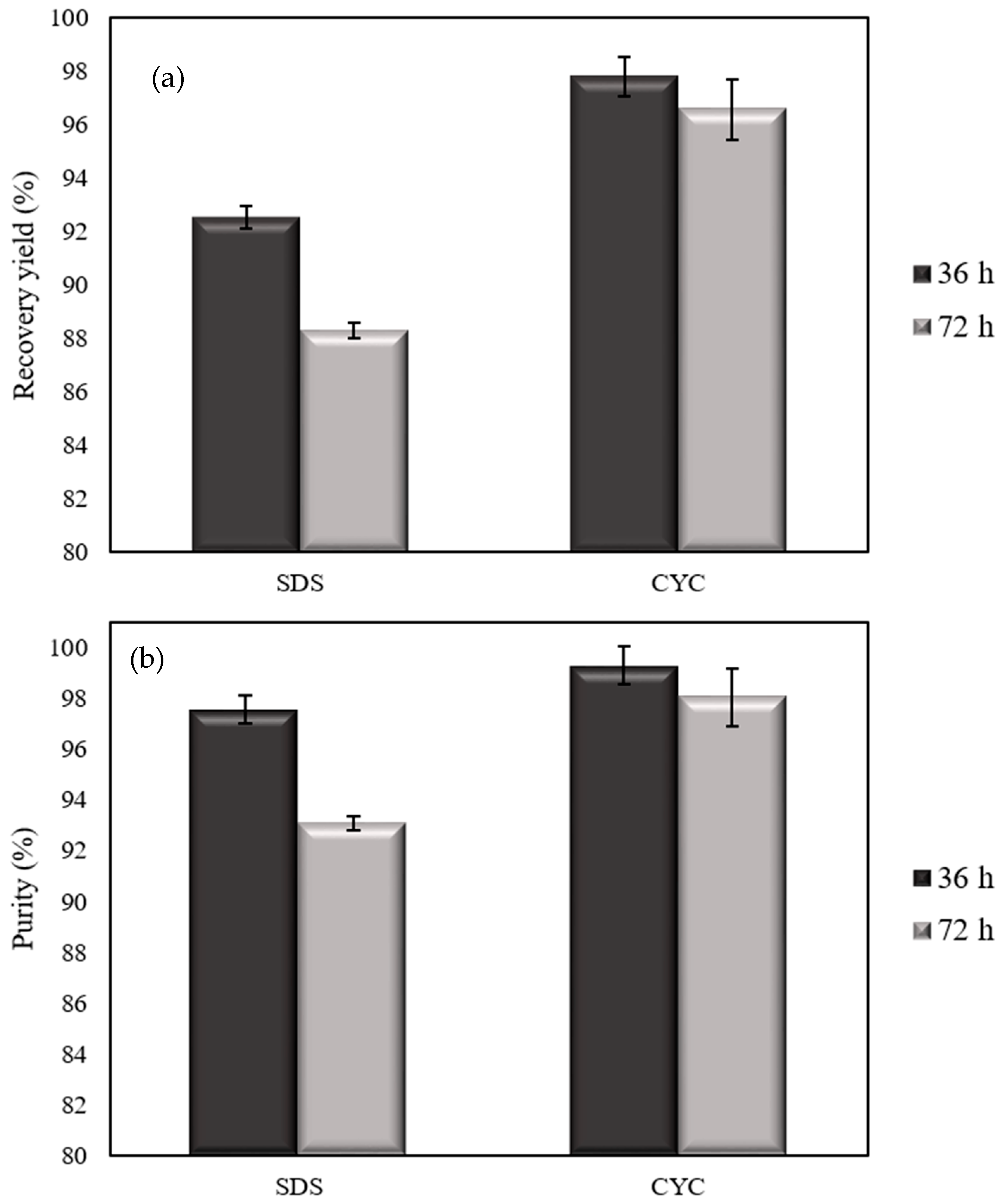
3.3. Yield and Purity of the Products Obtained from OP-PhbP3+ Using SDS and CYC Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and physical properties of polyhydroxyalkanoate (PHA)-based block copolymers: A review. Int. J. Biol. Macromol. 2024, 263 Pt 1, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. A review on established and emerging fermentation schemes for microbial production of polyhydroxyalkanoate (PHA) biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa, M.; Gerhart, B. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A. Recent advances in the use of waste as raw materials for polyhydroxyalkanoates production. Biotechnol. Adv. 2019, 54, 107871. [Google Scholar] [CrossRef]
- Koller, M. Switching from petro-plastics to microbial polyhydroxyalkanoates (PHA): The biotechnological escape route of choice out of the plastic predicament. EuroBiotech J. 2019, 3, 32–44. [Google Scholar] [CrossRef]
- Wong, Y.-M.; Brigham, C.J.; Rha, C.; Sinskey, A.J.; Sudesh, K. Biosynthesis and characterization of polyhydroxyalkanoate containing high 3-Hydroxyhexanoate monomer fraction from crude palm kernel oil by recombinant Cupriavidus necator. Bioresour. Technol. 2012, 121, 320–327. [Google Scholar] [CrossRef]
- Abate, T.; Amabile, C.; Muñoz, R.; Chianese, S.; Musmarra, D. Polyhydroxyalkanoate recovery overview: Properties, characterizations, and extraction strategies. Chemosphere 2024, 356, 141950. [Google Scholar] [CrossRef]
- Tamer, I.M.; Moo-Young, M.; Chisti, Y. Optimization of poly(β-hydroxybutyric acid) recovery from Alcaligenes latus: Combined mechanical and chemical treatments. Bioprocess. Eng. 1998, 19, 459–468. [Google Scholar] [CrossRef]
- Kurian, N.S.; Das, B. Comparative analysis of various extraction processes based on economy, eco-friendly, purity and recovery of polyhydroxyalkanoate: A review. Int. J. Biol. Macromol. 2021, 183, 1881–1890. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Chisti, Y. Recent Advances in the Production, Recovery and Applications of Polyhydroxyalkanoates. J. Polym. Environ. 2012, 21, 580–605. [Google Scholar] [CrossRef]
- Hahn, S.K.; Chang, Y.K.; Kim, B.S.; Chang, H.N. Optimization of microbial poly(3-hydroxybutyrate) recover using dispersions of sodium hypochlorite solution and chloroform. Biotechnol. Bioeng. 1994, 44, 256–261. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Keshavarz, T.; Bucke, C.; Roy, I. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J. Biotechnol. 2007, 132, 251–258. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Pérez, D.; Castro, M.; Utruvia, V.; Catillo, T.; Díaz-Barrera, A.; Espín, G.; Peña, C. Production and recovery of poly-3-hydroxybutyrate [P(3HB)] of ultra-high molecular weight using fed-batch cultures of Azotobacter vinelandii OPNA strain. J. Chem. Technol. Biotechnol. 2019, 94, 1853–1860. [Google Scholar] [CrossRef]
- García-Cerna, S.; Sánchez-Pacheco, U.; Meneses-Acosta, A.; Rojas-García, J.; Campillo-Illanes, B.; Segura-Gonzáles, D.; Peña-Malacara, C. Evaluation of Poly-3-Hydroxybutyrate (P3HB) Scaffolds Used for Epidermal Cells Growth as Potential Biomatrix. Polymers. 2022, 14, 4021. [Google Scholar] [CrossRef]
- Martínez, V.; García, P.; García, J.L.; Prieto, M.A. Controlled autolysis facilitates the polyhydroxyalkanoate recovery in Pseudomonas putida KT2440. Microb. Biotechnol. 2011, 4, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, W.; Wen, R.; Chen, G.-Q. An automatic lytic system for downstream purification of PHA produced by Halomonas. Chem. Eng. J. 2025, 517, 164425. [Google Scholar] [CrossRef]
- Borrero-de Acuña, J.M.; Hidalgo-Dumont, C.; Pacheco, N.; Cabrera, A.; Poblete-Castro, I. A novel programmable lysozyme-based lysis system in Pseudomonas putida for biopolymer production. Sci. Rep. 2017, 7, 4373. [Google Scholar] [CrossRef]
- Resch, S.; Gruber, K.; Wanner, G.; Slater, S.; Dennis, D.; Lubitz, W. Aqueous release and purification of poly(β-hydroxybutyrate) from Escherichia coli. J. Biotechnol. 1998, 65, 173–182. [Google Scholar] [CrossRef]
- Hori, K.; Kaneko, M.; Tanji, Y.; Xing, X.H.; Unno, H. Construction of self-disruptive Bacillus megaterium in response to substrate exhaustion for polyhydroxybutyrate production. Appl. Microbiol. Biotechnol. 2002, 59, 211–216. [Google Scholar] [CrossRef]
- Lacmata, S.T.; Yao, L.; Xian, M.; Liu, H.; Kuiate, J.-R.; Liu, H.; Feng, X.; Zhao, G. A novel autolysis system controlled by magnesium and its application to poly (3-hydroxypropionate) production in engineered Escherichia coli. Bioengineered 2017, 8, 594–599. [Google Scholar] [CrossRef]
- Quiroz-Cardoso, R.; Castillo, T.; Galindo, E.; Ruíz-Escobedo, J.; Segura, D.; Peña, C. Looking for improved strains of Azotobacter vineladii and favorable culture conditions yielding high-molecular-weight poly(3-hydoxybutyrate). J. Chem. Technol. Biotechnol. 2025, 100, 477–487. [Google Scholar] [CrossRef]
- Ruiz Escobedo, J. Estudio del Papel de las Proteínas Avin34710 y Avin34720 en el Metabolismo de Polihidroxibutirato (PHB) en la Bacteria Azotobacter vinelandii. Master’s Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2020. [Google Scholar]
- Doran, P.M. Mass Transfer. In Bioprocess Engineering Principles, 1st ed.; Elsevier Science & Technology Books: San Diego, CA, USA, 1995; pp. 191–217. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Gómez-Hernández, E.; Salgado-Lugo, H.; Segura, D.; García, A.; Díaz-Barrera, A.; Peña, C. Production of Poly-3-Hydroxybutyrate (P3HB) with Ultra-High Molecular Weight (UHMW) by Mutant Strains of Azotobacter vinelandii Under Microaerophilic Conditions. Appl. Biochem. Biotechnol. 2021, 193, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Ruíz, H.; Moreno, S.; Guzmán, J.; Nájera, R.; León, R.; Soberón-Chávez, G.; Espín, G. Isolation and characterization of an Azotobacter vinelandii algK mutant. FEMS Microbiol. Lett. 1997, 156, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Servain-Viel, S.; Aknin, M.L.; Domenichini, S.; Perlemuter, G.; Cassard, A.M.; Schlecht-Louf, G.; Lievin-Le Moal, V. A flow cytometry method for safe detection of bacterial viability. Cytom. Part A 2024, 105, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.F.; Anderlei, T.; Büchs, J.; Binder, M. Oxygen limitation is a pitfall during screening for industrial strains. Appl. Microbiol. Biotechnol. 2006, 72, 1157–1160. [Google Scholar] [CrossRef]
- Anderlei, T.; Zang, W.; Papaspyrou, M.; Büchs, J. Online respiration activity measurement (OTR, CTR, RQ) in shake flasks. Biochem. Eng. J. 2004, 17, 187–194. [Google Scholar] [CrossRef]
- García, A.; Ferrer, P.; Albiol, J.; Castillo, T.; Segura, D.; Peña, C. Metabolic flux analysis and the NAD(P)H/NAD(P)+ ratios in chemostat cultures of Azotobacter vinelandii. Microb. Cell Fact. 2018, 17, 10. [Google Scholar] [CrossRef]
- Rosengart, A.; Cesário, M.T.; de Almeida, C.D.; Raposo, R.S.; Espert, A.; Díaz de Apodaca, E.; da Fonseca, M.R. Efficient P(3HB) extraction from Burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Jiang, G.; Johnston, B.; Tonrow, D.E.; Radeka, I.; Koller, M.; Chaber, P.; Adamus, G.; Kowalczuk, M. Biomass Extraction Using Non-Chlorinated Solvents for Biocompatibility Improvement of Polyhydroxyalkanoates. Polymers 2018, 10, 731. [Google Scholar] [CrossRef]
- Van Walsem, J.; Zhong, L.; Shih, S.S. Polymer Extraction Methods 2010. U.S. Patent No. 7,713,720 B2, 7 August 2007. [Google Scholar]
- Filippi, S.; Cinelli, P.; Mezzetta, A.; Carlozzi, P.; Seggiani, M. Extraction of polyhydroxyalkanoates from purple non-sulfur bacteria by non-chlorinated solvents. Polymers 2021, 13, 4163. [Google Scholar] [CrossRef]

| Ref. | Bacterial Strain | Content of P(3HB) (%) | Temperature of Extraction (°C) | Time of Extraction (min) | Yield of Extraction (%) | Purity (%) |
|---|---|---|---|---|---|---|
| This research | A. vinelandii OP-PhbP3+ | 95 | 120–130 | 15 | 98 | 99 |
| [36] | Rhodovulum sulfidophilum DSM-1374 | 14 | 125 | 5 10 20 | 43 95 98 | N. A |
| [34] | Cupriavidus necator H16 | 82.3 | 80 100 120 | 1200 5 3 | 16 90 99 | 95 |
| [33] | Burkholderia sacchari DSM 17,165 | 57.7 | 120–130 | 15 15 30 | 98 87 89 | 98.2 91.9 92.3 |
| [35] | E. coli | 80 | 90 | 35 | 80 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, J.; Segura, D.; Aguirre-Zapata, C.; Galindo, E.; Peña, C. Release and Purification of Poly(3-hydroxybutyrate) P(3HB) via the Combined Use of an Autolytic Strain of Azotobacter vinelandii OP-PhbP3+ and Non-Halogenated Solvents. Fermentation 2025, 11, 571. https://doi.org/10.3390/fermentation11100571
Valencia J, Segura D, Aguirre-Zapata C, Galindo E, Peña C. Release and Purification of Poly(3-hydroxybutyrate) P(3HB) via the Combined Use of an Autolytic Strain of Azotobacter vinelandii OP-PhbP3+ and Non-Halogenated Solvents. Fermentation. 2025; 11(10):571. https://doi.org/10.3390/fermentation11100571
Chicago/Turabian StyleValencia, Joshua, Daniel Segura, Claudia Aguirre-Zapata, Enrique Galindo, and Carlos Peña. 2025. "Release and Purification of Poly(3-hydroxybutyrate) P(3HB) via the Combined Use of an Autolytic Strain of Azotobacter vinelandii OP-PhbP3+ and Non-Halogenated Solvents" Fermentation 11, no. 10: 571. https://doi.org/10.3390/fermentation11100571
APA StyleValencia, J., Segura, D., Aguirre-Zapata, C., Galindo, E., & Peña, C. (2025). Release and Purification of Poly(3-hydroxybutyrate) P(3HB) via the Combined Use of an Autolytic Strain of Azotobacter vinelandii OP-PhbP3+ and Non-Halogenated Solvents. Fermentation, 11(10), 571. https://doi.org/10.3390/fermentation11100571







