Abstract
The aim of this work was to study the effects of modification of the skin maceration process by adding ascorbic acid and a yeast culture of Saccharomyces cerevisiae on the quality and safety of Welschriesling wine. The pH, total acids, SO2, and alcohol content were significantly affected by the modification of the maceration (p < 0.001), except for the alcohol variable, which was not influenced by the skin maceration modifications of the measured values (p > 0.05). The antioxidant activity, total polyphenols, flavonoids, and ascorbic acid levels changed significantly during the experiment, including the maceration and maturation periods (p < 0.001). The observed histamine concentrations were below the recommended limit of 10 mg·L−1 in each analysed sample. Histamine (HIS) and tyramine (TYR) amounts were significantly affected by the experimental factors (p < 0.001). After nine months of maturation, wine samples with ascorbic acid had 2.23 ± 0.00 and 0.35 ± 0.00 mg·L−1 HIS and TYR content, respectively. On the other side, macerated wines without ascorbic acid had 3.05 ± 0.10 and 0.37 ± 0.05 mg·L−1 HIS and TYR content, respectively. Modified vinification procedure with ascorbic acid negatively affected wine samples in the overall sensory evaluation scores of the wines (p < 0.001).
1. Introduction
The quality of white wine produced in Slovakia is predominantly defined through its chemical composition, geographical origin, and sensory characteristics. A comprehensive analysis of various white wines from Slovakia’s distinct regions reveals that factors such as the concentration (glucose and fructose), organic acids (malic and tartaric), alcohol content, total phenolics, and antioxidant activity significantly influence wine quality perceptions [1,2].
Ascorbic acid acts as a reducing agent and can alter biogenic amine formation during wine production. While ascorbic acid may suppress certain pathways leading to biogenic amine production, it can also facilitate the transformation of other precursors that contribute to amine synthesis [3]. Thus, the net impact of ascorbic acid on biogenic amines (BA) in wine can seem both inhibitory and catalytic, depending on fermentation conditions such as pH and temperature, which influence microbial activity [4]. Skouroumounis et al. [5] claimed that the addition of 90 mg·L−1 of ascorbic acid during bottling had little impact on wine aroma in the first six months. In bottled wine, ascorbic acid has been shown to contribute to increased yellow, but not brown colouration of wines even when sulphur dioxide (SO2) is present [5]. In terms of appeal to consumers, brown and/or orange colouration is negative while yellow colouration is generally taken as an indicator of age [6,7]. Chardonnay and Riesling wines with ascorbic acid added at bottling (92 and 99 mg·L−1) became more yellow after three to five years after bottling, residual SO2 and ascorbic acid were still present [5]. Riesling wines with the addition of ascorbic acid increased yellow colour in previous published studies [5,8].
The overall reaction of the ascorbic acid in wines involves a metal ion-mediated reaction between ascorbic acid and molecular oxygen to form dehydroascorbic acid and hydrogen peroxide, which reacts with SO2 to form sulphuric acid [9]. Danilewicz and Wallbridge [10] observed that in the absence of ascorbic acid, the consumption of oxygen in wine matrices is accelerated by the presence of SO2, thereby accelerating the phenolic oxidation reaction. The efficiency of oxygen consumption by ascorbic acid is not specific to wine alone, and it can also be used in must or juice to consume oxygen in competition with oxidative enzymes (e.g., polyphenol oxidase, laccase), thereby lowering their activity [11]. Ascorbic acid has been described as being able to reduce oxidised components in wine back to their reduced state. This includes the reduction in o-quinone compounds back to their parent phenolic substance [9]. The antioxidant capacity derived from these phenolic compounds contributes not only to the perceived quality but also to the health aspects of wine, linking them to potential protective effects against chronic diseases [12]. A study by Kalhaa et al. [13] demonstrated that the addition of ascorbic acid significantly reduces histamine (HIS) levels in white wine [13], which suggests a potential suppressive impact of ascorbic acid on the microbial activity responsible for BA formation in wine and may affect the oxidative environment during fermentation, influencing the metabolic pathways of yeast and bacteria. It also acts as an antioxidant, thereby stabilising the wine matrix against oxidative stress that could promote the growth of BA-producing microorganisms [14,15]. The official limits for BAs in wine had not yet been established. For humans with HIS intolerance, consuming foods high in HIS or tyramine (TYR) can trigger severe toxic reactions, which include headaches, respiratory issues, heart palpitations, nausea, and dangerous shifts in blood pressure.
This experiment was conducted to evaluate the chemical, microbial, and sensory variables of experimental samples of Welschriesling wine produced using a modified grape skin maceration process with ascorbic acid and yeast Saccharomyces cerevisiae. The main aim of this study was to evaluate HIS and TYR content from the health point of view in wine samples.
2. Materials and Methods
2.1. Wine Preparation and Sampling
For this experiment, the monovarietal white grape variety Welschriesling (Vitis vinifera L.) from the eastern Slovak wine region (48°46′ N, 22°13′ E, 126 m above sea level) was selected. It was manually harvested (approximately 1.5 t of grapes) during the last week of September 2021 and transported to the experimental wine cellar of the University of Veterinary Medicine and Pharmacy in Košice (Košice, Slovakia). The grapes were collected in a good sanitary state and at maximum sugar content (soluble solids: avg. 19.4° Brix), and thanks to the optimal vinification process, the sugar content was increased by the addition of sugar to 21° Brix. The presence of Botrytis cinerea was evaluated by visual assessment.
The predominant soil type was volcanic andesite. The climate had a distinctive continental character (Central European steppes). The average annual temperature was 12 °C, annual rainfall was 682 mm, and the average length of sunshine was 2088 h per one-year period (from October 2020 to October 2021) (Slovak Hydrometeorological Institute in Košice). The soil area was fertilised using gravity with a pressure of 1 atmosphere. With a plough, the fertiliser was blown on two sides into a furrow dug below the surface of the soil 40 cm deep. The ploughshare distributed the granular fertiliser up to a width of 120 cm. The fertiliser consisted of N (12%), P (6%), and K (18%). The dose of fertiliser before sprouting was 50 g/m. Pest control, soil management, and other viticultural operations were conducted according to standard practices. Pest control was controlled according to the recommendations of the Central Control and Testing Institute of Agriculture in Bratislava, Slovakia. The spraying was carried out equally in both the control and the experimental groups. In the given year, spraying was used against Plasmopara viticola and Uncinula necator.
The grapes were destemmed and crushed using a DMCI Goma horizontal crusher and destemmer (Grifo Marchetti, Piadena Drizzona, Italy). The total volume of the grapes was destemmed and crushed at once. Subsequently, the entire volume was divided into four parts, each designated for separate treatment.
In the first group—control treatment (C)—the grape juice was pressed directly from the must on a Lancman VSX 80 water press (Lancman, Vransko, Slovenia) and distributed into 100 L stainless steel tanks. Potassium metabisulphite at a concentration of 40 mg·L1, and Saccharomyces cerevisiae yeast culture (O.K. SERVIS BioPro, s.r.o, Praha, Czech Republic) in an amount of 30 g·100 L−1 (approximately 3 × 103 CFU·mL−1) were added to must. The second experimental group of wines (M) underwent pre-fermentation maceration of the skins in a stainless steel tank for 72 h without access to oxygen at a temperature of 18 ± 3 °C, with the addition of 40 mg·L−1 of potassium metabisulphite. The third experimental wine treatment (MA) underwent the same process as the second experimental group M. with the addition of food-grade ascorbic acid (Sigma–Aldrich, St. Louis, MO, USA) in an amount of 20 g·100 L−1, and the must was subjected to a 3-day skin maceration treatment. The M and MA experimental groups underwent spontaneous maceration with only presence of natural microorganisms without addition of yeast culture. The fourth experimental wine samples group (MY) underwent the same process as the M experimental group, but additionally the yeast culture Saccharomyces cerevisiae (O.K. SERVIS BioPro, s.r.o, Praha, Czech Republic) was added in an amount of 30 g·100 L−1 (approximately 3 × 103 CFU·mL−1) during the 3-day skin maceration process.
After three days of skin maceration, the must of M, MA and MY experimental groups was pressed and transferred to stainless steel tanks, where the fermentation process was completed. Each experimental treatment was performed three times to improve the experimental design in terms of the technological process of vinification. The fermentation processes of each group took place at a controlled temperature (18 ± 2 °C) over a period of four weeks. The young wine was then removed from the yeast lees and transferred to clean stainless steel tanks, where the samples matured for 8 months. During the maturation process, potassium metabisulphite was added to the wine in doses of up to 40 mg L−1 to protect the wine from microbial contamination and oxidation. Wine samples were taken after the fermentation process (first month), and after three, five, and eight months of wine maturation into sterile, closable bottles with a volume of 750 mL and were analysed immediately. Standard wine analyses were performed according to the International Organization of Vine and Wine.
2.2. Physical and Chemical Analysis
The wine samples were assessed to determine pH, titratable acidity (TA; g·L−1), alcohol volume (AV; %) and SO2 content (mg·L−1). Physical and chemical analyses were performed according to the OIV method [16]. The pH was determined using a pH Pro2Go meter with a an InPro X1 Sensor (Mettler-Toledo Co., Columbus, OH, USA) with a pH scale calibrated in pH units and with the sample temperature set at 20 °C, according to the OIV-MA-AS313-15 method [16]. To determine the TA, a DS Titra analyser and Vola 2000 extractor were used (Dujardin-Salleron, Noizay, France). TA was determined via the titration method according to OIV-MA-AS313-01 [International Organisation of Vine and Wine]. The alcohol content in wines was determined using a DS Ebuliometer with a DS Thermometer (Dujardin-Salleron, Noizay, France). To determine the total SO2 content, we used a DS IODO automatic titrator (Dujardin-Salleron, Noizay, France) to perform iodometric titration accohe tording to OIV-MA-VI-09 [17]. The total SO2 content was expressed as mg of SO2 per L of the wine sample [16].
To measure the antioxidant activity, a simple method of DPPH (2,2-diphenyl-1-picrylhydrazyl) radical uptake was used. The magnitude of the antioxidant activity was expressed as the % inhibition of DPPH radicals according to Brand-Williams [18]. The total polyphenol content (TPC) was determined using the Folin–Ciocalteu spectrophotometric method [19]. The absorbance was measured using a UV-VIS spectrophotometer (Helios γ, Thermo Spectronic, Cambridge, UK) at 700 nm. The total content of polyphenols was expressed based on the gallic acid calibration curve in mg GAE.L−1 of the sample. The concentration of TPC was expressed as gallic acid equivalent (mg·L−1 GAE). The total flavonoid content (TFC) was determined by the spectrophotometric method according to Lamaison and Carnat [20] and the results were expressed as quercetin equivalent (mg·L−1 QE). Qualitative and quantitative analyses of ascorbic acid were performed using the Dionex UltiMate 3000 RS UHPLC (Thermo Scientific, Darmstadt, Germany) system equipped with a diode array detector (operated at 260 nm). Ascorbic acid was separated on a YMC-Triart C18 chromatography column (250 × 4.6 mm i.d., 5.0 µm particle size, 120 Å pore size; YMC Europe GmbH, Dinslaken, Germany) at a constant column temperature of 40 °C ± 0.5 °C. The mobile phase for isocratic elution consisted of phosphate buffer (component A) and acetonitrile (component B) in a 90:10 ratio. The flow rate was 0.8 mL·min−1, the injection volume was 10 μL, and the total analysis time was 30 min. Chromatographic data were evaluated using Chromeleon 7.2 Chromatography Data System software.
The concentrations of biogenic amines (BA): HIS and TYR concentrations in experimental samples were determined using ultra-high-performance liquid chromatography (UHPLC) according to Regecová et al. [21]. To analyse BA, a Thermo Scientific UHPLC system (Dionex UltiMate 3000 RS, Thermo Scientific, Darmstadt, Germany) coupled with a fluorescence detector (FLD) was used. A standard stock solution of the tested substances HIS and TYR was prepared by dissolving each standard in deionised water to a concentration of 1000 mg·L−1. HIS and TYR were identified by comparing their retention time with their corresponding standard. BA quantification in wine samples was performed using a standard curve generated by the ratio of the peak area to BA concentration. Each determination was performed in triplicate.
2.3. Microbial Analysis
A stock suspension and subsequent 10-fold dilutions were prepared according to the guidelines of ISO 6887-1 [22]. The prepared 10-fold dilutions were used in the subsequent microbiological cultivation sample examinations.
The microbiological cultivation examination of the grape juice, must and wine samples determined the total viable counts (TVCs) according to ISO 4833-1 [22], using the pouring method onto Plate Count Agar (incubation at 30 ± 1 °C for 72 h). After incubation, the colony counts were determined on the surface and within the agar medium in the Petri dishes. The resulting counts were converted to the amount per 1 mL of the sample. At the same time, the counts of lactic acid bacteria (LAB) were determined on the surface of the selective diagnostic agar medium of De Man, Rogosa, and Sharpe (Hi-Media, Maharashtra, India) according to ISO 6887-1 [23]. The inoculated plates were incubated under anaerobic conditions at 37 °C for 48 h using AnaeroGen (Oxoid, Basingstoke, UK). The resulting counts were similarly converted to the amount per 1 mL of the sample. Another microbiological cultivation examination was focused on the determination of the number of yeasts and moulds (YM) according to ISO 21527-1 [24], using the spread plate method and Dichloran Rose-Bengal Chloramphenicol agar (Hi-Media, Maharashtra, India). The inoculated plates were incubated at 25 °C for 5 days. After incubation, the counts of yeasts and moulds were obtained and converted to the amount per 1 mL of the sample.
2.4. Sensory Evaluation
The analysis of the sensory properties of the wine samples was carried out by a trained panel composed of 12 panellists (aged 26–49). The assessors received training involving 120 h of sensory analysis, which covered methodological, technical and tasting sessions. The tests were conducted twice during two consecutive weeks to ensure the reliability of the data. Two methods of sensory assessment were employed in the present study. The 100-point OIV system [25] and Quantitative Descriptive Analysis (QDA) were used to observe detailed differences between the experimental wine samples. Samples (100 mL each) were presented in standard, clear wine glasses at a usual consumption temperature of 10 ± 2 °C. Sensory assessment was performed according to the OIV/concours 332A/2009 [25], where assessors rated the samples based on four categories: appearance (limpidity–up to 5 points, other aspects–up to 10 points,), aroma (genuineness–up to 6 points, positive intensity–up to 8 points, quality–up to 16 points), taste (genuineness–up to 6 points, positive intensity–up to 8 points, harmonious persistence–up to 8 points, quality–up to 22 points) and harmony (the overall judgement–up to 11 points). The scores for each sensory attribute were recorded, and a total score was calculated as the sum of the individual attribute scores. The OIV point scoring method comprises the following wine categories: table wine without a geographical indication (scores 60 to 64.99); table wine with a controlled geographical indication (score 65 to 74.99); quality wine with a controlled geographical origin (score 75 to 84.99), and superior wine (scoring more than 85) [25]. The maximum score that a wine sample can obtain according to the OIV system with such an appraisal is 100.
QDA was used to thoroughly evaluate wine sensory variables using a 10-point structured scale (0 = attribute not perceptible, 10 = attribute strongly perceptible) including the following descriptors sorted into groups: appearance (yellow, green, golden); aroma (peach, apricot, citrus, fruit, floral, honey, petrol); and taste (acidic, spicy, exotic fruit, apricot, honey, sweet spice, mineral, and pear). Sensory attributes were defined during the initial QDA testing sessions by the assessors.
2.5. Statistical Analysis
All experiments were performed in triplicate and expressed as the mean value with standard deviation. Data analysis was carried out via R-statistics software version 4.2.3 [26]. The Analysis of Variance method and Tukey’s test for multiple comparisons of means at a significance level of p < 0.05 were conducted. The effects of the wine production technology (modification of the skin maceration process with ascorbic acid and yeast culture) were set as the main factors of the data analysis.
3. Results
The results of the analysis of the raw material, Welschriesling berries and must for the production of experimental wine samples are presented in Table 1. The increase in TVC, LAB and YM between berries and must was observed. The average pH measured in the initial phase of the wine production was 3.27, and it changed significantly in the next production phases in the control and experimental wine sample groups (Table 2).

Table 1.
Results of determination of selected raw material parameters in the presented experiment–variety Welschriesling (mean ± SD).

Table 2.
The results of the pH, total acids, total SO2 and alcohol content measurements of the control and experimental wine samples during the maturation of the samples (mean ± SD).
The individual results for the measurements of pH, TA, SO2 and alcohol content determined after one, four, six and nine months of maturation for the control (C) and experimental wine sample groups (M, MY and MA) are shown in Table 2.
The pH value increased between the first and last analysis in the ninth month of the experiment for the control (C) sample and the experimental macerated wine samples (M). The pH value decreased in samples with added ascorbic acid (MA) during the whole experimental period. The highest pH value was observed in the 6th month in the MY group, while the lowest value was observed in the C group at the beginning of the experiment. The total acid content in each group of wine samples decreased with increasing maturation time. The initial total acid content in the first month of the experiment was the highest in the MA experimental group. During the maturation of the wine, the wine samples in the stainless steel tanks were treated by applying SO2 during each phase of the experiment (when the wine samples were collected). The limit of 200 mg·L−1 was slightly increased in the M, MA, and MY experimental group in the ninth month of maturation, with the concentrations of 208.10 ± 2.91, 203.94 ± 9.43, and 202.02 ± 9.13 mg SO2·L−1, respectively. During this experiment, the alcohol content of control and experimental wine samples was relatively stable, ranging from 10.72 to 11.39%. After the ninth month of maturation, the alcohol content of wine samples did not significantly differ (p > 0.05).
Application of the different wine production technology (maceration of must with the addition of ascorbic acid, and Saccharomyces cerevisiae yeast wine culture) had a significant effect (p < 0.001) on pH, TA and SO2 content of wine samples.
Table 3 shows the results of the determination of antioxidant activity, total polyphenols and flavonoids content, and ascorbic acid concentration in the experimental wine samples. The lowest antioxidant activity was measured in the 1st month of wine maceration in the C group. During the production phases, the antioxidant activity increased significantly (p < 0.001) in each of the wine sample groups. The highest antioxidant activity (88.37 ± 0.16%) was observed in the MA group in the ninth month of maturation, while the highest increase within the experimental group was in M.

Table 3.
The results of the determination of antioxidant activity, total polyphenols and flavonoids and ascorbic acid concentration of the control and experimental wine samples during the maturation of the samples (mean ± SD).
TPC after the first month of wine maturation in the C group reached 387.33 mg·L−1 GAE, while M and MY had higher values, 491.26 and 508.33 mg·L−1 GAE, respectively. However, initial TPC in sample group with the ascorbic acid applied had the lowest TPC concentration at 343.52 mg·L−1 GAE. Between the wine sampling in the first month and the last measurements in the ninth month, a significant increase in TPC in the C group (final concentration 583.42 ± 47.50 mg·L−1 GAE) was observed. A significant increase was also observed in the experimental wine samples (M, MY and MA) during the wine maturation period (p < 0.001). The highest increase in TPC during the production process was observed in group C, while the lowest was observed in group MY. The skin maceration process applied during fermentation affects the extraction of polyphenols from the grapes into the wine, increasing their content and decreasing their colour intensity.
TFC increased significantly during different production phases in all groups (p < 0.001). Among all tested groups, the MA group consistently recorded the highest concentration in each measurement instance (102.21 ± 2.01 mg·L−1 QE < 105.7521 ± 3.23 mg·L−1 QE < 109.41 ± 0.93 mg·L−1 QE < 115.62 ± 11.54 mg·L−1 QE). The measurements showed that maceration processes significantly increased the TFC concentration in the wine samples where the maceration processes were applied.
Ascorbic acid concentration decreased significantly (p < 0.001) during different production phases in all sample groups. The wine samples from the C, M and MY groups had ascorbic content between 3.54 and 5.18 mg·L−1. The highest concentration of ascorbic acid was justifiably in the MA group, decreasing from a value of 180.91 ± 0.01 mg·L−1 measured on the first month of maturation, to a value of 126.31 ± 0.37 mg·L−1 measured in the ninth month of the winemaking period.
Figure 1 shows the results of the determination of the HIS and TYR content in the experimental wine samples during the various phases of production. The HIS concentration in group C decreased during the production phase from 3.26 ± 0.02 to 2.65 ± 0.01 mg·L−1. On the contrary, an increasing trend was observed during the production phases of the wine samples in the MA samples group, but the final HIS concentration in the MA samples group did not exceed 2.23 mg·L−1. The maximum HIS in the experimental groups was observed in the M samples group after the first month (9.43 mg·L−1). In each experimental group, TYR content decreased significantly during the production phases (p < 0.001). After nine months of maturation, the highest concentration of TYR was observed in the M group (0.37 ± 0.05 mg·L−1) and the lowest in the C group samples (0.22 ± 0.01 mg·L−1). The influence of maceration procedures and their modifications had significant effect on the concentration of HIS and TYR (p < 0.001).
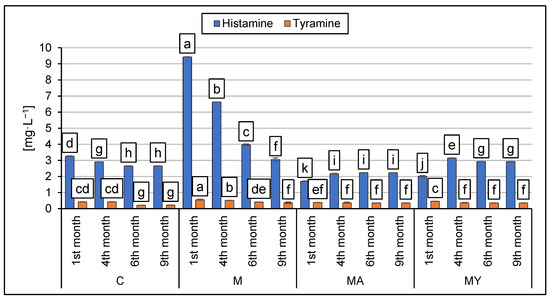
Figure 1.
The results of HIS and TYR determination in experimental wine samples during phases of production (mean ± SD). C: the control group of wine samples. M: wine samples that underwent maceration in wine production technology. MA: wine samples that underwent maceration in wine production technology with the addition of ascorbic acid. MY: wine samples that underwent maceration in wine production technology with an added yeast culture. a–j: the means in columns with a different superscript letter are statistically different (p < 0.05).
Table 4 shows the quantitative microbiological culture examination of the samples. In the control group C, a gradual decrease in all monitored microorganisms was observed over the course of 9 months. LAB were detected only during the first month of fermentation (4.05 ± 0.05 log CFU·mL−1). Similarly, the number of YM decreased gradually (from 4.55 ± 0.05 to 2.45 ± 0.05 log CFU·mL−1), as well as TVC (from 3.95 ± 0.05 to 1.95 ± 0.05 log CFU·mL−1), which is typical for spontaneous fermentation.

Table 4.
The quantitative microbiological culture examination of the control and experimental wine samples: total counts (log CFU·mL−1 mean ± SD).
In the next monitored group M, higher initial values of all microbial groups were recorded, especially TVC (5.83 ± 0.05 log CFU·mL−1) and YM (6.15 ± 0.05 log CFU·mL−1). LAB were detected up to month 6 (3.95 ± 0.05 log CFU·mL−1), which may be related to increased extraction of nutrients and nitrogen compounds during maceration. At month 9 of maturation, a slight increase in TVC and YM was detected again (3.95 ± 0.05 and 3.85 ± 0.05 log CFU·mL−1), which may be related to reactivation of sublethally damaged microorganisms or spores due to improved growth conditions in the later fermentation phase, nutrient release by yeast autolysis, or reduced competitive pressure after LAB disappearance.
In the MA group, LAB were detected until month 6 (3.65 ± 0.05 log CFU·mL−1) with the highest initial values among all groups (5.75 ± 0.05 log CFU·mL−1). Ascorbic acid may have contributed to maintaining favourable conditions for this microflora due to its antioxidant properties, which reduce oxidative stress in the fermentation environment. At the end of the maturation process, stable values of YM (3.35 ± 0.05 log CFU·mL−1) and TVC (3.95 ± 0.05 log CFU·mL−1) were recorded, similar to the M and MY samples.
In the MY group, LAB counts were detected at the highest level at month 1 (5.15 ± 0.05 log CFU·mL−1), while their presence decreased to undetectable levels by month 6 of maturation. YM counts remained relatively high until the end of the observation period (4.65 ± 0.05 log CFU·mL−1 at month 9), similar to the M group.
Figure 2 shows the results of the sensory analysis of the experimental wine samples according to the 100-point sensory evaluation method as a standardised approach for assessing wine quality. The sensory evaluation conducted through 100-points OIV method showed that wine samples of the C group after nine months of maturation were notably ranked with a higher point score (82.33 pt) in overall evaluation than experimental wine groups (M: 80.50 pt, MA: 68.00 pt and MY: 76.67 pt). On the other hand, the application of maceration with ascorbic acid in the winemaking of MA wine samples resulted in the lowest overall sensory score (68 pt). The experimental treatments of vinification procedures and the time of maturation had significant effects on the sensory score of wine samples (p < 0.001).
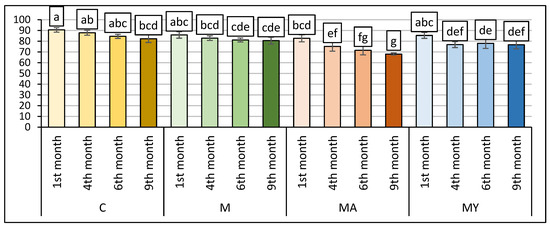
Figure 2.
The total score of sensory evaluation of the experimental wine samples with the hedonic 100-point OIV method (means ± SD). C: the control group of wine samples. M: wine samples that underwent maceration in wine production technology. MA: wine samples that underwent maceration in wine production technology with the addition of the ascorbic acid. MY: wine samples that underwent maceration in wine production technology with an added yeast culture. a–g: the means in columns with a different superscript letter are statistically different (p < 0.05).
These results indicate that, unlike the control vinification treatment (C), the applied vinification procedure negatively affected wine samples evaluated after nine months of maturation in terms of overall quality scores. This suggests that the use of advanced vinification procedures with ascorbic acid during maceration (MA) led to a decrease in wine quality.
The spider plots in Figure 3, Figure 4, Figure 5 and Figure 6 show the QDA results of the of experimental wine samples during maturation periods. The influence of maceration procedures and their modifications had significant effects on each sensory attribute of the performed QDA (p < 0.001). On the other hand, period of wine maturation affected significantly only the following attributes: yellow appearance (p < 0.01), apricot (p < 0.01), and citrus fruit aroma (p < 0.05), sour (p < 0.05), apricot (p < 0.01), mineral (p < 0.01), and pear taste (p < 0.01).
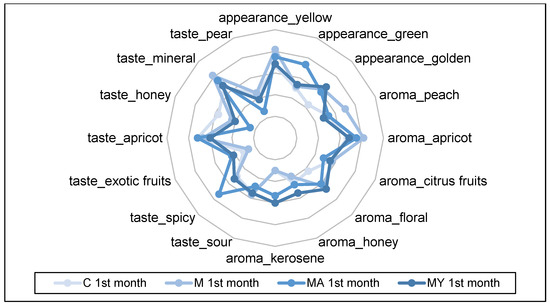
Figure 3.
The results of the Quantitative Descriptive Analysis of the experimental wine samples after one month of maturation (means). C: the control group of wine samples. M: wine samples that underwent maceration in wine production technology. MA: wine samples that underwent maceration in wine production technology with addition of the ascorbic acid. MY: wine samples that underwent maceration in wine production technology with an added yeast culture.
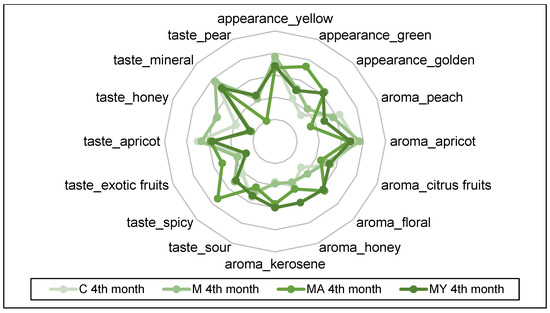
Figure 4.
The results of the Quantitative Descriptive Analysis of the experimental wine samples after fourth month of maturation (means). C: the control group of wine samples. M: wine samples that underwent maceration in wine production technology. MA: wine samples that underwent maceration in wine production technology with the addition of ascorbic acid. MY: wine samples that underwent maceration in wine production technology with an added yeast culture.
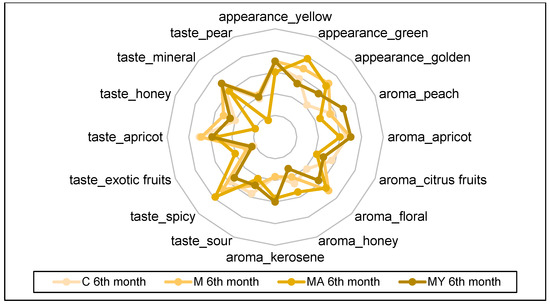
Figure 5.
The results of the Quantitative Descriptive Analysis of the experimental wine samples after the sixth month of maturation (means). C: the control group of wine samples. M: wine samples that underwent maceration in wine production technology. MA: wine samples that underwent maceration in wine production technology with addition of the ascorbic acid. MY: wine samples that underwent maceration in wine production technology with an added yeast culture.
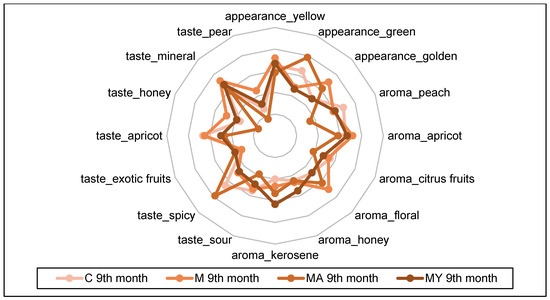
Figure 6.
The results of the Quantitative Descriptive Analysis of the experimental wine samples after ninth month of maturation (means). C: the control group of wine samples. M: wine samples that underwent maceration in wine production technology. MA: wine samples that underwent maceration in wine production technology with the addition of ascorbic acid. MY: wine samples that underwent maceration in wine production technology with an added yeast culture.
In the group of control wine samples (C), the intensities of yellow (6.50 ± 0.55) and green (6.50 ± 1.76) appearance dominated; peach (6.83 ± 0.98) and apricot (6.50 ± 0.55) aroma; spicy (6.50 ± 1.76), apricot (6.67 ± 0.52), and mineral (6.50 ± 0.55) taste. The macerated wine samples (M) without the use of ascorbic acid or yeast culture were characterised by the following sensory attributes: yellow (7.17 ± 0.75) and golden (4.83 ± 0.75) appearance; apricot (7.17 ± 0.75) and floral (7.00 ± 0.89) aroma; mineral (7.17 ± 0.75) taste.
On the other hand, macerated wine samples with the addition of ascorbic acid (MA) showed the highest intensities of the green appearance (7.83 ± 0.89) and spicy taste (7.83 ± 0.98).
Wine samples with the addition of yeast culture in the process of the skin maceration were evaluated with the highest intensities of the yellow (6.67 ± 0.82) colour; peach (6.00 ± 1.41), apricot (5.67 ± 0.82) and kerosene (6.33 ± 1.03) aroma; and mineral (6.67 ± 0.82) taste.
4. Discussion
The application of different winemaking techniques is crucial when considering the ability to produce wines with diversified organoleptic profiles and the potential to impart greater aroma complexity to the beverages, along with other sensory characteristics [27,28]. The sensory and physicochemical characteristics of white wines are strongly influenced by the methods employed during the various stages of winemaking [29,30]. Among these processes, pellicular maceration techniques play a key role in enhancing wine quality this process directly influences the sensory profile of the beverage [31], e.g., by the extraction of the various components from grapes, such as phenolic compounds, aroma precursors, proteins, amino acids and polysaccharides [32]. The chemical profiles of Slovak white wines vary by region, affecting their taste and overall quality. For instance, wines from different geographical regions exhibit diverse total polyphenol and flavonoid contents, which are directly correlated with their antioxidant properties, which are critical in determining the perceived health benefits associated with moderate wine consumption [12,33].
The relationship between ascorbic acid, pH, and total acid content in wine is significant, as these components interact to influence the stability, flavour profile, and overall quality of the wine [34,35]. Ascorbic acid, known for its antioxidant properties, plays an essential role in the preservation of wine, and its efficacy can be influenced by the pH and total acidity of the wine. Ascorbic acid is the most stable in acidic conditions; hence, lower pH values can enhance its effectiveness. The acidic environment of wine, typically maintained by various organic acids such as tartaric, malic, and citric acids, contributes to the overall sensory profile and affects the behaviour of ascorbic acid during storage and ageing [36,37]. A lower pH can facilitate the preservation of ascorbic acid, preventing its rapid degradation into dehydroascorbic acid, which is less effective as an antioxidant [4]. In the wine matrix, ascorbic acid may also react with oxygen to form hydrogen peroxide, potentially leading to undesired effects if not managed properly [9,38]. This statement is in agreement with our chemical determination, in which we found a lower pH value and an increasing antioxidant capacity of the experimental MA samples. However, the concentration of ascorbic acid after nine months of maturation was significantly decreased (p < 0.001). Based on the results, it can be concluded that macerated wine samples have increased their antioxidant capacity mainly by increasing the content of polyphenols and flavonoids, which are generally natural biological antioxidants [34]. Additionally, the presence of organic acids increases the overall acidity, which is critical for maintaining the integrity of ascorbic acid throughout the winemaking process. According to our measurements of pH and total acid content of experimental wine samples, we found decreasing values of pH and total acid content with increasing time of maturation. The changes were significantly affected by the modification of the maceration process and the maturation time (p < 0.001).
The increase in pH in the samples C and M during maturation can be explained by a combination of chemical–physical processes and previous microbial activity. In samples C, the increase in pH was influenced by previous microbial activity, which could have transformed certain acids and metabolites, affecting the overall acidity, while chemical interactions with phenols and added SO2 further reduced free acidity, thereby increasing the pH. In the M samples, which underwent pre-fermentative maceration with spontaneous fermentation, the pH after the first month was higher than in the C samples, related to more intense activity of natural yeasts and lactobacilli that produced metabolites capable of partially neutralising acids or altering their availability. After the fourth month, when LAB gradually disappeared, pH still slightly increased, likely due to chemical–physical processes such as acid binding to phenols and interaction with SO2, similarly to the C samples [39].
HIS and TYR are the most toxic BA found in wine [40]. Our findings are in accordance with Cinquanta et al. [41], who found that no significant HIS production was observed in white wines when the pH was adjusted to 3.2, and Zheng et al. [42] proposed a critical pH range of 3.5–3.6, above which BA levels can increase [42]. Our results of pH measurements of the wine samples after nine months of maturation showed that the pH of C and M samples was 3.3 on average, and 3.2 in MA and MY samples. However, HIS and TYR concentrations in control and experimental wine samples were affected by factors other than pH. International legislation has not yet placed restrictions on BAs. Some EU countries (Germany, the Netherlands, Belgium, France) have established maximum permissible limits for HIS in wine (in the range of 2–10 mg·L−1); however, these limits are optional and may cause serious issues in commercial transactions. According to the European Food Safety Authority, further research is needed on the toxicity and associated concentrations of HIS and TYR, as well as the related potentiating effects of putrescine and cadaverine, which may provide new insight into the development of new safety criteria for HIS in fermented foods other than fish [43,44,45,46,47]. Our observations of the HIS concentration in control and experimental samples were below 10 mg·L−1. After the first month of maturation, the highest concentration of BA was observed in the M samples (maceration with spontaneous fermentation). This phenomenon is caused by a combination of spontaneous microbial activity and pre-fermentative skin maceration, which releases more amino acids and nitrogenous compounds—substrates for the formation of histamine and tyramine. The naturally present strains of yeasts and lactic acid bacteria had optimal conditions for growth and BA production, as no added yeast culture was used to suppress their activity. In the MY samples, BA values were lower than in the M samples, although still higher than in the control group C. The added yeast quickly dominated the environment and limited the activity of natural microorganisms capable of producing BA, thereby reducing the formation of biogenic amines. The control group C, which underwent classic fermentation without maceration, showed the lowest BA values, likely due to the limited availability of amino acids during fermentation.
Another critical element is considering the influence of ascorbic acid on the microbiota of wine during fermentation and maturation. In our investigation, the addition of ascorbic acid caused a rise in TVC and YM, which declined gradually and then levelled off, just like in the other samples. LAB and yeasts, particularly Saccharomyces cerevisiae, significantly influence the quality of wine since the taste, aroma, and other chemical characteristics depend on these microorganisms. They are active during fermentation and generate different metabolites that define the wine’s sensory profile [48].
Furthermore, during maceration, yeast, nutrients and phenolic compounds become more available, stimulating microbial activity—particularly LAB—which can persist longer. This corresponds to the elevation of LAB and yeast in macerated samples [49]. The persistence of LAB, specifically in MA samples, was maintained during the fermentation process until the sixth month. This may explain the enhanced longevity due to the antioxidative properties of ascorbic acid, lessening oxidative stress, thereby making conditions more favourable to these microorganisms.
LAB also improve the sensory characteristics of wine by softening its acidity through the conversion of malic acid to lactic acid and carbon dioxide. However, uncontrolled or prolonged LAB activity can create unwanted metabolites such as BA, acetic acid, and other by-products. These can also lead to off-flavours and a decline in wine quality [50,51]. This could be a result of the addition of yeast culture during the maceration of MY experimental samples, where a higher HIS content (Figure 1), decreased sensory evaluation, and increased intensities of negative wine descriptors (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 respectively) were also observed.
During the malolactic fermentation process (MLF), LAB lower the total acidity and raise the pH of the wine [52]. In the control and macerated groups, we saw a rise in pH and a drop in titratable acidity, which is typical during MLF. In contrast, the MA group with added ascorbic acid showed a decrease in pH and a higher TA compared to the other samples. This is likely due to the antioxidant effects of ascorbic acid, which influence microbial activity and slow MLF [53].
Ascorbic acid acts as a strong reducing agent in wine by absorbing oxygen and reducing oxidative stress. This helps stabilise the fermentation environment [50]. This helps stabilise the fermentation environment and may extend the viability of LAB [51]. On the other hand, SO2 is a key oxidation inhibitor and antimicrobial agent. It not only reduces oxidation, but also effectively controls the growth of unwanted microorganisms, preventing off-flavour compounds [54].
The combination of ascorbic acid and SO2 creates a synergistic effect: ascorbic acid absorbs oxygen and prevents oxidative damage, while SO2 ensures microbial control and long-term stability of the wine [50]. The slight excess of the 200 mg·L−1 SO2 limit in macerated samples may reflect a response to increased microbial activity and more available nutrients during ageing [52].
Maceration boosts the extraction of polyphenols and flavonoids. These compounds have strong antioxidant properties and can affect microbial dynamics [49]. At the same time, the different maceration times during fermentation with Saccharomyces cerevisiae yeast are responsible for the various antioxidant and antiradical activities of the wine and the content of specific phenolic compounds present in the wine [55]. Moreover, the methodologies employed during production, such as limited maceration to prevent oxidative browning, affect both the sensory attributes and the long-term stability of these wines [56,57]. Higher TPC and TFC in the MA group are associated with the increased antioxidant activity, which lowers oxidative stress and extends microbial survival time. Ascorbic acid acts as an antioxidant, but it may also support LAB survival, leading to higher biogenic amine production [52], although this was not confirmed in our study.
Controlling the microbial population, particularly with SO2 and selective LAB strains, is necessary to reduce the risk of unwanted metabolites [53]. Accurate dosing of ascorbic acid and its use with SO2 is crucial for effective microbial management and maintaining the sensory qualities of wine [51,58]. Using ascorbic acid in winemaking effectively boosts antioxidant stability [34]. However, its impact on microbial populations, especially LAB, needs careful management alongside SO2 to prevent unfavourable changes in taste and quality [54].
Our findings also support this insight, as MA samples had the lowest sensory evaluation scores, likely due to increased microbial activity and the emergence of spicy and green notes, which are generally not favoured [59]. Although ascorbic acid lessens oxidative stress and extends microbial activity, it may raise the risk of sensory defects [60].
The microbial population and its interaction with chemical parameters are vital for wine quality. While maceration and ascorbic acid enhance antioxidant properties and stability, they also prolong LAB survival, which may raise the risk of biogenic amine formation and defects. Therefore, optimising SO2 levels and managing microbial activity are essential to ensure quality and safety of wine.
5. Conclusions
This study evaluated the impact of modifying the skin maceration process through the addition of ascorbic acid and yeast culture on the production of Welschriesling wine, with the specific aim of reducing BA levels and HIS content. At the same time, the impact on the sensory properties of the wine produced was evaluated. The chemical, microbial and sensory variables were analysed in wine samples over a nine-month maturation period. The formation of bioactive components increased the antioxidant activity and the total phenolic and total flavonoid content of the experimental macerated wine samples. These compounds may potentially reduce oxidative stress and the risk of inflammation, and may contribute to improved cardiovascular health and other positive health outcomes.
This study also confirmed that the modified maceration process promoted chemical reduction, resulting in decreased BA concentrations in the final stages of the Welschriesling wine maturation process. We can conclude that ascorbic acid has a positive effect on the chemical qualities of wine by reducing HIS formation; however, higher concentrations of ascorbic acid resulted in lower sensory evaluation scores and increased intensities of negative sensory descriptors in these wine samples. This study provides a basis for future research and development of macerated wines. However, it is necessary to investigate the optimal concentrations of ascorbic acid.
Author Contributions
Conceptualization, L.Š., B.S. and S.M.; methodology, M.B. and Z.M.E.; software, S.M.; validation, B.S., I.R. and Z.M.E.; formal analysis, P.O., I.R. and Z.M.E.; investigation, B.S., M.B., P.O., I.R., Z.M.E., and L.Š.; resources, B.S.; data curation, B.S.; writing—original draft preparation, B.S., I.R., Z.M.E. and L.Š.; writing—review and editing, B.S. and S.M.; visualisation, B.S.; supervision, S.M.; project administration, B.S. and S.M.; funding acquisition, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences under the project VEGA 1/0156/21.
Institutional Review Board Statement
Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
This study involved sensory evaluation by trained panellists and sensory evaluation with the participation of 12 evaluators (aged 26–49) from the staff of the University of Veterinary Medicine and Pharmacy in Košice. The assessors received training involving 120 h of sensory analysis, which covered methodological, technical and tasting sessions. In compliance with food science ethics regulations and data protection laws, including the General Data Protection Regulation (GDPR), the participants were thoroughly informed about the study’s purpose, procedures and risks and provided written consent prior to their involvement. They were also informed that they could withdraw from the study at any time without any consequences. The participants were not coerced to participate and received no monetary or other incentives. The data collected from the participants were anonymized and stored securely. As the study did not involve vulnerable populations or novel foods and given its low-risk nature, ethical committee approval was deemed unnecessary, in accordance with the Institutional Commission’s Ethics Review board.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Lots of gratitude to the company REGIA TT Ltd. Orechová (Slovakia) for their cooperation and granting us permission to take samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AV | Alcohol Volume |
| BA | Biogenic Amines |
| CFU | Colony-Forming Units |
| GAE | Gallic acid |
| HIS | Histamine |
| LAB | Lactic Acid Bacteria |
| MLF | Malolactic Fermentation Process |
| QDA | Quantitative Descriptive Analysis |
| QE | Quercetin |
| SO2 | Sulphur Dioxide |
| TA | Titratable Acidity |
| TFC | Total Flavonoids Content |
| TPC | Total Polyphenol Content |
| TVC | total viable counts |
| TYR | Tyramine |
| YM | yeasts and moulds |
References
- Jakabová, S.; Fikselová, M.; Mendelová, A.; Ševčík, M.; Jakab, I.; Aláčová, Z.; Kolačkovská, J.; Ivanova-Petropulos, V. Chemical Composition of White Wines Produced from Different Grape Varieties and Wine Regions in Slovakia. Appl. Sci. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Fikselová, M.; Šnirc, M.; Rybnikár, S.; Mezey, J.; Jakabová, S.; Zeleňáková, L.; Vlčko, T.; Baláška, M. The wine quality description of different origin evaluated by modern chemometric approach. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e9270. [Google Scholar] [CrossRef]
- Clark, A.; Vestner, J.; Barril, C.; Maury, C.; Prenzler, P.; Scollary, G. The influence of stereochemistry of antioxidants and flavanols on oxidation processes in a model wine system: Ascorbic acid, erythorbic acid, (+)-catechin and (−)-epicatechin. J. Agric. Food Chem. 2010, 58, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Pedretti, F.; Prenzler, P.; Scollary, G. Impact of ascorbic acid on the oxidative coloration and associated reactions of a model wine solution containing (+)−catechin, caffeic acid and iron. Aust. J. Grape Wine Res. 2008, 14, 238–249. [Google Scholar]
- Skouroumounis, G.; Kwiatkowski, M.; Francis, I.; Oakey, H.; Capone, D.; Peng, Z.; Duncan, .B.; Waters, E. The influence of ascorbic acid on the composition, colour and flavour properties of a riesling and a wooded chardonnay wine during five years’ storage. Aust. J. Grape Wine Res. 2005, 11, 355–368. [Google Scholar] [CrossRef]
- Blackman, J.W.; Saliba, A.J. Sensory characterisation of Hunter Valley Semillon using descriptive analysis. Flavour Fragr. J. 2009, 24, 238–244. [Google Scholar] [CrossRef]
- Blackman, J.W.; Hopfer, H.; Saliba, A.J.; Schmidtke, L.M.; Barril, C.; Scollary, G.R. Sensory characterization of Hunter Valley Semillon aged in bottle. Flavour Fragr. J. 2014, 29, 340–349. [Google Scholar] [CrossRef]
- Morozova, K.; Schmidt, O.; Schwack, W. Effect of headspace volume, ascorbic acid and sulphur dioxide on oxidative status and sensory profile of Riesling wine. Eur. Food Res. Technol. 2015, 240, 205–221. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef]
- Danilewicz, J.C.; Wallbridge, P.J. Further studies on the mechanism of the interaction of polyphenols, oxygen, and sulfite in wine. Am. J. Enol. Vitic. 2010, 61, 166–175. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking, 1st ed.; Springer: New York, NY, USA, 1999; pp. 448–473. [Google Scholar]
- Bajčan, D.; Árvay, J.; Vollmannová, A.; Bystrická, J.; Trebichalský, P.; Harangozo, Ľ.; Šimanský, V. Antioxidant properties, total phenolic and total flavonoid content of the slovak white wines—welschriesling and chardonnay. Potr. S. J. F. Sci. 2017, 11, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Kalhaa, R.; Damgude, G.; Dabade, A. Reduction of food histamine in white wine by bromelain, quercetin and ascorbic acid. Food Sci. Appl. Biotechnol. 2021, 4, 130–137. [Google Scholar] [CrossRef]
- Sonni, F.; Clark, A.; Prenzler, P.; Riponi, C.; Scollary, G. Antioxidant action of glutathione and the ascorbic acid/glutathione pair in a model white wine. J. Agric. Food Chem. 2011, 59, 3940–3949. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010, 75, 139–150. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Analysis; OIV: Paris, France, 2021; ISBN 9782850380334. Available online: https://www.oiv.int/public/medias/7788/oiv-compendium-of-international-methods-of-analysis-vol2-en.pdf (accessed on 9 July 2025).
- OIV-MA-VI-09; Compendium of International Methods of Analysis for Vinegars. OIV (International Organisation of Vine and Wine): Paris, France, 2023.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singelton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic—phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 6, 144–158. [Google Scholar] [CrossRef]
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 292, 695–698. [Google Scholar] [CrossRef]
- Regecová, I.; Semjon, B.; Jevinová, P.; Očenáš, P.; Výrostková, J.; Šuľáková, L.; Nosková, E.; Marcinčák, S.; Bartkovský, M. Detection of Microbiota during the Fermentation Process of Wine in Relation to the Biogenic Amine Content. Foods 2022, 11, 3061. [Google Scholar] [CrossRef]
- ISO 4833-1:2014; Microbiology of Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. Slovak Standards Institute: Bratislava, Slovakia, 2014.
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. Slovak Standards Institute: Bratislava, Slovakia, 2017.
- ISO 21527-1:2010; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater Than 0.95. Slovak Standards Institute: Bratislava, Slovakia, 2010.
- OIV/CONCOURS 332A/2009; OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions. International Organisation of Vine and Wine: Paris, France, 2009.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://cran.r-project.org/bin/windows/base/old/4.4.2/R-4.4.2-win.exe (accessed on 15 December 2024).
- dos Santos Gomes, W.; Partelli, F.L.; da Silva Oliveira, E.C.; Guimarães, C.V.; Simmer, M.M.B.; Filete, C.A.; Guarçoni, R.C.; da Luz, J.M.R.; Moreli, A.P.; Pereira, L.L. Fermentation time and temperature induce changes in the volatile and sensory profile of Coffea canephora var. Conilon subjected to carbonic maceration. J. Food Compos. Anal. 2025, 143, 107636. [Google Scholar] [CrossRef]
- Mercanti, N.; Macaluso, M.; Pieracci, Y.; Flamini, G.; Scappaticci, G.; Marianelli, A.; Zinnai, A. Towards Sulphite-Free Winemaking: A New Horizon of Vinification and Maturation. Foods 2024, 13, 1108. [Google Scholar] [CrossRef]
- Bajčan, D.; Vollmannová, A.; Šnirc, M.; Árvay, J.; Mezey, J.; Šimanský, V.; Trebichalský, P.; Stanovič, R.; Timoracká, M. Phenolic compounds and antiradical activity in tokaj wines. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 955–959. [Google Scholar] [CrossRef]
- Ladvenicová, J.; Čeryová, D.; Bajusová, Z. Analysis of the grape and wine market in slovakia. Visegr. J. Bioecon. Sustain. Dev. 2022, 11, 89–93. [Google Scholar] [CrossRef]
- Radeka, S.; Bestulić, E.; Rossi, S.; Orbanić, F.; Bubola, M.; Plavša, T.; Lukić, I.; Jeromel, A. Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation 2023, 9, 676. [Google Scholar] [CrossRef]
- Boulet, J.C.; Meudec, E.; Ducasse, M.A.; Abi-Habib, E.; Le Gall, S.; Falourd, X.; Bakan, B.; Traore, A.; Lucchi, G.; Gourrat, K.; et al. Insights into multiscale chemical characterisation for the understanding of berry maturation. OENO One 2025, 59, 1–13. [Google Scholar] [CrossRef]
- Čeryová, N.; Bajčan, D.; Lidiková, J.; Musilová, J.; Šnirc, M.; Jančo, I.; Franková, H.; Bláhová, M. Phenolic content and antioxidant activity of slovak varietal wines of muscat type. J. Microbiol. Biotechnol. Food Sci. 2021, 10, e4292. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Tsai, Y.C.; Fu, C.C.; Wu, J.S.B. Degradation of ascorbic acid in ethanolic solutions. J. Agric. Food Chem. 2012, 60, 10696–10701. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Królak, M.; Kalisz, S. Polyphenols, L-ascorbic acid, and antioxidant activity in wines from rose fruits (Rosa rugosa). Molecules 2021, 26, 2561. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Scollary, G.R.; Prenzler, P.D. Examination of the sulfur dioxide–ascorbic acid anti-oxidant system in a model white wine matrix. J. Sci. Food Agric. 2004, 84, 318–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Zhang, F.; Yuan, D.; Yi, L.; Min, Z. Effect of SO2, glutathione, and glutathione-rich inactive dry yeast on the color, phenolic compounds, ascorbic acid, and antioxidant activity of Roxburgh rose wine. J. Food Sci. 2024, 89, 2814–2826. [Google Scholar] [CrossRef]
- Pons-Mercadé, P.; Anguela, S.; Giménez, P.; Heras, J.M.; Sieczkowski, N.; Rozès, N.; Canals, J.M.; Zamora, F. Measuring the oxygen consumption rate of some inactivated dry yeasts: Comparison with other common wine antioxidants. OENO One 2021, 55, 147–158. [Google Scholar] [CrossRef]
- Alves, C.A.N.; Biasoto, A.C.T.C.T.; da Silva Nunes, G.; do Nascimento, H.O.; do Nascimento, R.F.; Oliveira, I.G.; de Leão, P.C.S.; de Vasconcelos, L.B. Exploring the Impact of Elevated pH and Short Maceration on the Deterioration of Red Wines: Physical and Chemical Perspectives. OENO One 2025, 59, 8111. [Google Scholar] [CrossRef]
- Moreira, L.; Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Exploring factors influencing the levels of biogenic amines in wine and microbiological strategies for controlling their occurrence in winemaking. Food Res. Int. 2024, 190, 114558. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; De Stefano, G.; Formato, D.; Niro, S.; Panfili, G. Effect of pH on malolactic fermentation in southern Italian wines. Eur. Food Res. Technol. 2018, 244, 1261–1268. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic Fermentation—Theoretical Advances and Practical Considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Míšková, Z.; Lorencová, E.; Salek, R.N.; Koláčková, T.; Trávníková, L.; Rejdlová, A.; Buňková, L.; Buňka, F. Occurrence of Biogenic Amines in Wines from the Central European Region (Zone B) and Evaluation of Their Safety. Foods 2023, 12, 1835. [Google Scholar] [CrossRef]
- La Torre, G.L.; Rotondo, A.; Salvo, A. Do vine cropping and breeding practices affect the biogenic amines’ content of produced wines? J. Food Compost. Anal. 2023, 115, 104901. [Google Scholar] [CrossRef]
- Esposito, F.; Montuori, P.; Schettino, M.; Velotto, S.; Stasi, T.; Romano, R.; Cirillo, T. Level of Biogenic Amines in Red and White Wines, Dietary Exposure, and Histamine-Mediated Symptoms upon Wine Ingestion. Molecules 2019, 24, 3629. [Google Scholar] [CrossRef]
- Marques, A.P.; Leitão, M.C.; San Romão, M.V. Biogenic amines in wines: Influence of oenological factors. Food Chem. 2008, 107, 853–860. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Zhu, L.; Wang, Y. Microorganisms: The Key Regulators of Wine Quality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70198. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Control of Malolactic Fermentation in Wine. A Review. S. Afr. J. Enol. Vitic. 2004, 25, 74–88. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Chen, J.; Wei, X.Y.; Lin, B.; Zheng, F.J.; Verma, K.K.; Chen, G.L. Response of alcohol fermentation strains, mixed fermentation and extremozymes interactions on wine flavor. Front. Microbiol. 2025, 16, 1532539. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, C.; Zhang, L.; Chu, R.; Yu, Q.; Cai, J.; Zhang, M. The impact of simultaneous inoculation with Torulaspora delbrueckii and Hanseniaspora uvarum combined with Saccharomyces cerevisiae on chemical and sensory quality of Sauvignon blanc wines. Front. Microbiol. 2024, 15, 1413650. [Google Scholar] [CrossRef]
- Krieger, S.; Arnink, K. Malolactic Fermentation—Timing of Inoculation and Yeast Bacteria Combinations. Internet J. Vitic. Enol. 2005, 10, 1–11. [Google Scholar]
- Lisov, N.; Petrović, A.; Čakar, U.; Jadranin, M.; Tešević, V.; Bukarica-Gojković, L. Extraction Kinetic of Some Phenolic Compounds during Cabernet Sauvignon Alcoholic Fermentation and Antioxidant Properties of Derived Wines. Maced. J. Chem. Chem. Eng. 2020, 39, 185–196. [Google Scholar] [CrossRef]
- Lapčíková, B.; Lapčík, L.; Hupková, J. Physico-chemical characterisation of Slovak wines. Potr. S. J. F. Sci. 2017, 11, 216–222. [Google Scholar] [CrossRef][Green Version]
- Ivanova-Petropulos, V.; Vojnoski, B.; Стефoва, М. Effect of the Winemaking Practices and Aging on Phenolic Content of Smederevka and Chardonnay Wines. Food Bioprocess. Tech. 2011, 4, 1512–1518. [Google Scholar] [CrossRef]
- Sumby, K.M.; Niimi, J.; Betteridge, A.L.; Jiranek, V. Ethanol tolerant lactic acid bacteria strains as a basis for efficient malolactic fermentation in wine: Evaluation of experimentally evolved lactic acid bacteria and winery isolates. Aust. J. Grape Wine Res. 2019, 25, 404–413. [Google Scholar] [CrossRef]
- Renouf, V.; Claisse, O.; Lonvaud Funel, A. Understanding the microbial ecosystem on the grape berry surface through numeration and identification of yeast and bacteria. Aust. J. Grape Wine Res. 2005, 11, 316–327. [Google Scholar] [CrossRef]
- Rodríguez González, Á.; Ramírez Lozano, D.; Carro Huerga, G.; Zanfaño, L.; Antolín Rodríguez, A.; Casquero, P.A. Phenology of Xylotrechus arvicola (Coleoptera: Cerambycidae) Adults in Spanish Vineyards. Aust. J. Grape Wine Res. 2025, 1, 5555011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).