The Effect of Modification of Wine Maceration Processes with the Addition of Ascorbic Acid and Yeast Culture on Biogenic Amine, Chemical, Microbial and Sensory Variables of Welschriesling Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Preparation and Sampling
2.2. Physical and Chemical Analysis
2.3. Microbial Analysis
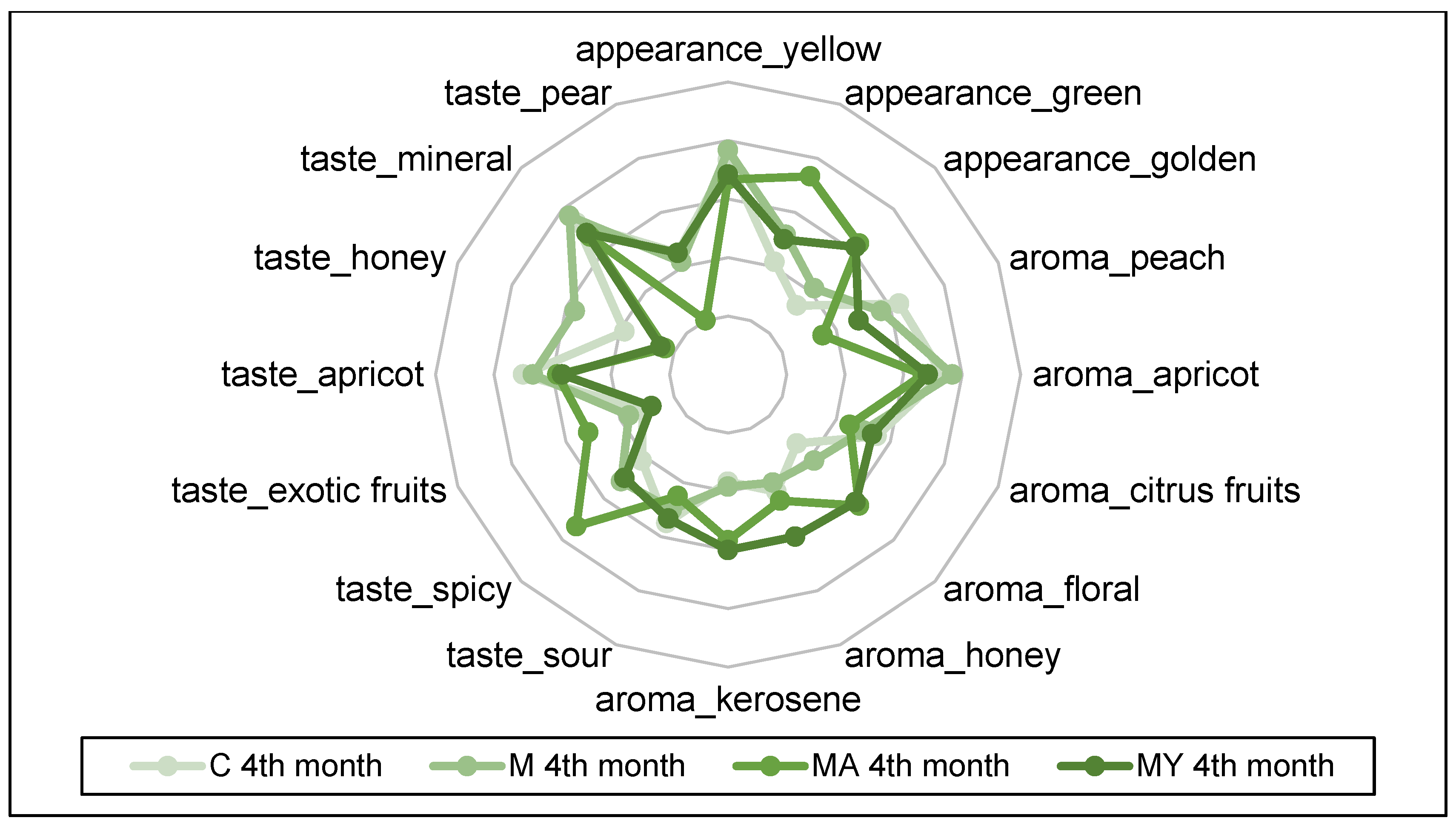
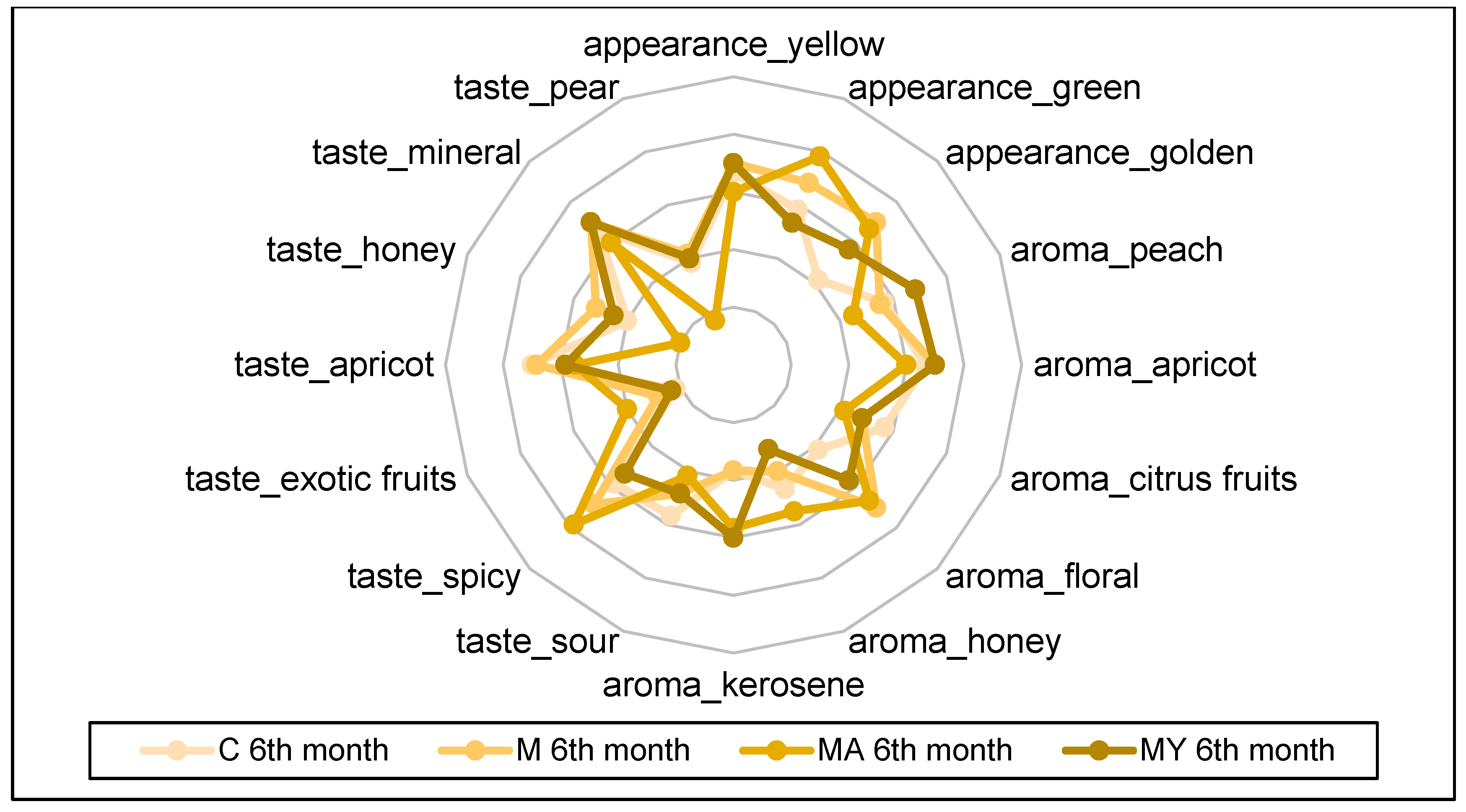
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Alcohol Volume |
| BA | Biogenic Amines |
| CFU | Colony-Forming Units |
| GAE | Gallic acid |
| HIS | Histamine |
| LAB | Lactic Acid Bacteria |
| MLF | Malolactic Fermentation Process |
| QDA | Quantitative Descriptive Analysis |
| QE | Quercetin |
| SO2 | Sulphur Dioxide |
| TA | Titratable Acidity |
| TFC | Total Flavonoids Content |
| TPC | Total Polyphenol Content |
| TVC | total viable counts |
| TYR | Tyramine |
| YM | yeasts and moulds |
References
- Jakabová, S.; Fikselová, M.; Mendelová, A.; Ševčík, M.; Jakab, I.; Aláčová, Z.; Kolačkovská, J.; Ivanova-Petropulos, V. Chemical Composition of White Wines Produced from Different Grape Varieties and Wine Regions in Slovakia. Appl. Sci. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Fikselová, M.; Šnirc, M.; Rybnikár, S.; Mezey, J.; Jakabová, S.; Zeleňáková, L.; Vlčko, T.; Baláška, M. The wine quality description of different origin evaluated by modern chemometric approach. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e9270. [Google Scholar] [CrossRef]
- Clark, A.; Vestner, J.; Barril, C.; Maury, C.; Prenzler, P.; Scollary, G. The influence of stereochemistry of antioxidants and flavanols on oxidation processes in a model wine system: Ascorbic acid, erythorbic acid, (+)-catechin and (−)-epicatechin. J. Agric. Food Chem. 2010, 58, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Pedretti, F.; Prenzler, P.; Scollary, G. Impact of ascorbic acid on the oxidative coloration and associated reactions of a model wine solution containing (+)−catechin, caffeic acid and iron. Aust. J. Grape Wine Res. 2008, 14, 238–249. [Google Scholar]
- Skouroumounis, G.; Kwiatkowski, M.; Francis, I.; Oakey, H.; Capone, D.; Peng, Z.; Duncan, .B.; Waters, E. The influence of ascorbic acid on the composition, colour and flavour properties of a riesling and a wooded chardonnay wine during five years’ storage. Aust. J. Grape Wine Res. 2005, 11, 355–368. [Google Scholar] [CrossRef]
- Blackman, J.W.; Saliba, A.J. Sensory characterisation of Hunter Valley Semillon using descriptive analysis. Flavour Fragr. J. 2009, 24, 238–244. [Google Scholar] [CrossRef]
- Blackman, J.W.; Hopfer, H.; Saliba, A.J.; Schmidtke, L.M.; Barril, C.; Scollary, G.R. Sensory characterization of Hunter Valley Semillon aged in bottle. Flavour Fragr. J. 2014, 29, 340–349. [Google Scholar] [CrossRef]
- Morozova, K.; Schmidt, O.; Schwack, W. Effect of headspace volume, ascorbic acid and sulphur dioxide on oxidative status and sensory profile of Riesling wine. Eur. Food Res. Technol. 2015, 240, 205–221. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef]
- Danilewicz, J.C.; Wallbridge, P.J. Further studies on the mechanism of the interaction of polyphenols, oxygen, and sulfite in wine. Am. J. Enol. Vitic. 2010, 61, 166–175. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking, 1st ed.; Springer: New York, NY, USA, 1999; pp. 448–473. [Google Scholar]
- Bajčan, D.; Árvay, J.; Vollmannová, A.; Bystrická, J.; Trebichalský, P.; Harangozo, Ľ.; Šimanský, V. Antioxidant properties, total phenolic and total flavonoid content of the slovak white wines—welschriesling and chardonnay. Potr. S. J. F. Sci. 2017, 11, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Kalhaa, R.; Damgude, G.; Dabade, A. Reduction of food histamine in white wine by bromelain, quercetin and ascorbic acid. Food Sci. Appl. Biotechnol. 2021, 4, 130–137. [Google Scholar] [CrossRef]
- Sonni, F.; Clark, A.; Prenzler, P.; Riponi, C.; Scollary, G. Antioxidant action of glutathione and the ascorbic acid/glutathione pair in a model white wine. J. Agric. Food Chem. 2011, 59, 3940–3949. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010, 75, 139–150. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Analysis; OIV: Paris, France, 2021; ISBN 9782850380334. Available online: https://www.oiv.int/public/medias/7788/oiv-compendium-of-international-methods-of-analysis-vol2-en.pdf (accessed on 9 July 2025).
- OIV-MA-VI-09; Compendium of International Methods of Analysis for Vinegars. OIV (International Organisation of Vine and Wine): Paris, France, 2023.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singelton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic—phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 6, 144–158. [Google Scholar] [CrossRef]
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 292, 695–698. [Google Scholar] [CrossRef]
- Regecová, I.; Semjon, B.; Jevinová, P.; Očenáš, P.; Výrostková, J.; Šuľáková, L.; Nosková, E.; Marcinčák, S.; Bartkovský, M. Detection of Microbiota during the Fermentation Process of Wine in Relation to the Biogenic Amine Content. Foods 2022, 11, 3061. [Google Scholar] [CrossRef]
- ISO 4833-1:2014; Microbiology of Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. Slovak Standards Institute: Bratislava, Slovakia, 2014.
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. Slovak Standards Institute: Bratislava, Slovakia, 2017.
- ISO 21527-1:2010; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater Than 0.95. Slovak Standards Institute: Bratislava, Slovakia, 2010.
- OIV/CONCOURS 332A/2009; OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions. International Organisation of Vine and Wine: Paris, France, 2009.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://cran.r-project.org/bin/windows/base/old/4.4.2/R-4.4.2-win.exe (accessed on 15 December 2024).
- dos Santos Gomes, W.; Partelli, F.L.; da Silva Oliveira, E.C.; Guimarães, C.V.; Simmer, M.M.B.; Filete, C.A.; Guarçoni, R.C.; da Luz, J.M.R.; Moreli, A.P.; Pereira, L.L. Fermentation time and temperature induce changes in the volatile and sensory profile of Coffea canephora var. Conilon subjected to carbonic maceration. J. Food Compos. Anal. 2025, 143, 107636. [Google Scholar] [CrossRef]
- Mercanti, N.; Macaluso, M.; Pieracci, Y.; Flamini, G.; Scappaticci, G.; Marianelli, A.; Zinnai, A. Towards Sulphite-Free Winemaking: A New Horizon of Vinification and Maturation. Foods 2024, 13, 1108. [Google Scholar] [CrossRef]
- Bajčan, D.; Vollmannová, A.; Šnirc, M.; Árvay, J.; Mezey, J.; Šimanský, V.; Trebichalský, P.; Stanovič, R.; Timoracká, M. Phenolic compounds and antiradical activity in tokaj wines. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 955–959. [Google Scholar] [CrossRef]
- Ladvenicová, J.; Čeryová, D.; Bajusová, Z. Analysis of the grape and wine market in slovakia. Visegr. J. Bioecon. Sustain. Dev. 2022, 11, 89–93. [Google Scholar] [CrossRef]
- Radeka, S.; Bestulić, E.; Rossi, S.; Orbanić, F.; Bubola, M.; Plavša, T.; Lukić, I.; Jeromel, A. Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation 2023, 9, 676. [Google Scholar] [CrossRef]
- Boulet, J.C.; Meudec, E.; Ducasse, M.A.; Abi-Habib, E.; Le Gall, S.; Falourd, X.; Bakan, B.; Traore, A.; Lucchi, G.; Gourrat, K.; et al. Insights into multiscale chemical characterisation for the understanding of berry maturation. OENO One 2025, 59, 1–13. [Google Scholar] [CrossRef]
- Čeryová, N.; Bajčan, D.; Lidiková, J.; Musilová, J.; Šnirc, M.; Jančo, I.; Franková, H.; Bláhová, M. Phenolic content and antioxidant activity of slovak varietal wines of muscat type. J. Microbiol. Biotechnol. Food Sci. 2021, 10, e4292. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Tsai, Y.C.; Fu, C.C.; Wu, J.S.B. Degradation of ascorbic acid in ethanolic solutions. J. Agric. Food Chem. 2012, 60, 10696–10701. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Królak, M.; Kalisz, S. Polyphenols, L-ascorbic acid, and antioxidant activity in wines from rose fruits (Rosa rugosa). Molecules 2021, 26, 2561. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Scollary, G.R.; Prenzler, P.D. Examination of the sulfur dioxide–ascorbic acid anti-oxidant system in a model white wine matrix. J. Sci. Food Agric. 2004, 84, 318–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Zhang, F.; Yuan, D.; Yi, L.; Min, Z. Effect of SO2, glutathione, and glutathione-rich inactive dry yeast on the color, phenolic compounds, ascorbic acid, and antioxidant activity of Roxburgh rose wine. J. Food Sci. 2024, 89, 2814–2826. [Google Scholar] [CrossRef]
- Pons-Mercadé, P.; Anguela, S.; Giménez, P.; Heras, J.M.; Sieczkowski, N.; Rozès, N.; Canals, J.M.; Zamora, F. Measuring the oxygen consumption rate of some inactivated dry yeasts: Comparison with other common wine antioxidants. OENO One 2021, 55, 147–158. [Google Scholar] [CrossRef]
- Alves, C.A.N.; Biasoto, A.C.T.C.T.; da Silva Nunes, G.; do Nascimento, H.O.; do Nascimento, R.F.; Oliveira, I.G.; de Leão, P.C.S.; de Vasconcelos, L.B. Exploring the Impact of Elevated pH and Short Maceration on the Deterioration of Red Wines: Physical and Chemical Perspectives. OENO One 2025, 59, 8111. [Google Scholar] [CrossRef]
- Moreira, L.; Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Exploring factors influencing the levels of biogenic amines in wine and microbiological strategies for controlling their occurrence in winemaking. Food Res. Int. 2024, 190, 114558. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; De Stefano, G.; Formato, D.; Niro, S.; Panfili, G. Effect of pH on malolactic fermentation in southern Italian wines. Eur. Food Res. Technol. 2018, 244, 1261–1268. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic Fermentation—Theoretical Advances and Practical Considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Míšková, Z.; Lorencová, E.; Salek, R.N.; Koláčková, T.; Trávníková, L.; Rejdlová, A.; Buňková, L.; Buňka, F. Occurrence of Biogenic Amines in Wines from the Central European Region (Zone B) and Evaluation of Their Safety. Foods 2023, 12, 1835. [Google Scholar] [CrossRef]
- La Torre, G.L.; Rotondo, A.; Salvo, A. Do vine cropping and breeding practices affect the biogenic amines’ content of produced wines? J. Food Compost. Anal. 2023, 115, 104901. [Google Scholar] [CrossRef]
- Esposito, F.; Montuori, P.; Schettino, M.; Velotto, S.; Stasi, T.; Romano, R.; Cirillo, T. Level of Biogenic Amines in Red and White Wines, Dietary Exposure, and Histamine-Mediated Symptoms upon Wine Ingestion. Molecules 2019, 24, 3629. [Google Scholar] [CrossRef]
- Marques, A.P.; Leitão, M.C.; San Romão, M.V. Biogenic amines in wines: Influence of oenological factors. Food Chem. 2008, 107, 853–860. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Zhu, L.; Wang, Y. Microorganisms: The Key Regulators of Wine Quality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70198. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Control of Malolactic Fermentation in Wine. A Review. S. Afr. J. Enol. Vitic. 2004, 25, 74–88. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Chen, J.; Wei, X.Y.; Lin, B.; Zheng, F.J.; Verma, K.K.; Chen, G.L. Response of alcohol fermentation strains, mixed fermentation and extremozymes interactions on wine flavor. Front. Microbiol. 2025, 16, 1532539. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, C.; Zhang, L.; Chu, R.; Yu, Q.; Cai, J.; Zhang, M. The impact of simultaneous inoculation with Torulaspora delbrueckii and Hanseniaspora uvarum combined with Saccharomyces cerevisiae on chemical and sensory quality of Sauvignon blanc wines. Front. Microbiol. 2024, 15, 1413650. [Google Scholar] [CrossRef]
- Krieger, S.; Arnink, K. Malolactic Fermentation—Timing of Inoculation and Yeast Bacteria Combinations. Internet J. Vitic. Enol. 2005, 10, 1–11. [Google Scholar]
- Lisov, N.; Petrović, A.; Čakar, U.; Jadranin, M.; Tešević, V.; Bukarica-Gojković, L. Extraction Kinetic of Some Phenolic Compounds during Cabernet Sauvignon Alcoholic Fermentation and Antioxidant Properties of Derived Wines. Maced. J. Chem. Chem. Eng. 2020, 39, 185–196. [Google Scholar] [CrossRef]
- Lapčíková, B.; Lapčík, L.; Hupková, J. Physico-chemical characterisation of Slovak wines. Potr. S. J. F. Sci. 2017, 11, 216–222. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Vojnoski, B.; Стефoва, М. Effect of the Winemaking Practices and Aging on Phenolic Content of Smederevka and Chardonnay Wines. Food Bioprocess. Tech. 2011, 4, 1512–1518. [Google Scholar] [CrossRef]
- Sumby, K.M.; Niimi, J.; Betteridge, A.L.; Jiranek, V. Ethanol tolerant lactic acid bacteria strains as a basis for efficient malolactic fermentation in wine: Evaluation of experimentally evolved lactic acid bacteria and winery isolates. Aust. J. Grape Wine Res. 2019, 25, 404–413. [Google Scholar] [CrossRef]
- Renouf, V.; Claisse, O.; Lonvaud Funel, A. Understanding the microbial ecosystem on the grape berry surface through numeration and identification of yeast and bacteria. Aust. J. Grape Wine Res. 2005, 11, 316–327. [Google Scholar] [CrossRef]
- Rodríguez González, Á.; Ramírez Lozano, D.; Carro Huerga, G.; Zanfaño, L.; Antolín Rodríguez, A.; Casquero, P.A. Phenology of Xylotrechus arvicola (Coleoptera: Cerambycidae) Adults in Spanish Vineyards. Aust. J. Grape Wine Res. 2025, 1, 5555011. [Google Scholar] [CrossRef]



| Variable | Berries | Must |
|---|---|---|
| TVC [log CFU·mL−1] | 3.75 ± 0.06 | 4.05 ± 0.06 |
| LAB [log CFU·mL−1] | 3.05 ± 0.06 | 3.95 ± 0.06 |
| YM [log CFU·mL−1] | 3.85 ± 0.06 | 4.23 ± 0.05 |
| pH | - | 3.27 ± 0.00 |
| Dry matter refractometrically [°Brix] | - | 19.41 ± 0.05 |
| Density [g·cm−3] | - | 1.07 ± 0.00 |
| Total sugars [g·L−1] | - | 178.9 ± 0.19 |
| Total acids [g·L−1] | - | 5.72 ± 0.02 |
| Samples | Period (Months) | pH | Total Acids [g·L−1] | Total SO2 [mg·L−1] | Total Alcohol [%] |
|---|---|---|---|---|---|
| C | 1 | 3.05 ± 0.04 j | 13.65 ± 0.05 a | 121.79 ± 9.45 gh | 10.90 ± 0.41 bc |
| 4 | 3.10 ± 0.02 i | 10.85 ± 0.11 c | 132.49 ± 0.49 fg | 11.30 ± 0.19 ab | |
| 6 | 3.41 ± 0.01 b | 10.21 ± 0.01 def | 141.43 ± 0.66 f | 11.25 ± 0.15 ab | |
| 9 | 3.32 ± 0.04 cd | 10.19 ± 0.03 def | 186.90 ± 2.91 c | 11.23 ± 0.16 ab | |
| M | 1 | 3.26 ± 0.01 e | 13.21 ± 0.02 a | 93.96 ± 13.23 i | 11.25 ± 0.16 ab |
| 4 | 3.28 ± 0.01 e | 10.51 ± 0.01 cde | 114.46 ± 8.37 h | 11.25 ± 0.23 ab | |
| 6 | 3.35 ± 0.01 c | 10.14 ± 0.02 ef | 182.58 ± 9.34 cd | 11.32 ± 0.10 ab | |
| 9 | 3.34 ± 0.02 c | 9.81 ± 0.11 fg | 202.02 ± 9.13 a | 11.32 ± 0.07 ab | |
| MA | 1 | 3.21 ± 0.01 f | 13.24 ± 0.32 a | 139.17 ± 4.60 f | 10.72 ± 0.46 c |
| 4 | 3.16 ± 0.01 g | 12.29 ± 0.82 b | 167.17 ± 1.08 de | 11.23 ± 0.21 ab | |
| 6 | 3.11 ± 0.01 hi | 12.11 ± 0.01 b | 169.13 ± 3.14 de | 11.39 ± 0.01 a | |
| 9 | 3.17 ± 0.02 fg | 11.88 ± 0.01 b | 203.94 ± 9.43 b | 11.31 ± 0.13 ab | |
| MY | 1 | 3.29 ± 0.01 de | 12.02 ± 0.02 b | 137.21 ± 3.69 fg | 11.10 ± 0.17 abc |
| 4 | 3.17 ± 0.01 fg | 10.63 ± 0.02 cd | 158.24 ± 15.77 e | 11.23 ± 0.20 ab | |
| 6 | 3.56 ± 0.01 a | 10.25 ± 0.03 def | 184.72 ± 4.82 c | 11.39 ± 0.04 a | |
| 9 | 3.15 ± 0.02 gh | 9.56 ± 0.01 g | 208.10 ± 2.91 b | 11.27 ± 0.08 ab | |
| p-values | T | p < 0.001 | p < 0.001 | p < 0.001 | p > 0.05 |
| R | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Samples | Period (Months) | Antioxidant Activity [% Inhibition of DPPH] | Total Polyphenols [mg·L−1 GAE] | Total Flavonoids [mg·L−1 QE] | Ascorbic Acid [mg·L−1] |
|---|---|---|---|---|---|
| C | 1 | 58.19 ± 0.58 d | 387.33 ± 19.44 f | 11.46 ± 0.26 i | 4.51 ± 0.10 d |
| 4 | 60.44 ± 1.72 cd | 457.64 ± 20.37 de | 30.14 ± 3.15 h | 4.16 ± 0.01 d | |
| 6 | 62.79 ± 0.16 cd | 462.00 ± 31.00 de | 43.75 ± 1.80 g | 3.87 ± 0.01 d | |
| 9 | 63.23 ± 0.76 cd | 583.42 ± 47.50 b | 50.78 ± 9.77 g | 3.77 ± 0.01 d | |
| M | 1 | 61.19 ± 3.63 cd | 491.26 ± 21.81 cd | 16.11 ± 1.51 i | 5.18 ± 0.00 d |
| 4 | 63.57 ± 1.93 c | 498.67 ± 15.76 cd | 74.54 ± 7.20 f | 5.08 ± 0.00 d | |
| 6 | 73.47 ± 6.13 b | 513.33 ± 22.88 c | 85.42 ± 1.78 de | 4.77 ± 0.01 d | |
| 9 | 76.91 ± 1.95 b | 598.50 ± 2.07 ab | 86.05 ± 0.47 de | 3.54 ± 0.08 d | |
| MA | 1 | 86.41 ± 1.16 a | 343.52 ± 12.17 g | 102.21 ± 2.01 bc | 180.91 ± 0.01 a |
| 4 | 87.55 ± 0.53 a | 362.53 ± 24.88 fg | 105.75 ± 3.23 ab | 131.21 ± 0.21 c | |
| 6 | 87.74 ± 0.16 a | 431.75 ± 4.01 e | 109.41 ± 0.93 ab | 131.21 ± 0.21 b | |
| 9 | 88.37 ± 0.16 a | 629.86 ± 15.08 a | 115.62 ± 11.54 a | 126.31 ± 0.37 b | |
| MY | 1 | 63.24 ± 1.43 cd | 508.33 ± 9.85 c | 15.82 ± 0.61 i | 4.18 ± 0.01 d |
| 4 | 75.36 ± 5.97 b | 559.04 ± 24.82 b | 82.12 ± 7.84 ef | 4.18 ± 0.01 d | |
| 6 | 76.72 ± 0.97 b | 563.50 ± 16.49 b | 84.48 ± 2.04 def | 4.76 ± 0.03 d | |
| 9 | 78.29 ± 0.70 b | 627.00 ± 4.85 a | 93.47 ± 4.25 cd | 3.76 ± 0.06 d | |
| p-values | T | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| R | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
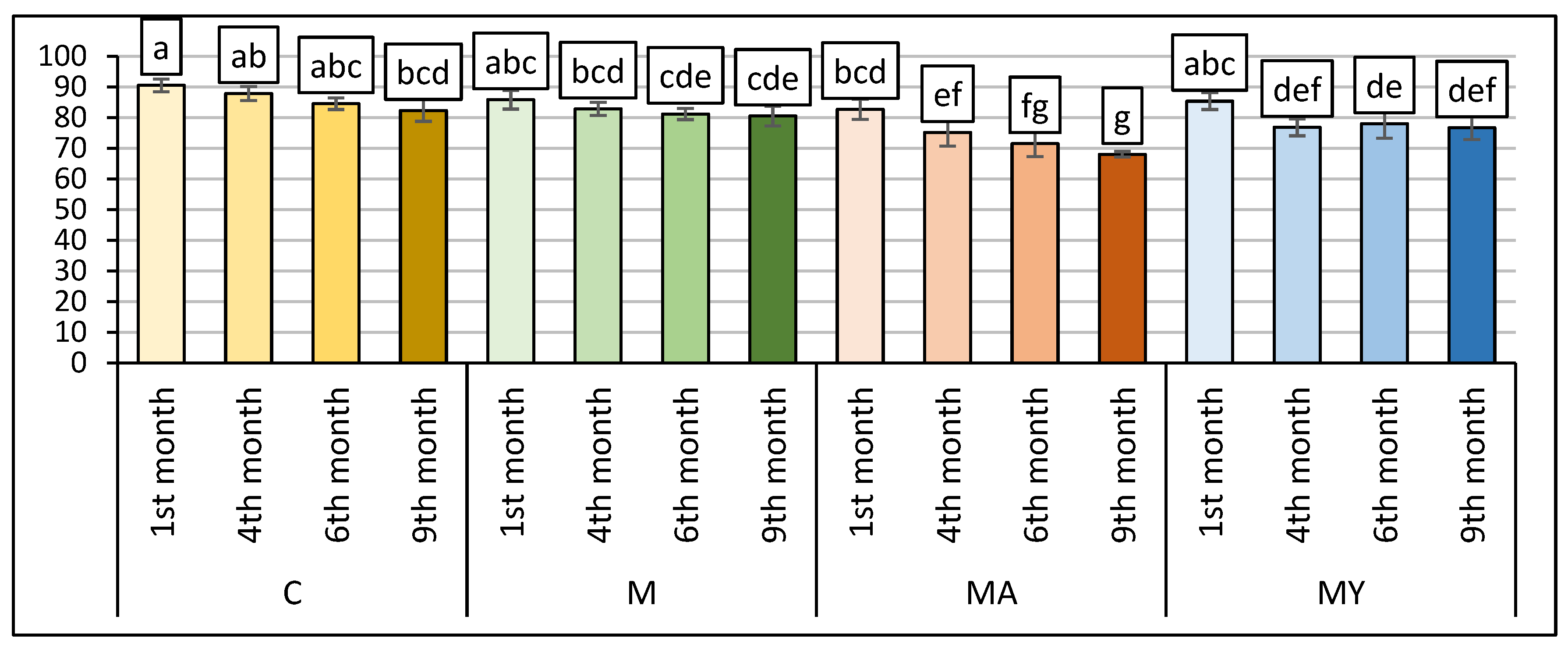
| Samples | Period | Total Viable Count | Lactic Acid Bacteria | Yeasts and Moulds |
|---|---|---|---|---|
| C | 1st month | 3.95 ± 0.05 c | 4.05 ± 0.05 d | 4.55 ± 0.05 c |
| 4th month | 3.25 ± 0.05 e | 0.00 ± 0.00 h | 3.25 ± 0.05 g | |
| 6th month | 2.25 ± 0.05 f | 0.00 ± 0.00 h | 2.05 ± 0.05 j | |
| 9th month | 1.95 ± 0.05 g | 0.00 ± 0.00 h | 2.45 ± 0.05 h | |
| M | 1st month | 5.83 ± 0.05 a | 4.05 ± 0.05 d | 6.15 ± 0.05 a |
| 4th month | 3.45 ± 0.05 d | 4.05 ± 0.05 d | 3.55 ± 0.05 f | |
| 6th month | 2.15 ± 0.05 f | 3.95 ± 0.05 e | 2.25 ± 0.05 i | |
| 9th month | 3.95 ± 0.05 c | 0.00 ± 0.00 h | 3.85 ± 0.05 e | |
| MA | 1st month | 5.10 ± 0.05 b | 5.75 ± 0.05 a | 5.95 ± 0.05 b |
| 4th month | 3.45 ± 0.05 d | 3.45 ± 0.05 g | 4.05 ± 0.05 d | |
| 6th month | 2.15 ± 0.05 f | 3.65 ± 0.05 f | 3.35 ± 0.05 g | |
| 9th month | 3.95 ± 0.05 c | 0.00 ± 0.00 h | 3.35 ± 0.05 g | |
| MY | 1st month | 3.95 ± 0.05 c | 5.15 ± 0.05 b | 5.95 ± 0.05 b |
| 4th month | 3.55 ± 0.05 d | 4.25 ± 0.05 c | 4.55 ± 0.05 c | |
| 6th month | 3.95 ± 0.05 c | 0.00 ± 0.00 h | 3.25 ± 0.05 g | |
| 9th month | 3.85 ± 0.05 c | 0.00 ± 0.00 h | 4.65 ± 0.05 c | |
| p-values | T | p < 0.001 | p < 0.001 | p < 0.001 |
| R | p < 0.001 | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuľáková, L.; Semjon, B.; Regecová, I.; Očenáš, P.; Bartkovský, M.; Megyesy Eftimová, Z.; Marcinčák, S. The Effect of Modification of Wine Maceration Processes with the Addition of Ascorbic Acid and Yeast Culture on Biogenic Amine, Chemical, Microbial and Sensory Variables of Welschriesling Wine. Fermentation 2025, 11, 570. https://doi.org/10.3390/fermentation11100570
Šuľáková L, Semjon B, Regecová I, Očenáš P, Bartkovský M, Megyesy Eftimová Z, Marcinčák S. The Effect of Modification of Wine Maceration Processes with the Addition of Ascorbic Acid and Yeast Culture on Biogenic Amine, Chemical, Microbial and Sensory Variables of Welschriesling Wine. Fermentation. 2025; 11(10):570. https://doi.org/10.3390/fermentation11100570
Chicago/Turabian StyleŠuľáková, Lucia, Boris Semjon, Ivana Regecová, Peter Očenáš, Martin Bartkovský, Zuzana Megyesy Eftimová, and Slavomír Marcinčák. 2025. "The Effect of Modification of Wine Maceration Processes with the Addition of Ascorbic Acid and Yeast Culture on Biogenic Amine, Chemical, Microbial and Sensory Variables of Welschriesling Wine" Fermentation 11, no. 10: 570. https://doi.org/10.3390/fermentation11100570
APA StyleŠuľáková, L., Semjon, B., Regecová, I., Očenáš, P., Bartkovský, M., Megyesy Eftimová, Z., & Marcinčák, S. (2025). The Effect of Modification of Wine Maceration Processes with the Addition of Ascorbic Acid and Yeast Culture on Biogenic Amine, Chemical, Microbial and Sensory Variables of Welschriesling Wine. Fermentation, 11(10), 570. https://doi.org/10.3390/fermentation11100570







