Comparison of Gelatin and Plant Proteins in the Clarification of Grape Musts Using Flotation Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Processing and Must Production
2.2. Preliminary Small-Scale Static Must Sedimentation Studies
2.3. Clarification of the Grape Must Through Flotation
2.4. Chemical Analysis After Flotation Experiments
2.5. Sensory Analysis
3. Results
3.1. Results of Gravitational Sedimentation Trials
3.2. Chemical Analysis of the Musts Related to Flotation Processes
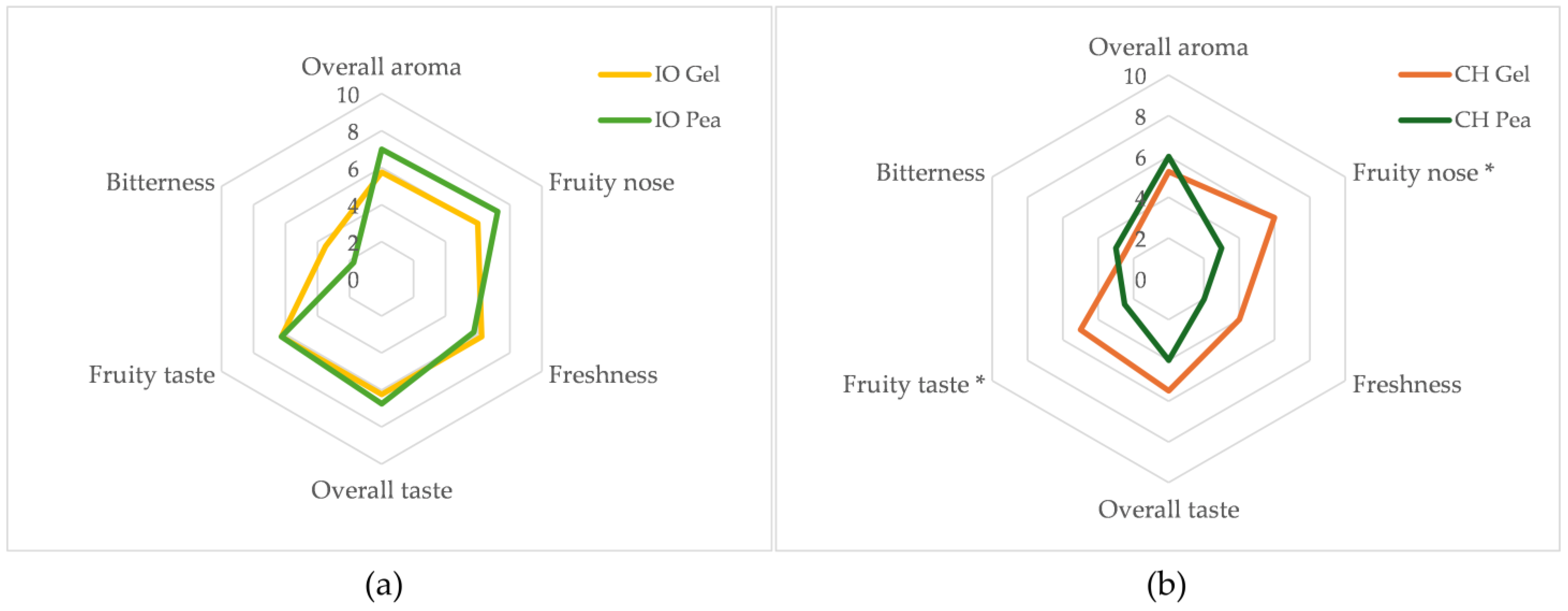
3.3. Sensory Analysis of the Wines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, Y.; Suhag, R. Impact of Fining Agents on Color, Phenolics, Aroma, and Sensory Properties of Wine: A Review. Beverages 2024, 10, 71. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science. Principles and Applications, 3rd ed.; Elsevier: Cambridge, MA, USA, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Bate-Smith, E.C. Usefulness of Chemistry in Plant Taxonomy as Illustrated by the Flavonoid Constituents. In Chemical Plant Taxonomy, 1st ed.; Swain, T., Ed.; Academic Press: London, UK, 1963; pp. 127–139. ISBN 9780124143081. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Dubourdieu, D.; Maujean, A. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; Wiley: Chichester, UK, 2000; ISBN 0-471-97363-7. [Google Scholar]
- Maury, C.; Sarni-Manchado, P.; Lefebvre, S.; Cheynier, V.; Moutounet, M. Influence of Fining with Plant Proteins on Proanthocyanidin Composition of Red Wines. Am. J. Enol. Vitic. 2003, 54, 105–111. [Google Scholar] [CrossRef]
- Tschiersch, C.; Nikfardjam, M.P.; Schmidt, O.; Schwack, W. Degree of Hydrolysis of Some Vegetable Proteins Used as Fining Agents and Its Influence on Polyphenol Removal from Red Wine. Eur. Food Res. Technol. 2010, 231, 65–74. [Google Scholar] [CrossRef]
- Puig-Deu, M.; López-Tamames, E.; Buxaderas, S.; Torre-Boronat, M.C. Quality of Base and Sparkling Wines as Influenced by the Type of Fining Agent Added Pre-Fermentation. Food Chem. 1999, 66, 35–42. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef]
- Kirschner, S.; Belloni, B.; Kugler, C.; Ring, J.; Brockow, K. Allergenicity of Wine Containing Processing Aids: A Double-Blind, Placebo-Controlled Food Challenge. J. Investig. Allergol. Clin. Immunol. 2009, 19, 210–217. [Google Scholar] [PubMed]
- Protein Plant Origin. In International Oenological Codex; International Organisation of Vine and Wine (OIV): Paris, France; Available online: https://www.oiv.int/node/1764/download/pdf (accessed on 11 August 2025).
- Council Regulation (EC) No 2165/2005 of 20 December 2005 Amending Regulation (EC) No 1493/1999 on the Common Organisation of the Market in Wine. Available online: https://eur-lex.europa.eu/eli/reg/2005/2165/oj/eng (accessed on 11 August 2025).
- Hüfner, E.; Sobe, M. Pretty in Pink. Roséweinbereitung. Der Dtsch. Weinbau 2019, 18, 30–32. [Google Scholar]
- Marchal, R.; Marchal-Delahaut, L.; Lallement, A.; Jeandet, P. Wheat Gluten Used as a Clarifying Agent of Red Wines. J. Agric. Food. Chem. 2001, 50, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rihak, Z.; Prusova, B.; Prokes, K.; Baron, M. The Effect of Different Fining Treatments on Phenolic and Aroma Composition of Grape Musts and Wines. Fermentation 2022, 8, 737. [Google Scholar] [CrossRef]
- Chitin-Glucan. In International Oenological Codex; International Organisation of Vine and Wine (OIV): Paris, France; Available online: https://www.oiv.int/public/medias/4122/e-coei-1-chitgl.pdf (accessed on 5 September 2025).
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef]
- Cosme, F.; Vilela, A. Chitin and Chitosan in the Alcoholic and Non-Alcoholic Beverage Industry: An Overview. Appl. Sci. 2021, 11, 11427. [Google Scholar] [CrossRef]
- Sobe, M.; Bausinger, S. Vegane Mostvorklärung. Das Dtsch. Weinmagazin 2020, 15, 34–35. Available online: https://erbsloeh.com/deutsch/download/120/articles/4296/vegane-mostvorklaerung-klaeren-mit-erbsenprotein-und-chitin-glucan.pdf (accessed on 10 September 2025).
- Vincenzi, S.; Polesani, M.; Curioni, A. Removal of Specific Protein Components by Chitin Enhances Protein Stability in a White Wine. Am. J. Enol. Vitic. 2005, 56, 246–254. [Google Scholar] [CrossRef]
- Ndlovu, T.; Divol, B.; Bauer, F.F. Yeast Cell Wall Chitin Reduces Wine Haze Formation. Appl. Environ. Microbiol. 2018, 84, e00668-18. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, P.L. Applications and Interest of Chitin, Chitosan and Their Derivatives in Enology. OENO One 2005, 39, 199–207. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, P.L. Chitosan, Chitin-Glucan and Chitin Effects on Minerals (Iron, Lead, Cadmium) and Organic (Ochratoxin A) Contaminants in Wines. Eur. Food. Res. Technol. 2008, 226, 681–689. [Google Scholar] [CrossRef]
- Quintela, S.; Villarán, M.C.; López de Armentia, I.; Elejalde, E. Ochratoxin A Removal in Wine: A Review. Food Control 2013, 30, 439–445. [Google Scholar] [CrossRef]
- Vázquez-Pateiro, I.; Mirás-Avalos, J.M.; Falqué, E. Influence of Must Clarification Technique on the Volatile Composition of Albariño and Treixadura Wines. Molecules 2022, 27, 810. [Google Scholar] [CrossRef] [PubMed]
- Ailer, Š.; Benešová, L.; Janás, M.; Mankovecký, J. Influence of Clarification Method of Must on Oxygen Regime and the Content of Phenolic Acids in White Wines. Cogent Food Agric. 2025, 11, 2527451. [Google Scholar] [CrossRef]
- Sindou, E.; Vaimakis, V.; Vaimakis, T.; Roussis, I.G. Effect of Juice Clarification by Flotation on the Quality of White Wine and Orange Juice and Drink. Czech J. Food Sci. 2008, 26, 223–228. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Current State of Knowledge and Challenges in Wine Clarification. Aust. J. Grape Wine Res. 2015, 21, 615–626. [Google Scholar] [CrossRef]
- Pettinelli, S.; Pollon, M.; Costantini, L.; Bellincontro, A.; Segade, S.R.; Rolle, L.; Mencarelli, F. Effect of Flotation and Vegetal Fining Agents on the Aromatic Characteristics of Malvasia del Lazio (Vitis vinifera L.) Wine. J. Sci. Food Agric. 2020, 100, 5269–5275. [Google Scholar] [CrossRef]
- Tscholl, S.; Candiago, S.; Marsoner, T.; Fraga, H.; Giupponi, C.; Egarter, L. Climate Resilience of European Wine Regions. Nat. Commun. 2024, 15, 6254. [Google Scholar] [CrossRef]
- Szenteleki, K.; Ladányi, M.; Gaál, M.; Zanathy, G.; Bisztray, G.Y. Climatic Risk Factors of Central Hungarian Grape Growing Regions. Appl. Ecol. Environm. Res. 2012, 10, 87–105. [Google Scholar] [CrossRef]
- Lennert, J.; Kovács, K.; Koós, B.; Swain, N.; Bálint, C.; Hamza, E.; Király, G.; Rácz, K.; Váradi, M.M.; Kovács, A.D. Climate Change, Pressures, and Adaptation Capacities of Farmers: Empirical Evidence from Hungary. Horticulturae 2024, 10, 56. [Google Scholar] [CrossRef]
- Kovács, E.; Puskás, J.; Pozsgai, A.; Kozma, K. Shift in the Annual Growth Cycle of Grapevines (Vitis vinifera L.) in West Hungary. Appl. Ecol. Environm. Res. 2018, 16, 2029–2042. [Google Scholar] [CrossRef]
- Ladányi, M.; Hlaszny, E.; Pernesz, G.; Bisztray, G. Climate Change Impact Study Based on Grapevine Phenology Modelling. In Proceedings of the VIIIth International Terroir Congress, Soave, Italy, 14–18 June 2010; Volume 3, pp. 65–71. [Google Scholar]
- Mesterházy, I.; Mészáros, R.; Pongrácz, R. The Effects of Climate Change on Grape Production in Hungary. Időjárás-Quart. J. Hung. Meteorol. Serv. 2014, 118, 193–206. [Google Scholar]
- Zsófi, Z.; Tóth, E.; Rusjan, D.; Bálo, B. Terroir Aspects of Grape Quality in a Cool Climate Wine Region: Relationship Between Water Deficit, Vegetative Growth and Berry Sugar Concentration. Sci. Hortic. 2011, 127, 494–499. [Google Scholar] [CrossRef]
- Teszlák, P.; Mika, J.; Csikasz-Krizsics, A.; Werner, J.; Kozma, P. Impact of Climate Change on Biological Characteristics, Yield and Quality of Grapevine (Review). Kertgazdaság 2009, 41, 24–40. [Google Scholar]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry Size and Vine Water Deficits as Factors in Winegrape Composition: Anthocyanins and Tannins. Austr. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Ferrero-del-Teso, S.; Arias, I.; Escudero, A.; Ferreira, V.; Fernández-Zurbano, P.; Sáenz-Navajas, M.-P. Effect of Grape Maturity on Wine Sensory and Chemical Features: The Case of Moristel Wines. LWT 2020, 118, 108848. [Google Scholar] [CrossRef]
- Fenyo, K.; Komuves, A.; Szakacs, G. Hungary’s White Wine Under Threat as Heatwave Forces Early Harvest|Reuters. Available online: https://www.reuters.com/business/environment/hungarys-white-wine-under-threat-heatwave-forces-early-harvest-2024-08-07/ (accessed on 10 September 2025).
- Szövényi, Á.P.; Sólyom-Leskó, A.; Szabó, A.; Nagy, B.; Varga, Z.; Nyitrainé Sárdy, D.Á. Influence of Plant Protein Fining Agents on the Phenolic Composition of Organic Grape Musts. Fermentation 2024, 10, 642. [Google Scholar] [CrossRef]
- Kielmayer, K.; Herczeg, Á. Irsai Olivér. Available online: https://winesofhungary.hu/grape-varieties/white-grape-varieties-white-wine-styles/irsai-oliver (accessed on 12 September 2025).
- Kielmayer, K.; Herczeg, Á. Chardonnay. Available online: https://winesofhungary.hu/grape-varieties/white-grape-varieties-white-wine-styles/chardonnay (accessed on 12 September 2025).
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Siuta, M. The Impact of Oxygen at Various Stages of Vinification on the Chemical Composition and the Antioxidant and Sensory Properties of White and Red Wines. Int. J. Food Sci. 2020, 7902974. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, W.J.; Marais, J.; Pretorius, I.S.; Du Toit, M. Oxygen in Must and Wine: A Review. S. Afr. J. Enol. Vitic. 2006, 27, 76–94. [Google Scholar] [CrossRef]
- Schneider, V. Must Hyperoxidation: A Review. Am. J. Enol. Vitic. 1998, 49, 65–73. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of Nitrogen Nutrition for Grapes, Fermentation and Wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of Condensed Tannins: A Review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Reactivity of Flavanols: Their Fate in Physical Food Processing and Recent Advances in Their Analysis by Depolymerization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4841–4880. [Google Scholar] [CrossRef]
- Folin–Ciocalteu Index (Type-IV). In Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine (OIV): Paris, France, 2025; Available online: https://www.oiv.int/node/1975/download/pdf (accessed on 11 August 2025).
- Flanzy, M.; Aubert, S.; Marinos, M. New Technique for Determination of Leucoanthocyanic Tannins. Ann. Technol. Agric. 1969, 18, 327–328. [Google Scholar]
- Rebelein, H. Beitrag zur Bestimmung des Catechingehaltes in Wein. Dtsch. Lebensm. Rundsch. 1965, 61, 182–183. [Google Scholar]
- MSZ ISO 11035:2001; Érzékszervi Vizsgálat. A Leíró Kifejezések Azonosítása és Kiválasztása Érzékszervi Profilhoz Többdimenziós Eljárással. Magyar Szabványügyi Testület: Budapest, Hungary, 2001.
- International Organisation of Vine and Wine (OIV). Review Document on Sensory Analysis of Wine. Available online: https://www.oiv.int/sites/default/files/2022-09/review-on-sensory-analysis-of-wine_en_3.pdf (accessed on 3 May 2025).
- Noble, A.C. Bitterness in Wine. Physiol. Behav. 1994, 56, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
| Compound/Measure | Irsai Olivér | Chardonnay |
|---|---|---|
| Reducing Sugars (g/L) | 171.5 | 165.6 |
| Titratable Acidity (g/L) | 6.00 | 8.55 |
| pH | 3.54 | 3.40 |
| YAN (mg/L) | 112 | 118 |
| TP (mg/L) | 434 | 774 |
| ABA (mg/L) | 573 | 809 |
| VA (mg/L) | 711 | 792 |
| Batch | Composition and Formula of the Clarifying Agent |
|---|---|
| IO 0 | reference batch (no processing aid used) |
| IO 1 | 12% gelatin, citric acid, 10% PVPP, 1% isinglass, SO2 (liquid) |
| IO 2 | 40% gelatin, 10% cellulose, 35% bentonite, 15% PVPP (powder) |
| IO 3 | 2% isinglass, citric acid, SO2 (liquid) |
| IO 4 | 10% cellulose, 60% pea protein, 10% PVPP, 20% bentonite (powder) |
| IO 5 | 100% pea protein (powder) |
| IO 6 | 10% pea protein, chitin–glucan, tartaric acid, SO2 (liquid) |
| IO 7 | 60% pea protein, 20% bentonite, 20% yeast protein (powder) |
| Variety/Harvest Date | Batch | Processing Aid | Average Batch Quantity (hL) | Sediment (Loss) (%) * |
|---|---|---|---|---|
| Irsai Olivér/5 August | IO Gel | gelatin | 25.3 (24–26) | 2.6 (2.5–2.6) a |
| IO Pea | pea protein + chitin–glucan | 26.7 (25–28) | 5.9 (5.9–6.0) b | |
| Chardonnay/26 August | CH Gel | gelatin | 30.3 (29–31) | 4.2 (4.0–4.4) a |
| CH Pea | pea protein + chitin–glucan | 30.0 (28–31) | 4.4 (4.3–4.4) a |
| Batch | Sediment (%) | NTU | Decrease in YAN (%) |
|---|---|---|---|
| IO 0 | 21.6 (21.5–21.6) f | 29 (27–31) a | 3.2 (2.7–3.9) b |
| IO 1 | 20.1 (20.0–20.2) c | 42 (40–44) b | 3.7 (3.5–4.0) b |
| IO 2 | 20.6 (20.5–20.7) d | 32 (30–34) a | 2.9 (2.6–3.2) b |
| IO 3 | 20.6 (20.5–20.6) d | 41 (38–42) b | 7.6 (7.1–8.6) d |
| IO 4 | 16.6 (16.5–16.7) a | 28 (26–30) a | 3.7 (3.0–4.3) b |
| IO 5 | 21.0 (20.9–21.0) e | 31 (30–32) a | 19.9 (19.5–20.3) e |
| IO 6 | 21.8 (21.7–22.0) f | 33 (30–35) a | 0.0 (−0.2–0.4) a |
| IO 7 | 19.4 (19.3–19.5) b | 66 (64–68) c | 6.0 (5.8–6.4) c |
| Compound | IO Gel | IO Pea |
|---|---|---|
| YAN | 7.9 (7.5–8.2) b | 2.9 (2.1–3.6) a |
| TP | 19.7 (17.1–22.6) a | 20.8 (20.0–21.9) a |
| ABA | 9.4 (9.0–10.0) a | 5.9 (3.0–7.7) a |
| VA | 19.1 (18.4–20.1) a | 3.1 (2.3–3.7) b |
| Compound | CH Gel | CH Pea |
|---|---|---|
| YAN | 5.8 (5.1–7.0) b | 0 (0–1.7) a |
| TP | 1.8 (0–3.1) a | 0 (0–0.5) a |
| ABA | 18.9 (18.0–19.5) a | 8.6 (5.1–11.5) b |
| VA | 0.3 (0–0.6) a | 3.6 (1.8–4.7) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szövényi, Á.P.; Sólyom-Leskó, A.; Nagy, B.; Varga, Z.; Németh, N.A.; Nyitrainé Sárdy, D.Á. Comparison of Gelatin and Plant Proteins in the Clarification of Grape Musts Using Flotation Techniques. Fermentation 2025, 11, 569. https://doi.org/10.3390/fermentation11100569
Szövényi ÁP, Sólyom-Leskó A, Nagy B, Varga Z, Németh NA, Nyitrainé Sárdy DÁ. Comparison of Gelatin and Plant Proteins in the Clarification of Grape Musts Using Flotation Techniques. Fermentation. 2025; 11(10):569. https://doi.org/10.3390/fermentation11100569
Chicago/Turabian StyleSzövényi, Áron Pál, Annamária Sólyom-Leskó, Balázs Nagy, Zsuzsanna Varga, Noémi Aletta Németh, and Diána Ágnes Nyitrainé Sárdy. 2025. "Comparison of Gelatin and Plant Proteins in the Clarification of Grape Musts Using Flotation Techniques" Fermentation 11, no. 10: 569. https://doi.org/10.3390/fermentation11100569
APA StyleSzövényi, Á. P., Sólyom-Leskó, A., Nagy, B., Varga, Z., Németh, N. A., & Nyitrainé Sárdy, D. Á. (2025). Comparison of Gelatin and Plant Proteins in the Clarification of Grape Musts Using Flotation Techniques. Fermentation, 11(10), 569. https://doi.org/10.3390/fermentation11100569







