Fungal Microbiota of Malbec Grapes and Fermenting Must Under Different Sanitary Conditions in the Southern Oasis of Mendoza Winemaking Region
Abstract
1. Introduction
2. Materials and Methods
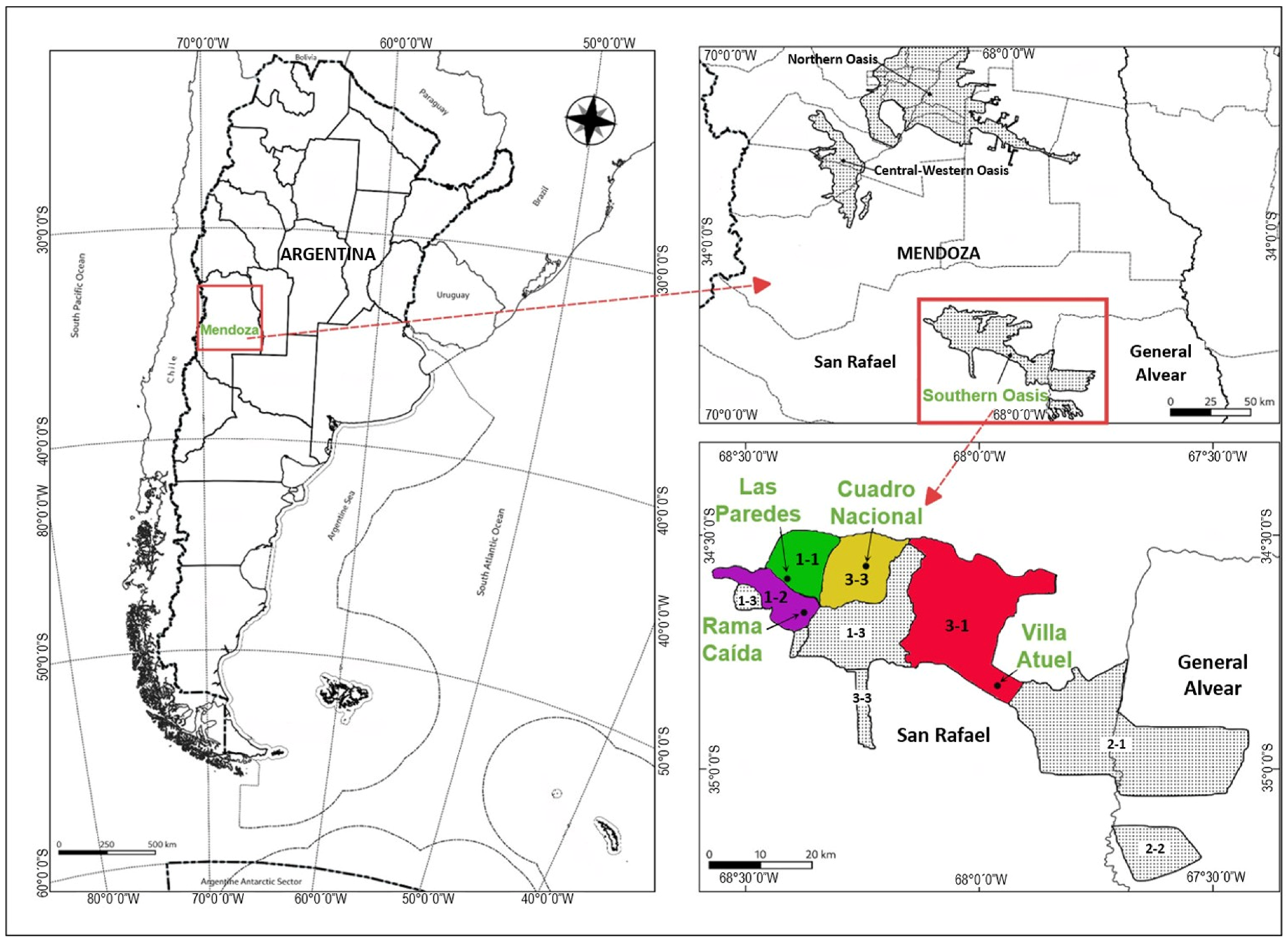
2.1. Studied Area and Sampling
2.2. Fermentation Trials
2.3. Enumeration and Isolation of Filamentous Fungi and Yeasts
2.4. Fungal and Yeast Identification
2.5. General Wine Composition
3. Results and Discussion
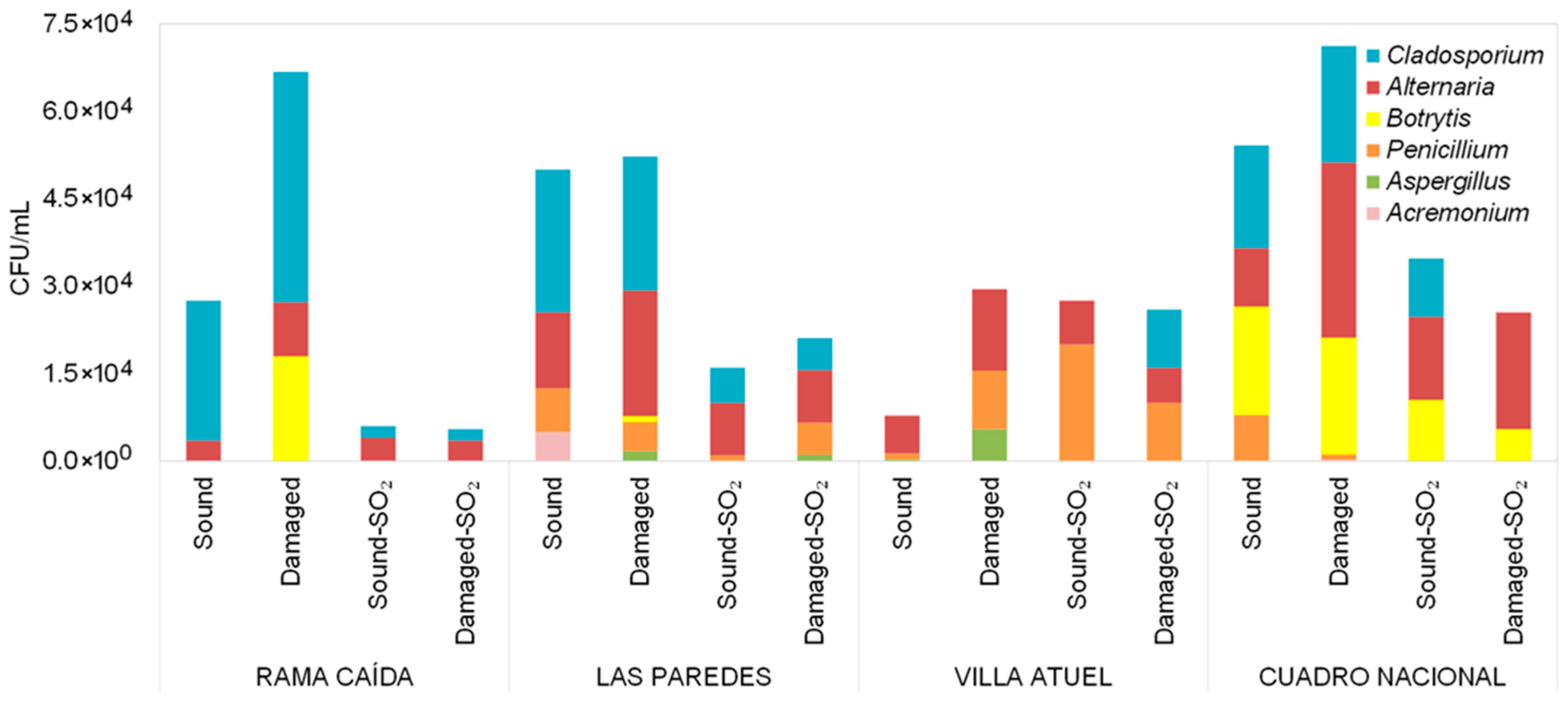
3.1. Diversity of Filamentous Fungi Detected in Malbec Grape Must
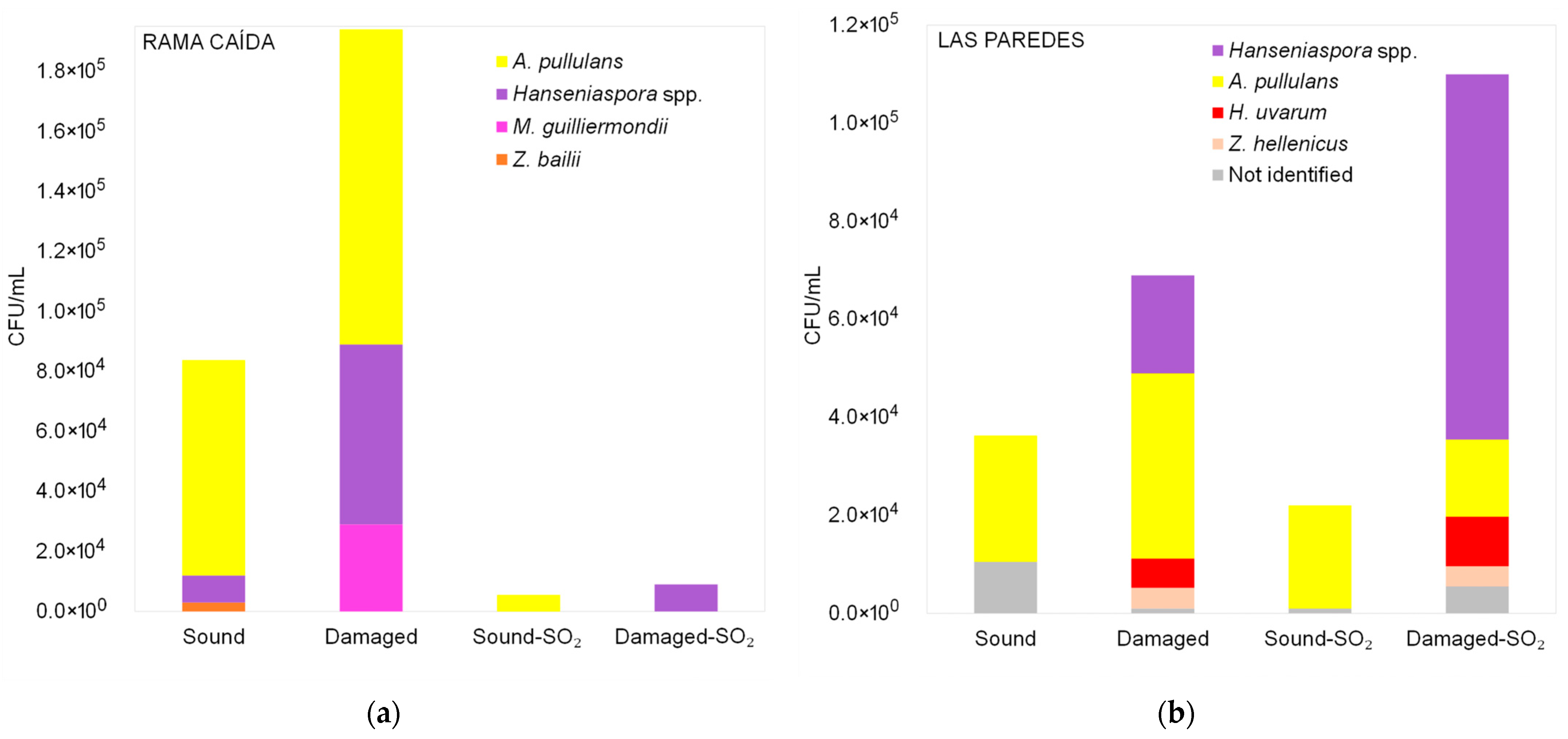
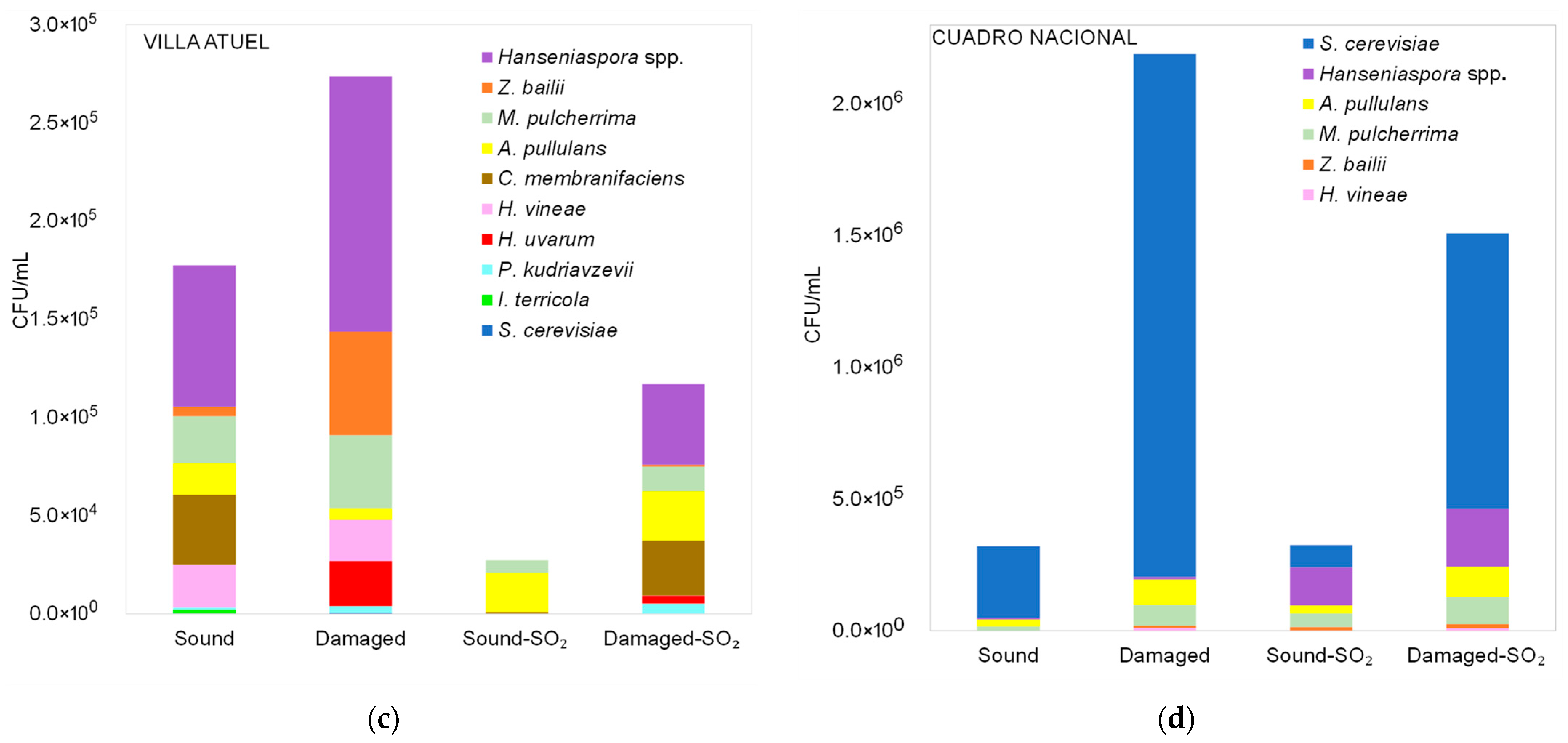
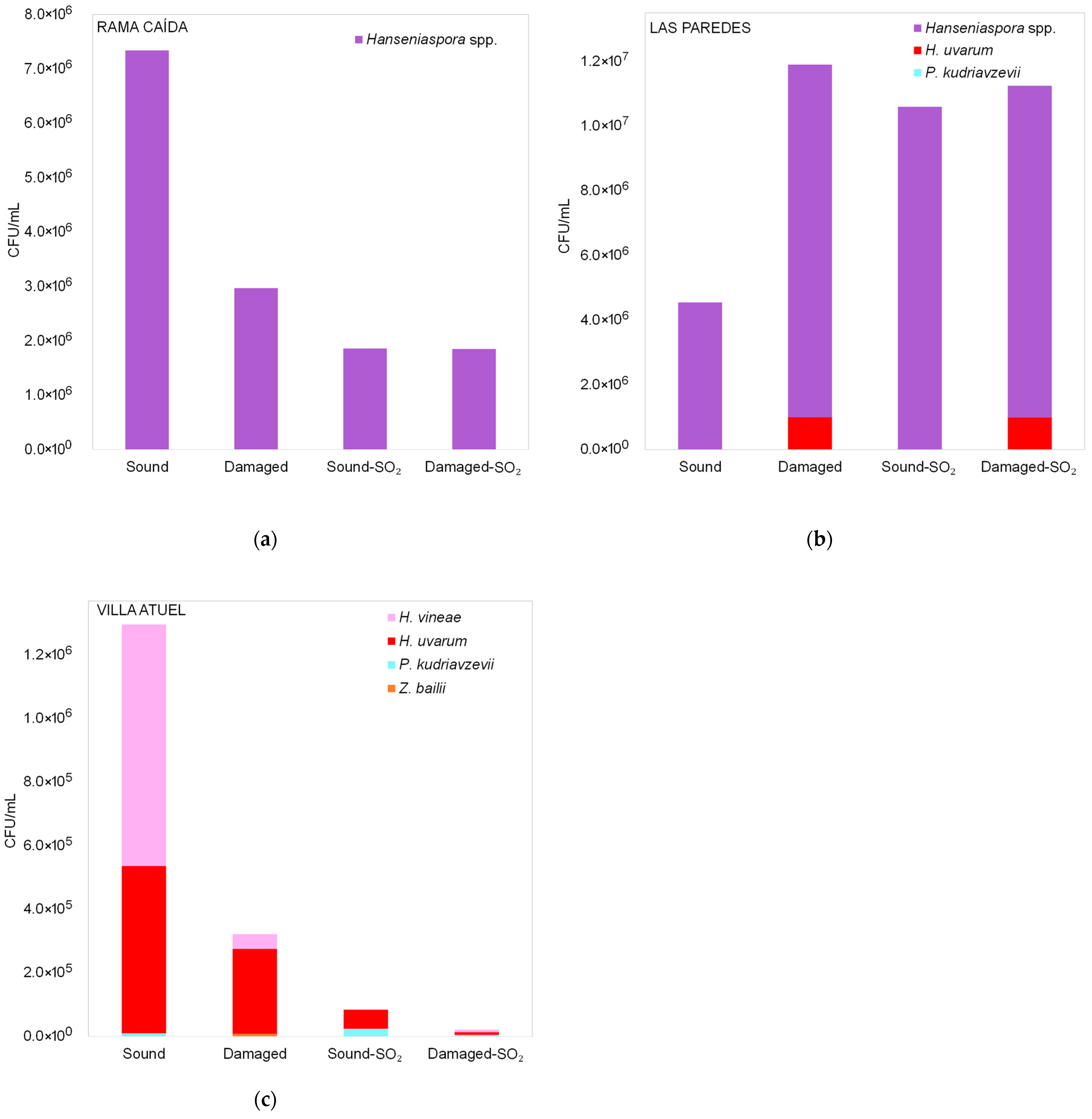
3.2. Diversity of Yeasts Detected in Malbec Grape Must During Fermentation
3.2.1. Diversity of Yeasts Detected in Malbec Grape Must Before Fermentation
3.2.2. Diversity of Non-Saccharomyces Yeasts Detected in Malbec Grape Must at the Middle and End of Fermentation
3.3. Physicochemical Composition of Malbec Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organization Internationale de la Vigne et du Vine (OIV). Base de Datos OIV. Available online: https://www.oiv.int/es/que-hacemos/giao-database-report?oiv= (accessed on 23 July 2025).
- Corporación Vitivinícola Argentina (COVIAR)—Estudios de Caracterización de Zonas Vitivinícolas. Available online: https://caracterizacion-fisico-ambiental-coviar.hub.arcgis.com/ (accessed on 23 July 2025).
- Instituto Nacional de Vitivinicultura (INV)—Informe Variedad Malbec 2025. Available online: https://www.argentina.gob.ar/sites/default/files/2018/10/informe_malbec-2025-inv.pdf (accessed on 23 July 2025).
- Boban, A.; Milanović, V.; Veršić Bratinčević, M.; Botta, C.; Ferrocino, I.; Cardinali, F.; Ivić, S.; Rampanti, G.; Budić-Leto, I. Spontaneous Fermentation of Maraština Wines: The Correlation between Autochthonous Mycobiota and Phenolic Compounds. Food Res. Int. 2024, 180, 114072. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial Biogeography of Wine Grapes Is Conditioned by Cultivar, Vintage, and Climate. Proc. Natl. Acad. Sci. USA 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and Diversity of Non-Saccharomyces Yeasts during the Early Stages in Winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, N.; Mi, L.; Peng, S.; Marie-Colette, A.K.; Zhao, X.; Wang, J. From Microbial Communities to Aroma Profiles: A Comparative Study of Spontaneous Fermentation in Merlot and Cabernet Sauvignon Wines. Food Chem. X 2025, 26, 102317. [Google Scholar] [CrossRef] [PubMed]
- García-Izquierdo, I.; Colino-Rabanal, V.J.; Tamame, M.; Rodríguez-López, F. Microbiota Ecosystem Services in Vineyards and Wine: A Review. Agronomy 2024, 14, 131. [Google Scholar] [CrossRef]
- Labarga, D.; Mairata, A.; Puelles, M.; Tronchoni, J.; Eichmeier, A.; de Toro, M.; Gramaje, D.; Pou, A. Vineyard Mycobiota Shows a Local and Long-Term Response to the Organic Mulches Application. Agric. Ecosyst. Environ. 2025, 382, 109506. [Google Scholar] [CrossRef]
- Englezos, V.; Mota-Gutierrez, J.; Giacosa, S.; Río Segade, S.; Pollon, M.; Gambino, G.; Rolle, L.; Ferrocino, I.; Rantsiou, K. Effect of Alternative Fungicides and Inoculation Strategy on Yeast Biodiversity and Dynamics from the Vineyard to the Winery. Food Res. Int. 2022, 162, 111935. [Google Scholar] [CrossRef]
- Perpetuini, G.; Rossetti, A.P.; Battistelli, N.; Zulli, C.; Cichelli, A.; Arfelli, G.; Tofalo, R. Impact of Vineyard Management on Grape Fungal Community and Montepulciano d’Abruzzo Wine Quality. Food Res. Int. 2022, 158, 111577. [Google Scholar] [CrossRef]
- Milanović, V.; Cardinali, F.; Ferrocino, I.; Boban, A.; Franciosa, I.; Gajdoš Kljusurić, J.; Mucalo, A.; Osimani, A.; Aquilanti, L.; Garofalo, C.; et al. Croatian White Grape Variety Maraština: First Taste of Its Indigenous Mycobiota. Food Res. Int. 2022, 162, 111917. [Google Scholar] [CrossRef]
- Ferrocino, I.; Pagiati, L.; Soulis, K.X.; Kazou, M.; Anastasiou, R.; Kalivas, D.; Tsakalidou, E. The Microbial Terroir of Agiorgitiko Cv. in the Nemea PDO Zone. Int. J. Food Microbiol. 2025, 433, 111111. [Google Scholar] [CrossRef] [PubMed]
- Cureau, N.; Threlfall, R.; Savin, M.; Marasini, D.; Lavefve, L.; Carbonero, F. Year, Location, and Variety Impact on Grape-, Soil-, and Leaf-Associated Fungal Microbiota of Arkansas-Grown Table Grapes. Microb. Ecol. 2021, 82, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Valentini, B.; Gatti, N.; Bossi, S.; Vigani, G.; Stefanini, I. Environmental Characteristics and Weather Impact Yeast Populations and Their Dynamics in Spontaneous Fermentations. Curr. Res. Microb. Sci. 2025, 9, 100410. [Google Scholar] [CrossRef]
- Wang, X.; Schlatter, D.C.; Glawe, D.A.; Edwards, C.G.; Weller, D.M.; Paulitz, T.C.; Abatzoglou, J.T.; Okubara, P.A. Native Yeast and Non-Yeast Fungal Communities of Cabernet Sauvignon Berries from Two Washington State Vineyards, and Persistence in Spontaneous Fermentation. Int. J. Food Microbiol. 2021, 350, 109225. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Changes in Sour Rotten Grape Berry Microbiota during Ripening and Wine Fermentation. Int. J. Food Microbiol. 2012, 154, 152–161. [Google Scholar] [CrossRef]
- Pinto, L.; Caputo, L.; Quintieri, L.; de Candia, S.; Baruzzi, F. Efficacy of Gaseous Ozone to Counteract Postharvest Table Grape Sour Rot. Food Microbiol. 2017, 66, 190–198. [Google Scholar] [CrossRef]
- Arpellino, A.; Englezos, V.; Giacosa, S.; Di Gianvito, P.; Ferrero, L.; Rantsiou, K.; Cocolin, L.S.; Spadaro, D.; Gonella, E.; Alma, A. Use of Antagonistic Yeasts for Multi-Purpose Grape and Wine Protection: Attraction for Drosophila Suzukii in Pre-Harvest and Bioprotection during Red Winemaking. J. Agric. Food Res. 2025, 22, 102067. [Google Scholar] [CrossRef]
- Gao, H.; Yin, X.; Jiang, X.; Shi, H.; Yang, Y.; Wang, C.; Dai, X.; Chen, Y.; Wu, X. Diversity and Spoilage Potential of Microbial Communities Associated with Grape Sour Rot in Eastern Coastal Areas of China. PeerJ 2020, 2020, e9376. [Google Scholar] [CrossRef]
- Mas, A.; Portillo, M.C. Strategies for Microbiological Control of the Alcoholic Fermentation in Wines by Exploiting the Microbial Terroir Complexity: A Mini-Review. Int. J. Food Microbiol. 2022, 367, 109592. [Google Scholar] [CrossRef] [PubMed]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Biogeographical Regionalization of Wine Yeast Communities in Greece and Environmental Drivers of Species Distribution at a Local Scale. Front. Microbiol. 2021, 12, 705001. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; García-Fernández, D.; González, B.; Izidoro, I.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Yeast Biodiversity from DOQ Priorat Uninoculated Fermentations. Front. Microbiol. 2016, 7, 930. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Q.; Zhang, P.; Chen, D.; Howell, K.S. The Fungal Microbiome Is an Important Component of Vineyard Ecosystems and Correlates with Regional Distinctiveness of Wine. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.A.; Albertin, W.; Masneuf-Pomarede, I.; Randazzo, C.L.; Caggia, C. Structure of Culturable Indigenous Yeast Population and Genetic Diversity of Saccharomyces Cerevisiae and Non-Saccharomyces Yeasts during Spontaneous Fermentation of Etna Vineyards Grapes. Int. J. Food Microbiol. 2025, 440, 111282. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “Terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef]
- Kamilari, E.; Mina, M.; Karallis, C.; Tsaltas, D. Metataxonomic Analysis of Grape Microbiota During Wine Fermentation Reveals the Distinction of Cyprus Regional Terroirs. Front. Microbiol. 2021, 12, 726483. [Google Scholar] [CrossRef]
- Temperini, C.; Kemppainen, M.; Moya, M.; Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic Fungi, OTA and Fumonisin B2 Production by Aspergillus Section Nigri Isolated from Wine Grapes and Natural Occurrence of OTA in Wines of Northern Argentinean Patagonia. Int. J. Food Microbiol. 2025, 433, 111135. [Google Scholar] [CrossRef]
- Rivas, G.A.; Semorile, L.; Delfederico, L. Microbial Diversity of the Soil, Rhizosphere and Wine from an Emerging Wine-Producing Region of Argentina. LWT 2022, 153, 112429. [Google Scholar] [CrossRef]
- Mercado, L.; Sturm, M.E.; Rojo, M.C.; Ciklic, I.; Martínez, C.; Combina, M. Biodiversity of Saccharomyces Cerevisiae Populations in Malbec Vineyards from the “Zona Alta Del Río Mendoza” Region in Argentina. Int. J. Food Microbiol. 2011, 151, 319–326. [Google Scholar] [CrossRef]
- González, M.L.; Sturm, M.E.; Lerena, M.C.; Rojo, M.C.; Chimeno, S.V.; Combina, M.; Mercado, L.A. Persistence and Reservoirs of Saccharomyces Cerevisiae Biodiversity in Different Vineyard Niches. Food Microbiol. 2020, 86, 103328. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Fariña, L.; Carrau, F.; Rosa, A.L. Grape-Specific Native Microbial Communities Influence the Volatile Compound Profiles in Fermenting Grape Juices. Food Chem. 2025, 466, 142155. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of Non-Saccharomyces Yeasts to Be Used in Grape Musts with High Alcoholic Potential: A Strategy to Obtain Wines with Reduced Ethanol Content. FEMS Yeast Res. 2017, 17, fox010. [Google Scholar] [CrossRef]
- Longhi, S.J.; Martín, M.C.; Merín, M.G.; Morata de Ambrosini, V.I. Yeast Multi-Enzymatic Systems for Improving Colour Extraction, Technological Parameters and Antioxidant Activity of Wine. Food Technol. Biotechnol. 2022, 60, 556–570. [Google Scholar] [CrossRef]
- Merín, M.G.; Mendoza, L.M.; Farías, M.E.; Morata de Ambrosini, V.I. Isolation and Selection of Yeasts from Wine Grape Ecosystem Secreting Cold-Active Pectinolytic Activity. Int. J. Food Microbiol. 2011, 147, 144–148. [Google Scholar] [CrossRef]
- Merín, M.G.; Mendoza, L.M.; Morata de Ambrosini, V.I. Pectinolytic Yeasts from Viticultural and Enological Environments: Novel Finding of Filobasidium Capsuligenum Producing Pectinases. J. Basic Microbiol. 2014, 54, 835–842. [Google Scholar] [CrossRef]
- Merín, M.G.; Martín, M.C.; Rantsiou, K.; Cocolin, L.; De Ambrosini, V.I.M. Characterization of Pectinase Activity for Enology from Yeasts Occurring in Argentine Bonarda Grape. Braz. J. Microbiol. 2015, 46, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Prendes, L.P.; Merín, M.G.; Andreoni, M.A.; Ramirez, M.L.; Morata de Ambrosini, V.I. Mycobiota and Toxicogenic Alternaria spp. Strains in Malbec Wine Grapes from DOC San Rafael, Mendoza, Argentina. Food Control 2015, 57, 122–128. [Google Scholar] [CrossRef]
- Prendes, L.P.; Merín, M.G.; Fontana, A.R.; Bottini, R.A.; Ramirez, M.L.; Morata de Ambrosini, V.I. Isolation, Identification and Selection of Antagonistic Yeast against Alternaria Alternata Infection and Tenuazonic Acid Production in Wine Grapes from Argentina. Int. J. Food Microbiol. 2018, 266, 14–20. [Google Scholar] [CrossRef]
- Prendes, L.P.; Merín, M.G.; Zamora, F.A.; Courtel, C.; Vega, G.A.; Ferreyra, S.G.; Fontana, A.R.; Ramirez, M.L.; Morata, V.I. Alternaria, Tenuazonic Acid and Spoilage Yeasts Associated with Bunch Rots of the Southern Oasis of Mendoza (Argentina) Winegrowing Region. Fermentation 2025, 11, 536. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2021, 10, 13. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, J.; Kioroglou, D.; Mas, A.; Portillo, M.D.C. Microbiome Dynamics during Spontaneous Fermentations of Sound Grapes in Comparison with Sour Rot and Botrytis Infected Grapes. Int. J. Food Microbiol. 2018, 281, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; O’Bryon, I.; Wilcox, W.F.; Osier, M.V.; Cadle-Davidson, L. The Epiphytic Microbiota of Sour Rot-Affected Grapes Differs Minimally from That of Healthy Grapes, Indicating Causal Organisms Are Already Present on Healthy Berries. PLoS ONE 2019, 14, e0211378. [Google Scholar] [CrossRef]
- Iorizzo, M.; Bagnoli, D.; Vergalito, F.; Testa, B.; Tremonte, P.; Succi, M.; Pannella, G.; Letizia, F.; Albanese, G.; Lombardi, S.J.; et al. Diversity of Fungal Communities on Cabernet and Aglianico Grapes from Vineyards Located in Southern Italy. Front. Microbiol. 2024, 15, 1399968. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Yeast-like Fungi and Yeasts in Withered Grape Carposphere: Characterization of Aureobasidium Pullulans Population and Species Diversity. Int. J. Food Microbiol. 2019, 289, 223–230. [Google Scholar] [CrossRef]
- Barata, A.; González, S.; Malfeito-Ferreira, M.; Querol, A.; Loureiro, V. Sour Rot-Damaged Grapes Are Sources of Wine Spoilage Yeasts. FEMS Yeast Res. 2008, 8, 1008–1017. [Google Scholar] [CrossRef]
- Rodrigues, N.; Gonçalves, G.; Pereira-Da-Silva, S.; Malfeito-Ferreira, M.; Loureiro, V. Development and Use of a New Medium to Detect Yeasts of the Genera Dekkera/Brettanomyces. J. Appl. Microbiol. 2001, 90, 588–599. [Google Scholar] [CrossRef]
- Schuller, D.; Corte-Real, M.; Leao, C. A Differential Medium for the Enumeration of the Spoilage Yeast Zygosaccharomyces Bailii in Wine. J. Food Prot. 2000, 63, 1570–1575. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-92207-2. [Google Scholar]
- Querol, A.; Barrio, E.; Huerta, T.; Ramón, D. Molecular Monitoring of Wine Fermentations Conducted by Active Dry Yeast Strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of Yeasts by RFLP Analysis of the 5.8S rRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Jiang, C.; Shi, J.; Chen, X.; Liu, Y. Effect of Sulfur Dioxide and Ethanol Concentration on Fungal Profile and Ochratoxin a Production by Aspergillus Carbonarius during Wine Making. Food Control 2015, 47, 656–663. [Google Scholar] [CrossRef]
- Kostelnikova, K.; Heralecka, R.; Krpatova, A.; Matousek, F.; Sochor, J.; Baron, M. Inhibitory Effects of Selected Chemical Substances on the Growth of Filamentous Fungi Occurring in Cellar Management. Microbiol. Res. 2025, 16, 182. [Google Scholar] [CrossRef]
- Africa, A.J.; Setati, M.E.; Hitzeroth, A.C.; Blancquaert, E.H. Exploring the Evolution of Microbial Communities from the Phyllosphere and Carposphere to the Grape Must of Vitis Vinifera L. Cv’s Chardonnay and Pinot Noir. Food Microbiol. 2025, 130, 104780. [Google Scholar] [CrossRef] [PubMed]
- Kakalíková, Ľ.; Jankura, E.; Šrobárová, A. First Report of Alternaria Bunch Rot of Grapevines in Slovakia. Australas. Plant Dis. Notes 2009, 4, 68–69. [Google Scholar] [CrossRef]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine Bunch Rots: Impacts on Wine Composition, Quality, and Potential Procedures for the Removal of Wine Faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef]
- Rousseaux, S.; Diguta, C.F.; Radoï-Matei, F.; Alexandre, H.; Guilloux-Bénatier, M. Non-Botrytis Grape-Rotting Fungi Responsible for Earthy and Moldy off-Flavors and Mycotoxins. Food Microbiol. 2014, 38, 104–121. [Google Scholar] [CrossRef]
- Lorenzini, M.; Cappello, M.S.; Logrieco, A.; Zapparoli, G. Polymorphism and Phylogenetic Species Delimitation in Filamentous Fungi from Predominant Mycobiota in Withered Grapes. Int. J. Food Microbiol. 2016, 238, 56–62. [Google Scholar] [CrossRef]
- Medina, A.; Mateo, R.; López-Ocaña, L.; Valle-Algarra, F.M.; Jiménez, M. Study of Spanish Grape Mycobiota and Ochratoxin A Production by Isolates of Aspergillus Tubingensis and Other Members of Aspergillus Section Nigri. Appl. Environ. Microbiol. 2005, 71, 4696–4702. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma Characteristics of Volatile Compounds Brought by Variations in Microbes in Winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef]
- Bubeck, A.M.; Preiss, L.; Jung, A.; Dörner, E.; Podlesny, D.; Kulis, M.; Maddox, C.; Arze, C.; Zörb, C.; Merkt, N.; et al. Bacterial Microbiota Diversity and Composition in Red and White Wines Correlate with Plant-Derived DNA Contributions and Botrytis Infection. Sci. Rep. 2020, 10, 13828. [Google Scholar] [CrossRef]
- Cadez, N.; Raspor, P.; De Cock, A.W.A.M.; Boekhout, T.; Smith, M.T. Molecular Identification and Genetic Diversity within Species of the Genera Hanseniaspora and Kloeckera. FEMS Yeast Res. 2002, 1, 279–289. [Google Scholar] [CrossRef]
- Smith, M.T. Hanseniaspora Zikes. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 1998; pp. 214–220. ISBN 978-0-444-81312-1. [Google Scholar]
- Albertin, W.; Miot-Sertier, C.; Bely, M.; Marullo, P.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Masneuf-Pomarede, I. Oenological Prefermentation Practices Strongly Impact Yeast Population Dynamics and Alcoholic Fermentation Kinetics in Chardonnay Grape Must. Int. J. Food Microbiol. 2014, 178, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Grangeteau, C.; Gerhards, D.; Von Wallbrunn, C.; Alexandre, H.; Rousseaux, S.; Guilloux-Benatier, M. Persistence of Two Non-Saccharomyces Yeasts (Hanseniaspora and Starmerella) in the Cellar. Front. Microbiol. 2016, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Chiara, M.; Capece, A.; Romano, P.; Pietrafesa, R.; Siesto, G.; Pesole, G. Genome Sequencing and Comparative Analysis of Three Hanseniaspora uvarum Indigenous Wine Strains Reveal Remarkable Biotechnological Potential. Front. Microbiol. 2020, 10, 3133, Erratum in Front. Microbiol. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Mančić, S.; Stamenković Stojanović, S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological Characterization of Native Hanseniaspora uvarum Strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- Onetto, C.A.; Borneman, A.R.; Schmidt, S.A. Investigating the Effects of Aureobasidium Pullulans on Grape Juice Composition and Fermentation. Food Microbiol. 2020, 90, 103451. [Google Scholar] [CrossRef]
- Merín, M.G.; Morata De Ambrosini, V.I. Highly Cold-Active Pectinases under Wine-like Conditions from Non-Saccharomyces Yeasts for Enzymatic Production during Winemaking. Lett. Appl. Microbiol. 2015, 60, 467–474. [Google Scholar] [CrossRef]
- Longhi, S.J.; Martín, M.C.; Fontana, A.; de Ambrosini, V.I.M. Different Approaches to Supplement Polysaccharide-Degrading Enzymes in Vinification: Effects on Color Extraction, Phenolic Composition, Antioxidant Activity and Sensory Profiles of Malbec Wines. Food Res. Int. 2022, 157, 111447. [Google Scholar] [CrossRef]
- Nisiotou, A.A.; Spiropoulos, A.E.; Nychas, G.J.E. Yeast Community Structures and Dynamics in Healthy and Botrytis-Affected Grape Must Fermentations. Appl. Environ. Microbiol. 2007, 73, 6705–6713. [Google Scholar] [CrossRef]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Reynoso, C.; Lauret, S.C.; Rosa, A.L. Isolation and Identification of the Indigenous Yeast Population during Spontaneous Fermentation of Isabella (Vitis labrusca L.) Grape Must. Front. Microbiol. 2017, 8, 532. [Google Scholar] [CrossRef]
- Hierro, N.; González, Á.; Mas, A.; Guillamón, J.M. Diversity and Evolution of Non-Saccharomyces Yeast Populations during Wine Fermentation: Effect of Grape Ripeness and Cold Maceration. FEMS Yeast Res. 2006, 6, 102–111. [Google Scholar] [CrossRef]
- Englezos, V.; Jolly, N.P.; Di Gianvito, P.; Rantsiou, K.; Cocolin, L. Microbial Interactions in Winemaking: Ecological Aspects and Effect on Wine Quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M.; Silva, A.C. Spoilage Yeasts in Wine Production. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 375–394. ISBN 978-1-4939-9780-0. [Google Scholar]
- Martín, M.; Prendes, L.; Morata, V.; Merín, M. Biocontrol and Enzymatic Activity of Non-Saccharomyces Wine Yeasts: Improvements in Winemaking. Fermentation 2024, 10, 218. [Google Scholar] [CrossRef]
- Shimizu, H.; Kamada, A.; Koyama, K.; Iwashita, K.; Goto-Yamamoto, N. Yeast Diversity during the Spontaneous Fermentation of Wine with Only the Microbiota on Grapes Cultivated in Japan. J. Biosci. Bioeng. 2023, 136, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Grangeteau, C.; Roullier-Gall, C.; Rousseaux, S.; Gougeon, R.D.; Schmitt-Kopplin, P.; Alexandre, H.; Guilloux-Benatier, M. Wine Microbiology Is Driven by Vineyard and Winery Anthropogenic Factors. Microb. Biotechnol. 2017, 10, 354–370. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Vigentini, I.; Foschino, R.; Maghradze, D.; Ruiz-Muñoz, M.; Benitez-Trujillo, F.; Cantoral, J.M. Culturable Yeast Diversity of Grape Berries from Vitis vinifera ssp. Sylvestris (Gmelin) Hegi. J. Fungi 2022, 8, 410. [Google Scholar] [CrossRef]
- Tarko, T.; Duda, A. Volatilomics of Fruit Wines. Molecules 2024, 29, 2457. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Merín, M.G.; Morata, V.I.; Farías, M.E. Characterization of Wines Produced by Mixed Culture of Autochthonous Yeasts and Oenococcus oeni from the Northwest Region of Argentina. J. Ind. Microbiol. Biotechnol. 2011, 38, 1777–1785. [Google Scholar] [CrossRef]
| Sub-Region | Raw Materials | Total Soluble Solids (°Brix) | pH |
|---|---|---|---|
| Rama Caída | Sound grape | 19.9 | 3.74 |
| Damaged grape | 20.0 | 3.65 | |
| Las Paredes | Sound grape | 19.6 | 3.55 |
| Damaged grape | 17.5 | 3.66 | |
| Villa Atuel | Sound grape | 24.4 | 3.78 |
| Damaged grape | 24.9 | 3.82 | |
| Cuadro Nacional | Sound grape | 22.8 | 4.23 |
| Damaged grape | 25.5 | 4.21 |
| Treatment | Grape Must Composition |
|---|---|
| Sound | 100% sound grape |
| Damaged | 20% damaged grape, 80% sound grape |
| Sound-SO2 | 100% sound grape, with SO2 * |
| Damaged-SO2 | 20% damaged grape, 80% sound grape, with SO2 * |
| Species | Restriction Fragments (bp) | Amplified Product (bp) e | |||
|---|---|---|---|---|---|
| CfoI a | HaeIII b | HinfI c | DdeI d | ||
| Aureobasidium pullulans | 190 + 180 + 100 | 450 + 150 | 290 + 180 + 130 | ND | 600 |
| Candida membranifaciens | 290 + 290 + 100 | 400 + 120 + 80 | 340 + 340 | ND | 600 |
| Hanseniaspora uvarum | 350 + 330 + 120 | 800 | 380 + 180 + 150 | 300 + 180 + 100 + 50 | 800 |
| Hanseniaspora vineae | 280 + 180 + 150 + 90 | 680 + 90 | 400 + 370 | 460 + 220 + 80 | 800 |
| Issatchenkia terricola | 130 + 100 + 90 + 85 + 45 | 300 + 120 | 250 + 100 + 100 | ND | 450 |
| Metschnikowia pulcherrima | 205 + 100 + 95 | 280 + 100 | 200 + 190 | ND | 400 |
| Meyerozyma guilliermondii | 320 + 270 + 50 + 10 | 420 + 120 + 50 + 20 + 5 | 300 + 300 + 10 | ND | 600 |
| Pichia kudriavzevii | 210 + 180 + 70 + 50 + 6 | 400 + 100 + 40 | 230 + 160 + 140 | ND | 500 |
| Saccharomyces cerevisiae | 380 + 330 + 150 | 350 + 250 + 150 + 125 | 380 + 370 + 120 | ND | 850 |
| Zygoascus hellenicus | 330 + 330 | 630 | 350 + 170 + 130 | ND | 650 |
| Zygosaccharomyces bailii | 320 + 280 + 100 + 100 | 700 + 90 | 350 + 230 + 160 + 60 | ND | 800 |
| Species | Sound Grape | Damaged Grape (20%) | SO2 Tolerance (80 mg/L) | Degree of Grape Maturity | |
|---|---|---|---|---|---|
| 19–20 °Bx a | 23–25 °Bx b | ||||
| A. pullulans | + | + | + | + | + |
| C. membranifaciens | + | + | + | − | + |
| Hanseniaspora spp. | + | + | + | + | + |
| H. uvarum | − | + | + | + | + |
| H. vineae | + | + | − | − | + |
| I. terricola | + | − | − | − | + |
| M. pulcherrima | + | + | + | − | + |
| M. guilliermondii | − | + | − | + | − |
| P. kudriavzevii | + | + | + | − | + |
| S. cerevisiae | + | + | + | − | + |
| Z. hellenicus | − | + | + | + | − |
| Z. bailii | + | + | + | + | + |
| Parameters | Rama Caída | Las Paredes | Villa Atuel | Cuadro Nacional | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | D | S-SO2 | D-SO2 | S | D | S-SO2 | D-SO2 | S | D | S-SO2 | D-SO2 | S | D | S-SO2 | D-SO2 | |
| Acetic Acid (g/L) | 0.60 | 0.54 | 0.61 | 0.61 | 0.57 | 0.62 | 0.62 | 0.63 | 0.76 | 0.58 | 0.62 | 0.71 | 0.75 | 0.72 | 0.70 | 0.80 |
| Alcohol % | 10.1 | 10.3 | 10.0 | 10.6 | 10.3 | 11.0 | 10.0 | 10.0 | 13.9 | 13.0 | 13.4 | 12.5 | 12.6 | 12.4 | 12.8 | 12.2 |
| Citric Acid (g/L) | 0.35 | 0.20 | 0.43 | 0.24 | 0.09 | 0.23 | 0.23 | 0.04 | 0.58 | 0.00 | 0.00 | 0.01 | 0.70 | 0.53 | 0.11 | 0.52 |
| Fructose (g/L) | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.3 | 0.1 | 0.0 | 0.5 | 0.1 | 0.0 |
| Glucose (g/L) | 1.3 | 2.4 | 1.5 | 1.6 | 1.7 | 1.4 | 1.6 | 2.1 | 1.4 | 1.6 | 1.5 | 0.9 | 0.4 | 1.5 | 0.4 | 1.2 |
| Glycerol (g/L) | 9.3 | 9.1 | 9.2 | 9.5 | 8.6 | 9.5 | 8.3 | 8.5 | 12.4 | 11.8 | 12.1 | 12.6 | 12 | 11.7 | 12.6 | 13.4 |
| Lactic Acid (g/L) | 0.55 | 0.43 | 0.81 | 0.91 | 0.50 | 0.50 | 0.57 | 0.49 | 0.63 | 1.12 | 1.41 | 1.08 | 0.31 | 0.26 | 0.90 | 0.26 |
| Malic Acid (g/L) | 2.7 | 3.3 | 2.3 | 2.4 | 1.9 | 1.9 | 2.1 | 2.3 | 1.1 | 2.5 | 1.6 | 2.8 | 1.6 | 1.5 | 1.8 | 1.7 |
| pH | 3.52 | 3.70 | 3.65 | 3.73 | 3.39 | 3.43 | 3.40 | 3.37 | 3.42 | 4.12 | 3.97 | 4.20 | 3.49 | 3.43 | 3.64 | 3.50 |
| Sucrose (g/L) | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.4 | 0.6 | 0.2 | 0.4 | 0.7 | 0.0 | 0.0 | 0.4 | 0.2 | 0.0 | 0.2 |
| Tartaric Acid (g/L) | 1.43 | 1.19 | 1.29 | 1.27 | 2.00 | 2.03 | 1.77 | 1.92 | 2.37 | 0.92 | 0.99 | 0.74 | 2.30 | 2.54 | 1.36 | 2.36 |
| Total acidity (g/L) | 5.9 | 5.8 | 5.8 | 5.3 | 5.7 | 5.8 | 6.0 | 5.9 | 6.2 | 5.0 | 4.8 | 5.0 | 6.2 | 6.0 | 5.7 | 6.2 |
| Total sugar (g/L) | 1.6 | 3.3 | 2.1 | 2.4 | 1.7 | 1.7 | 2.1 | 2.4 | 1.3 | 3.6 | 2.2 | 2.4 | 0.0 | 1.6 | 0.3 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garau, J.; Bignert, M.d.C.; Morata, V.I.; Merín, M.G. Fungal Microbiota of Malbec Grapes and Fermenting Must Under Different Sanitary Conditions in the Southern Oasis of Mendoza Winemaking Region. Fermentation 2025, 11, 553. https://doi.org/10.3390/fermentation11100553
Garau J, Bignert MdC, Morata VI, Merín MG. Fungal Microbiota of Malbec Grapes and Fermenting Must Under Different Sanitary Conditions in the Southern Oasis of Mendoza Winemaking Region. Fermentation. 2025; 11(10):553. https://doi.org/10.3390/fermentation11100553
Chicago/Turabian StyleGarau, Juliana, Marianela del Carmen Bignert, Vilma Inés Morata, and María Gabriela Merín. 2025. "Fungal Microbiota of Malbec Grapes and Fermenting Must Under Different Sanitary Conditions in the Southern Oasis of Mendoza Winemaking Region" Fermentation 11, no. 10: 553. https://doi.org/10.3390/fermentation11100553
APA StyleGarau, J., Bignert, M. d. C., Morata, V. I., & Merín, M. G. (2025). Fungal Microbiota of Malbec Grapes and Fermenting Must Under Different Sanitary Conditions in the Southern Oasis of Mendoza Winemaking Region. Fermentation, 11(10), 553. https://doi.org/10.3390/fermentation11100553







