The Impact of Fermented Quinoa Sourdough with Enterococcus Strains on the Nutritional, Textural, and Sensorial Features of Gluten-Free Muffins
Abstract
1. Introduction
2. Material and Methods
2.1. Strains and Culture Conditions
2.2. Screening for Amylolytic Activity
2.3. Fermentation of Quinoa
2.3.1. Sourdough Preparation
2.3.2. Cell Growth and pH Determination of Sourdoughs
2.3.3. Organic Acid and Sugar Determination Using HPLC-RID (Refractive Index Detector)
2.3.4. Phenolic Compound Determination Using HPLC-DAD-MS-ESI+
2.3.5. Rheological Parameters
2.4. Preparation and Analysis of Gluten-Free Muffins
2.4.1. Muffin Preparation
2.4.2. Organic Acid Determination Using HPLC-RID (Refractive Index Detector)
2.4.3. Phenolic Compound Determination Using HPLC-DAD-MS-ESI+
2.4.4. Textural Analysis
2.4.5. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Screening for Amylolytic Activity
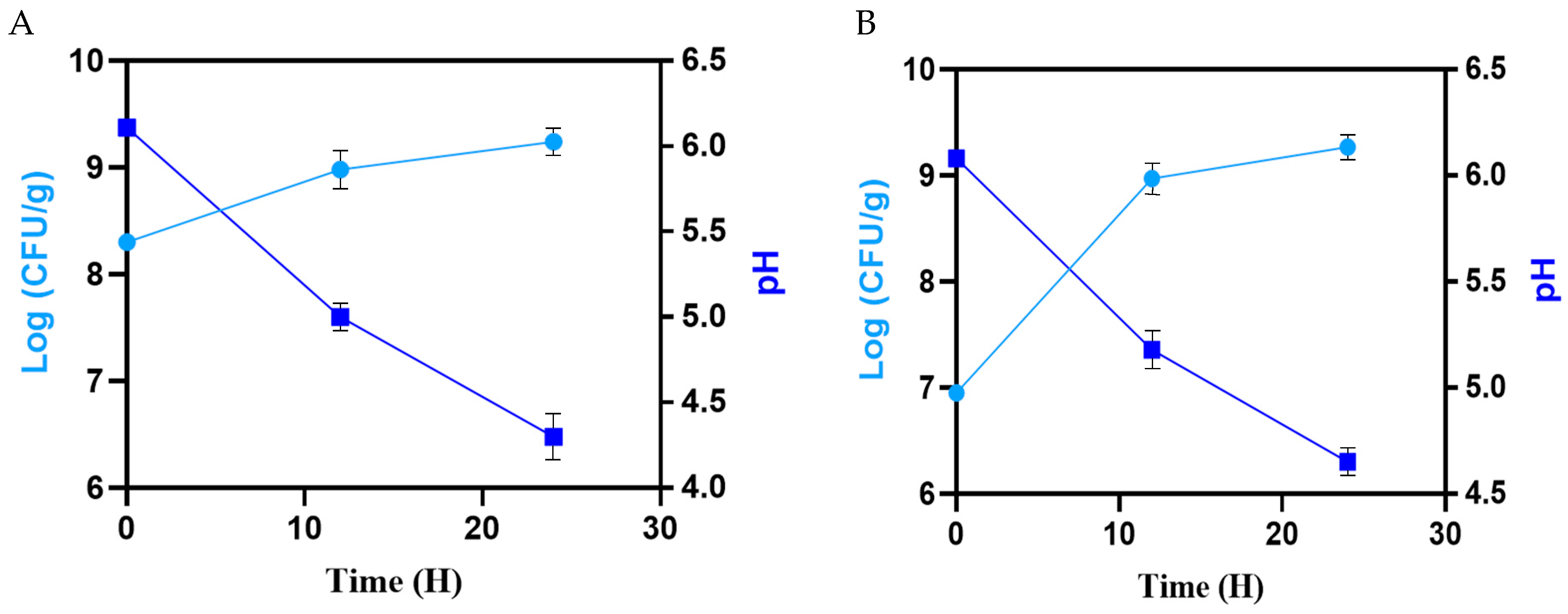
3.2. Cell Growth and pH Determination of Sourdoughs
3.3. Organic Acid and Sugar Determination Using HPLC-RID (Refractive Index Detector)
3.4. Phenolic Compound Determination Using HPLC-DAD-MS-ESI+
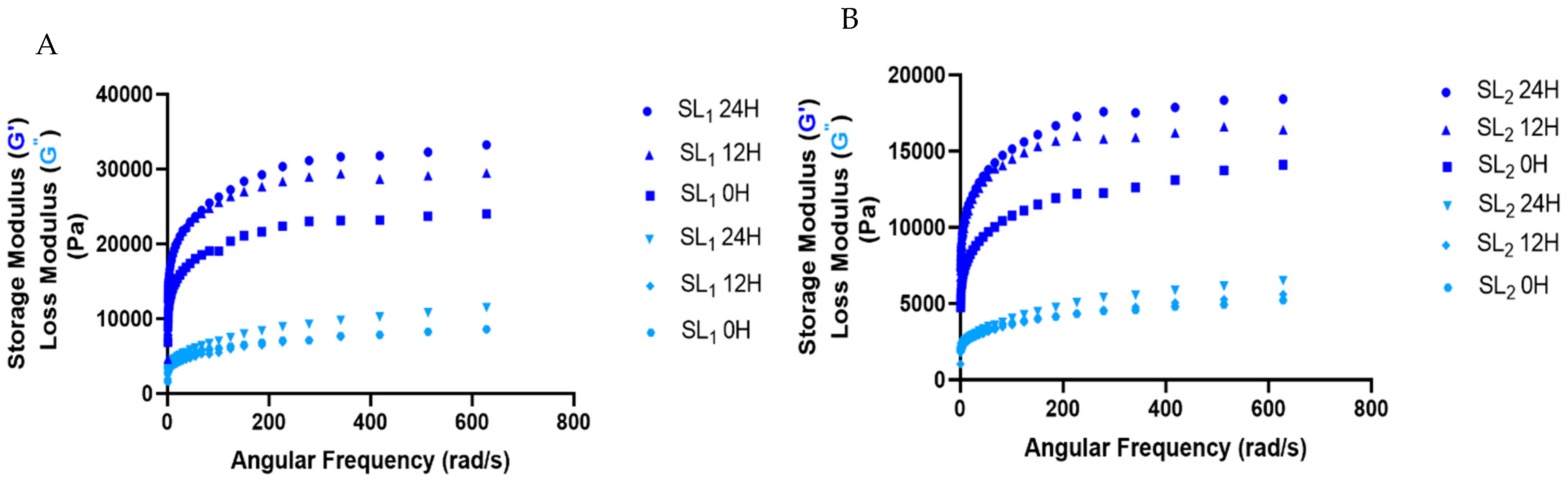
3.5. Rheological Parameters
3.6. Organic Acid and Carbohydrate Analysis in Muffins Using HPLC-RID
3.7. Phenolic Compound Determination Using HPLC-DAD-MS-ESI+
3.8. Textural Analysis
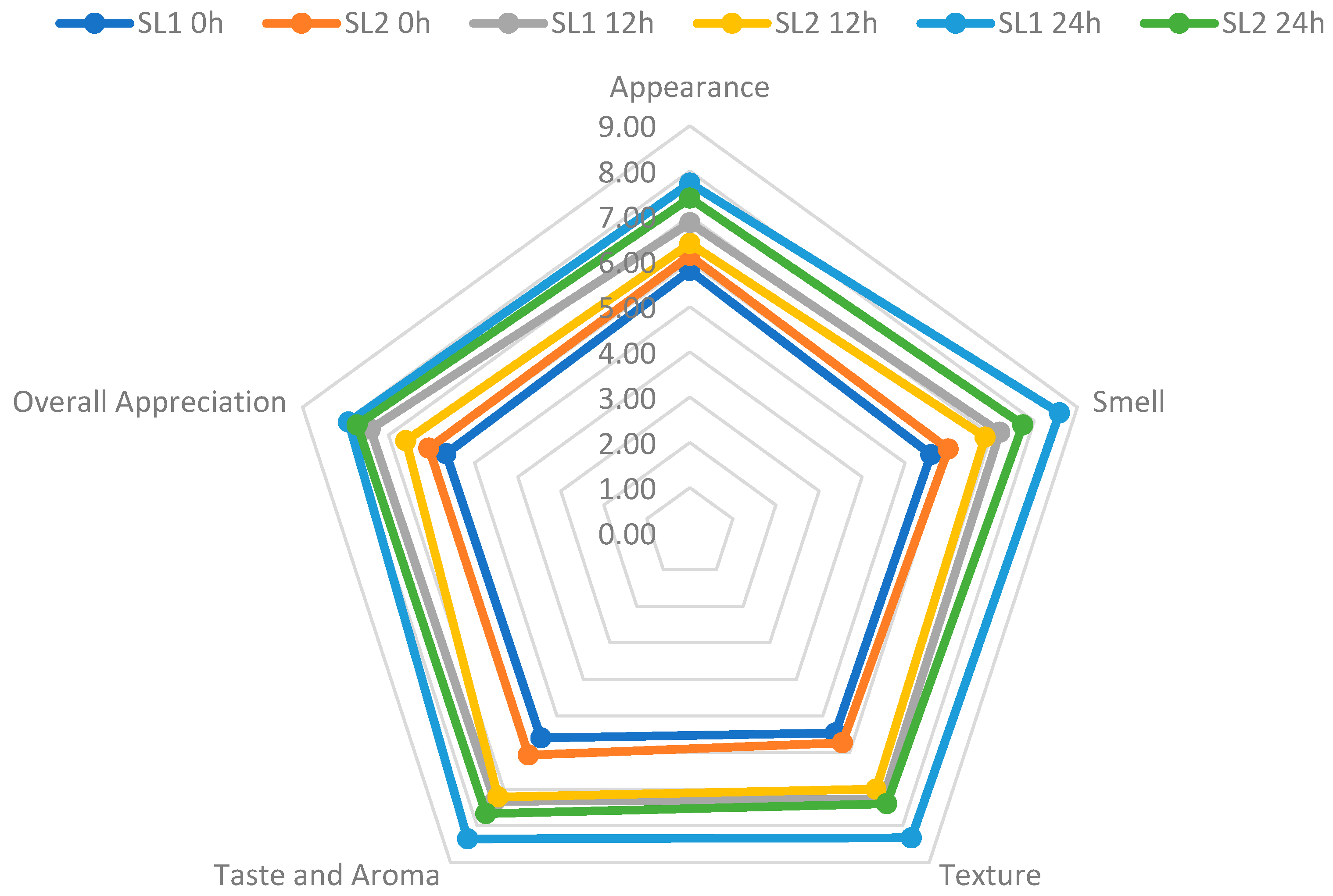
3.9. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalid, A.; Hameed, A.; Tahir, M.F. Wheat quality: A review on chemical composition, nutritional attributes, grain anatomy, types, classification, and function of seed storage proteins in bread making quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef]
- Xhaferaj, M.; Muskovics, G.; Schall, E.; Bugyi, Z.; Tömösközi, S.; Scherf, K.A. Development of a barley reference material for gluten analysis. Food Chem. 2023, 424, 136414. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, D.; Xiang, J.; Xu, W.; Xu, B.; Li, P.; Huang, J. Structural Variations of Wheat Proteins under ultrasound treatment. J. Cereal Sci. 2021, 99, 103219. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Pérez-Andrés, J.; Martínez-Blanco, H.; Ferrero, M.A.; Vaquero, L.; Vivas, S.; Casqueiro, J.; Rodríguez-Aparicio, L.B. The human digestive tract has proteases capable of gluten hydrolysis. Mol. Metab. 2017, 6, 693–702. [Google Scholar] [CrossRef]
- Chiş, M.S.; Păucean, A.; Man, S.M.; Mureşan, V.; Socaci, S.A.; Pop, A.; Stan, L.; Rusu, B.; Muste, S. Textural and Sensory Features Changes of Gluten Free Muffins Based on Rice Sourdough Fermented with Lactobacillus spicheri DSM 15429. Foods 2020, 9, 363. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Tonutti, E.; Bizzaro, N. Diagnosis and classification of celiac disease and gluten sensitivity. Autoimmun. Rev. 2014, 13, 472–476. [Google Scholar] [CrossRef]
- Foschia, M.; Horstmann, S.; Arendt, E.K.; Zannini, E. Nutritional therapy—Facing the gap between coeliac disease and gluten-free food. Int. J. Food Microbiol. 2016, 239, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tack, G.J.; Verbeek, W.H.M.; Schreurs, M.W.J.; Mulder, C.J.J. The spectrum of celiac disease: Epidemiology, clinical aspects and treatment. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 204–213. [Google Scholar] [CrossRef]
- Chiş, M.S.; Păucean, A.; Man, S.M.; Vodnar, D.C.; Teleky, B.E.; Pop, C.R.; Stan, L.; Borsai, O.; Kadar, C.B.; Urcan, A.C.; et al. Quinoa Sourdough Fermented with Lactobacillus plantarum ATCC 8014 Designed for Gluten-Free Muffins—A Powerful Tool to Enhance Bioactive Compounds. Appl. Sci. 2020, 10, 7140. [Google Scholar] [CrossRef]
- Saed, B.; El-Waseif, M.; Ali, H.; Alsulami, T.; Ban, Z.; Farouk, A. Improving the Nutritional Value and Physical Properties of Gluten-Free Mushroom Soup by Substituting Rice Flour with Quinoa Seed Flour. Processess 2023, 11, 3287. [Google Scholar] [CrossRef]
- Zhang, G.; Tu, J.; Sadiq, F.A.; Zhang, W.; Wang, W. Prevalence, Genetic Diversity, and Technological Functions of the Lactobacillus sanfranciscensis in Sourdough: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Plessas, S.; Mantzourani, I.; Alexopoulos, A.; Alexandri, M.; Kopsahelis, N.; Adamopoulou, V.; Bekatorou, A. Nutritional Improvements of Sourdough Breads Made with Freeze-Dried Functional Adjuncts Based on Probiotic Lactiplantibacillus plantarum subsp. plantarum and Pomegranate Juice. Antioxidants 2023, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Arouna, N.; Árvay, J.; Longo, V.; Pucci, L. Sourdough Fermentation Improves the Antioxidant, Antihypertensive, and Anti-Inflammatory Properties of Triticum dicoccum. Int. J. Mol. Sci. 2023, 24, 6283. [Google Scholar] [CrossRef]
- Teleky, B.E.; Martău, A.G.; Ranga, F.; Chețan, F.; Vodnar, D.C. Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour. Biomolecules 2020, 10, 778. [Google Scholar] [CrossRef]
- Bourekoua, H.; Benatallah, L.; Zidoune, M.N.; Rosell, C.M. Developing gluten free bakery improvers by hydrothermal treatment of rice and corn flours. LWT 2016, 73, 342–350. [Google Scholar] [CrossRef]
- Goswami, D.; Gupta, R.K.; Mridula, D.; Sharma, M.; Tyagi, S.K. Barnyard millet based muffins: Physical, textural and sensory properties. LWT Food Sci. Technol. 2015, 64, 374–380. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Bozoudi, D.; Tsaltas, D. Enterococci Isolated from Cypriot Green Table Olives as a New Source of Technological and Probiotic Properties. Fermentation 2018, 4, 48. [Google Scholar] [CrossRef]
- Falade, A.T.; Emmambux, M.N.; Buys, E.M.; Taylor, J.R.N. Improvement of maize bread quality through modification of dough rheological properties by lactic acid bacteria fermentation. J. Cereal Sci. 2014, 60, 471–476. [Google Scholar] [CrossRef]
- Yao Hou, C.; Lee, B.-H.; Shih, M.-K.; Hameed, A.; Condò, C.; Tauseef, I.; Idrees, M.; Ghazanfar, S.; Farid, A.; Muzammal, M.; et al. Isolation and Characterization of a Cholesterol-Lowering Bacteria from Bubalus bubalis Raw Milk. Fermentation 2022, 8, 163. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Barbera, M.; Franciosi, E.; Francesca, N.; Moschetti, G.; Settanni, L. Persistence of a mixed lactic acid bacterial starter culture during lysine fortification of sourdough breads by addition of pistachio powder. Food Microbiol. 2020, 86, 103349. [Google Scholar] [CrossRef]
- Gerez, C.L.; Dallagnol, A.; Rollán, G.; Font de Valdez, G. A combination of two lactic acid bacteria improves the hydrolysis of gliadin during wheat dough fermentation. Food Microbiol. 2012, 32, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Gholamhosseinpour, A.; Mousavi Khaneghah, A. Fermentation of acorn dough by lactobacilli strains: Phytic acid degradation and antioxidant activity. LWT 2019, 100, 144–149. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Dallagnol, A.M.; Pescuma, M.; De Valdez, G.F.; Rollán, G. Fermentation of quinoa and wheat slurries by Lactobacillus plantarum CRL 778: Proteolytic activity. Appl. Microbiol. Biotechnol. 2013, 97, 3129–3140. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torino, M.I.; Rollán, G.; Font de Valdez, G. Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 2009, 20, 144–148. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In vitro investigation of bioactivities of solid-state fermented lupin, quinoa and wheat using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef]
- Derdak, R.; Sakoui, S.; Pop, O.L.; Vodnar, D.C.; Addoum, B.; Teleky, B.E.; Elemer, S.; Elmakssoudi, A.; Suharoschi, R.; Soukri, A.; et al. Optimisation and characterization of α-D-glucan produced by Bacillus velezensis RSDM1 and evaluation of its protective effect on oxidative stress in Tetrahymena thermophila induced by H2O2. Int. J. Biol. Macromol. 2022, 222, 3229–3242. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. From Microbial Ecology to Innovative Applications in Food Quality Improvements: The Case of Sourdough as a Model Matrix. J 2020, 3, 9–19. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, J.; Ceballos-González, C.; Ramírez-Toro, C.; Bolívar, G.A. Reduction in saponin content and production of gluten-free cream soup base using quinoa fermented with Lactobacillus plantarum. J. Food Process. Preserv. 2018, 42, e13495. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.M.; Rochette, I.; Guyot, J.P.; Humblot, C. Influence of fermentation and other processing steps on the folate content of a traditional African cereal-based fermented food. Int. J. Food Microbiol. 2018, 266, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Thompson, T. Folate, iron, and dietary fiber contents of the gluten-free diet. J. Am. Diet. Assoc. 2000, 100, 1389–1396. [Google Scholar] [CrossRef]
- Campo, E.; del Arco, L.; Urtasun, L.; Oria, R.; Ferrer-Mairal, A. Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour. J. Cereal Sci. 2016, 67, 75–82. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Novotni, D.; Čukelj, N.; Smerdel, B.; Ćurić, D. Quality attributes and firming kinetics of partially baked frozen wholewheat bread with sourdough. Int. J. Food Sci. Technol. 2013, 48, 2133–2142. [Google Scholar] [CrossRef]
| (A) | ||||||
| Samples | Glucose | Maltose | Citric Acid | Lactic Acid | Acetic Acid | |
| SL1 | 0 h | 36.560 ± 0.12 a | 1.203 ± 0.32 a | 2.446 ± 0.01 a | n.d. | n.d. |
| 12 h | 43.266 ± 0.11 a,b | 1.333 ± 0.21 a,b | 3.949 ± 0.01 b | 1.564 ± 0.02 a | 1.103 ± 0.01 a | |
| 24 h | 54.415 ± 0.35 b | 2.201 ± 0.01 b | 4.426 ± 0.02 c | 4.642 ± 0.01 b | 2.345 ± 0.03 b | |
| SL2 | 0 h | 44.185 ± 0.10 a | 1.474 ± 0.05 a | 2.396 ± 0.01 a | n.d. | n.d. |
| 12 h | 47.186 ± 0.11 a,b | 2.065 ± 0.10 a,b | 3.849 ± 0.05 b | 0.663 ± 0.02 a | 0.564 ± 0.01 a | |
| 24 h | 60.383 ± 0.23 b | 2.133 ± 0.11 b | 4.105 ± 0.01 c | 3.866 ± 0.02 b | 1.901 ± 0.02 b | |
| (B) | ||||||
| Quinoa Flour (mg/g) | Cell-Free Supernatant (mg/mL) | |||||
| SL1 | SL2 | |||||
| Citric acid | 2.59 ± 0.1 a | 1.941 ± 0.2 b | 1.901 ± 0.2 b | |||
| Lactic acid | n.d. | 4.596 ± 0.3 a | 3.383 ± 0.2 a | |||
| Acetic acid | n.d. | 2.070 ± 0.2 a | 1.907 ± 0.1 a | |||
| Rt (min) | Phenolic Compound | SL1 | SL2 | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 0 h | 12 h | 24 h | ||
| 3.01 | 2-Hydroxybenzoic acid | 1664.444 | 2006.462 | 2542.151 | 1584.264 | 1865.134 | 2441.033 |
| 3.89 | 2,3-Dihydroxybenzoic acid | 359.662 | 477.316 | 662.898 | 343.602 | 438.296 | 603.060 |
| 4.57 | Gallic acid | 237.250 | 324.568 | 433.776 | 218.097 | 273.295 | 383.931 |
| 9.47 | Protocatechuic acid | 106.272 | 111.387 | 230.469 | 117.692 | 124.116 | 250.573 |
| 13.42 | Vanillic acid | 88.784 | 72.010 | 68.917 | 128.280 | 126.257 | 150.407 |
| 11.89 | Chlorogenic acid | 20.899 | 23.869 | 40.353 | 30.552 | 33.819 | 37.532 |
| 14.21 | Quercetin-rhamnosyl-rhamnosyl-glucoside | 189.164 | 183.669 | 176.987 | 275.446 | 260.447 | 225.994 |
| 14.54 | Quercetin-xylosyl-rutinoside | 110.159 | 90.259 | 75.854 | 165.255 | 169.561 | 142.236 |
| 14.83 | Kaempferol-rhamnosyl-rhamnosyl-glucoside | 551.963 | 461.672 | 417.863 | 445.336 | 404.349 | 353.411 |
| 15.24 | Quercetin-xylosyl-glucoside | 109.862 | 116.545 | 122.931 | 101.546 | 105.110 | 107.189 |
| 15.54 | Quercetin-rutinoside (Rutin) | 111.644 | 100.209 | 97.833 | 130.950 | 101.546 | 100.655 |
| 16.23 | Kaempferol-rutinoside | 142.088 | 72.290 | 54.172 | 123.822 | 67.092 | 54.024 |
| 16.72 | Quercetin-glucuronide | 60.113 | 56.252 | 54.234 | 98.427 | 97.685 | 94.566 |
| 21.39 | Quercetin | 5.017 | 7.690 | 15.116 | 7.542 | 11.848 | 32.788 |
| 23.27 | Kaempferol | 11.106 | 14.224 | 27.590 | 11.403 | 14.373 | 29.818 |
| Total phenolics | 3768.427 | 4118.422 | 5021.144 | 3788.087 | 4092.928 | 5007.217 | |
| Sample | Glucose | Maltose | Citric Acid | Lactic Acid | Acetic Acid | |
|---|---|---|---|---|---|---|
| SL1 | 0 h | 36.374 ± 0.02 a | 10.317 ± 0.43 a | 1.434 ± 0.01 a | n.d. | n.d. |
| 12 h | 33.585 ± 0.21 b | 8.465 ± 0.14 b | 1.290 ± 0.01 b | 0.515 ± 0.05 b | 0.332 ± 0.01 b | |
| 24 h | 30.398 ± 0.15 c | 7.317 ± 0.10 c | 0.634 ± 0.02 c | 0.669 ± 0.01 a | 1.543 ± 0.03 a | |
| SL2 | 0 h | 31.647 ± 0.30 a | 10.154 ± 0.39 a | 1.303 ± 0.01 a | n.d. | n.d. |
| 12 h | 17.192 ± 0.13 b | 6.812 ± 0.22 b | 0.444 ± 0.05 b | 0.399 ± 0.02 b | 0.846 ± 0.01 b | |
| 24 h | 19.357 ± 0.10 b | 6.271 ± 0.12 c | 0.205 ± 0.01 c | 0.406 ± 0.02 b | 0.541 ± 0.02 c | |
| Rt (min) | Phenolic Compound | SL1 | SL2 | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 0 h | 12 h | 24 h | ||
| 3.01 | 2-Hydroxybenzoic acid | 213.933 | 405.106 | 578.434 | 293.281 | 344.911 | 526.805 |
| 3.89 | 2,3-Dihydroxybenzoic acid | 27.756 | 60.352 | 98.777 | 46.671 | 55.475 | 84.739 |
| 4.57 | Gallic acid | 42.508 | 62.137 | 101.275 | 45.839 | 53.571 | 70.702 |
| 9.47 | Protocatechuic acid | 18.953 | 26.924 | 67.609 | 28.470 | 27.756 | 32.872 |
| 13.42 | Vanilic acid | 7.771 | 12.053 | 29.660 | 11.696 | 10.031 | 13.481 |
| 11.89 | Chlorogenic acid | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 14.21 | Quercetin-rhamnosyl-rhamnosyl-glucoside | 20.907 | 20.462 | 18.828 | 28.630 | 21.501 | 23.580 |
| 14.54 | Quercetin-xylosyl-rutinoside | 14.076 | 11.403 | 12.591 | 13.630 | 9.472 | 11.106 |
| 14.83 | Kaempferol-rhamnosyl-rhamnosyl-glucoside | 40.658 | 42.738 | 37.391 | 44.074 | 31.303 | 37.243 |
| 15.24 | Quercetin-xylosyl-glucoside | 8.136 | 10.957 | 12.888 | 15.710 | 8.136 | 13.630 |
| 15.54 | Quercetin-rutinoside (Rutin) | 22.838 | 30.263 | 30.709 | 37.540 | 25.956 | 40.807 |
| 16.23 | Kaempferol-rutinoside | 6.799 | 13.482 | 20.313 | 14.373 | 9.769 | 21.353 |
| 16.72 | Quercetin-glucuronide | 9.621 | 9.769 | 12.888 | 16.155 | 13.036 | 17.343 |
| 21.39 | Quercetin | 7.987 | 9.918 | 11.403 | 11.551 | 12.294 | 14.967 |
| 23.27 | Kaempferol | 14.076 | 17.195 | 17.789 | 13.036 | 13.779 | 15.413 |
| Total phenolics | 456.019 | 732.758 | 1050.555 | 620.657 | 636.991 | 924.040 | |
| Hardness (N) | Resilience (mJ) | Elasticity (mm) | Cohesion | Chewiness (N) | Mastication (kg·mm) | ||
|---|---|---|---|---|---|---|---|
| SL2 | 0 h | 26.28 ± 1.3 a | 0.08 ± 0.0 a | 5.01 ± 0.1 a | 0.33 ± 0.2 a | 8.67 ± 0.2 a | 4.41 ± 0.01 a |
| 12 h | 13.97 ± 1.9 b | 0.10 ± 0.3 a | 5.50 ± 0.2 a | 0.41 ± 0.1 a | 5.72 ± 0.6 b | 3.20 ± 0.02 b | |
| 24 h | 11.38 ± 0.6 c | 0.17 ± 0.2 b | 7.36 ± 0.1 b | 0.65 ± 0.1 b | 7.39 ± 0.4 a | 5.54 ± 0.01 c | |
| SL1 | 0 h | 27.74 ± 0.0 a | 0.07 ± 0.2 a | 4.76 ± 0.2 a | 0.31 ± 0.1 a | 8.59 ± 0.2 a | 4.16 ± 0.07 a |
| 12 h | 14.19 ± 0.2 b | 0.11 ± 0.2 a | 5.01 ± 0.0 a | 0.42 ± 0.2 a | 5.95 ± 0.5 b | 3.03 ± 0.02 b | |
| 24 h | 07.40 ± 1.4 c | 0.12 ± 0.4 a | 5.30 ± 0.1 a | 0.56 ± 0.1 b | 4.14 ± 0.6 c | 2.22 ± 0.01 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakoui, S.; Derdak, R.; Jouga, F.; Dagni, A.; Pop, O.L.; Vodnar, D.C.; Teleky, B.-E.; Chis, M.S.; Pop, C.R.; Stan, L.; et al. The Impact of Fermented Quinoa Sourdough with Enterococcus Strains on the Nutritional, Textural, and Sensorial Features of Gluten-Free Muffins. Fermentation 2025, 11, 26. https://doi.org/10.3390/fermentation11010026
Sakoui S, Derdak R, Jouga F, Dagni A, Pop OL, Vodnar DC, Teleky B-E, Chis MS, Pop CR, Stan L, et al. The Impact of Fermented Quinoa Sourdough with Enterococcus Strains on the Nutritional, Textural, and Sensorial Features of Gluten-Free Muffins. Fermentation. 2025; 11(1):26. https://doi.org/10.3390/fermentation11010026
Chicago/Turabian StyleSakoui, Souraya, Reda Derdak, Fatimazahra Jouga, Amal Dagni, Oana Lelia Pop, Dan Cristian Vodnar, Bernadette-Emőke Teleky, Maria Simona Chis, Carmen Rodica Pop, Laura Stan, and et al. 2025. "The Impact of Fermented Quinoa Sourdough with Enterococcus Strains on the Nutritional, Textural, and Sensorial Features of Gluten-Free Muffins" Fermentation 11, no. 1: 26. https://doi.org/10.3390/fermentation11010026
APA StyleSakoui, S., Derdak, R., Jouga, F., Dagni, A., Pop, O. L., Vodnar, D. C., Teleky, B.-E., Chis, M. S., Pop, C. R., Stan, L., Ranga, F., Suharoschi, R., Soukri, A., & El Khalfi, B. (2025). The Impact of Fermented Quinoa Sourdough with Enterococcus Strains on the Nutritional, Textural, and Sensorial Features of Gluten-Free Muffins. Fermentation, 11(1), 26. https://doi.org/10.3390/fermentation11010026













