Effect of Skimmed Milk Powder and Fruit Jams Addition on the Physicochemical Characteristics of Yogurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Materials
2.1.1. Raw and Pasteurized Milk and SMP
2.1.2. Fruit Collection
2.2. Preparation
2.2.1. Yogurt Samples
2.2.2. Jam Samples
2.3. Analysis of Milk and Yogurt Samples
2.3.1. Raw and Pasteurized Milk Samples
2.3.2. Yogurts
2.3.3. Jams
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Properties of Natural Yogurts
3.2. Properties of Fruits and Jams
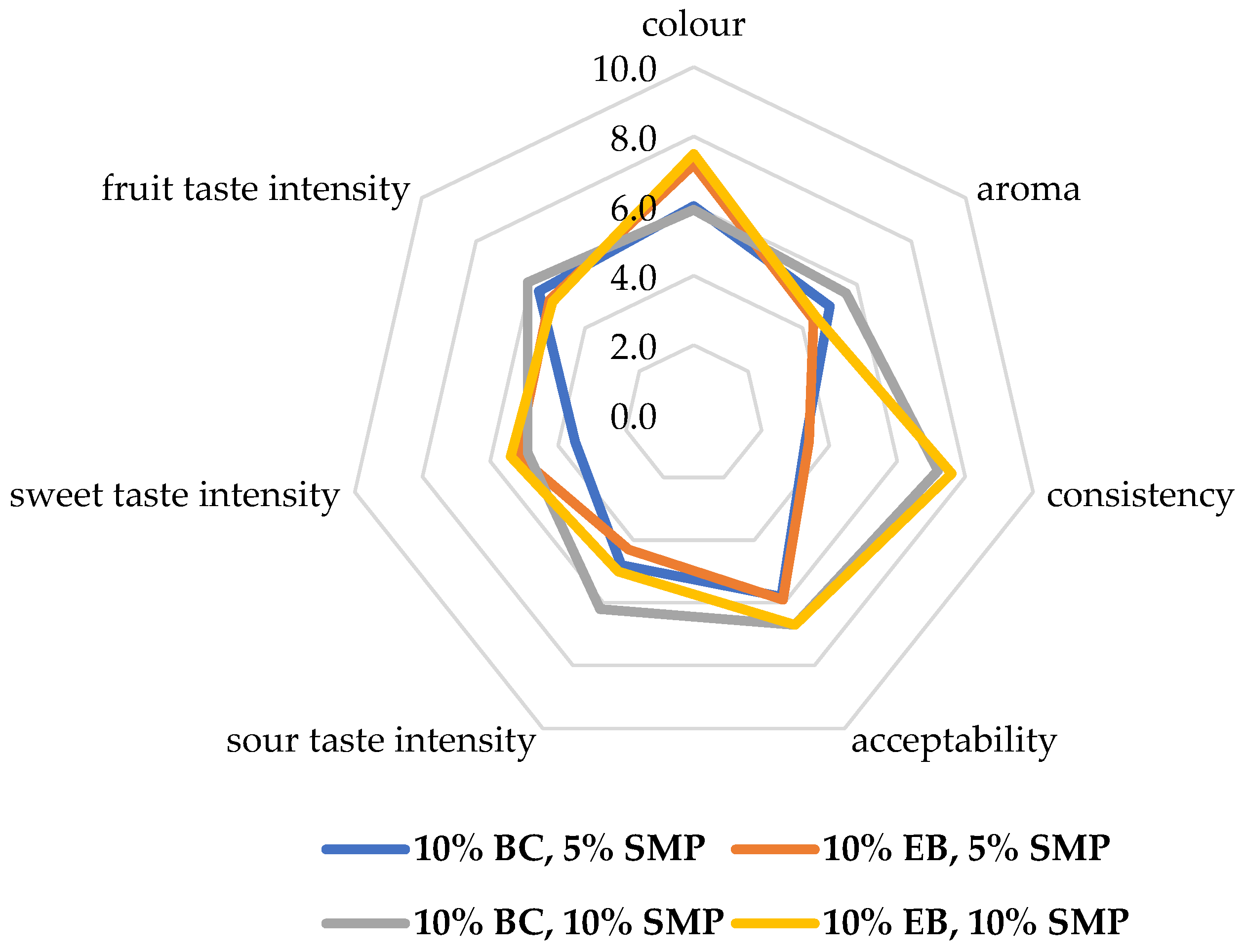
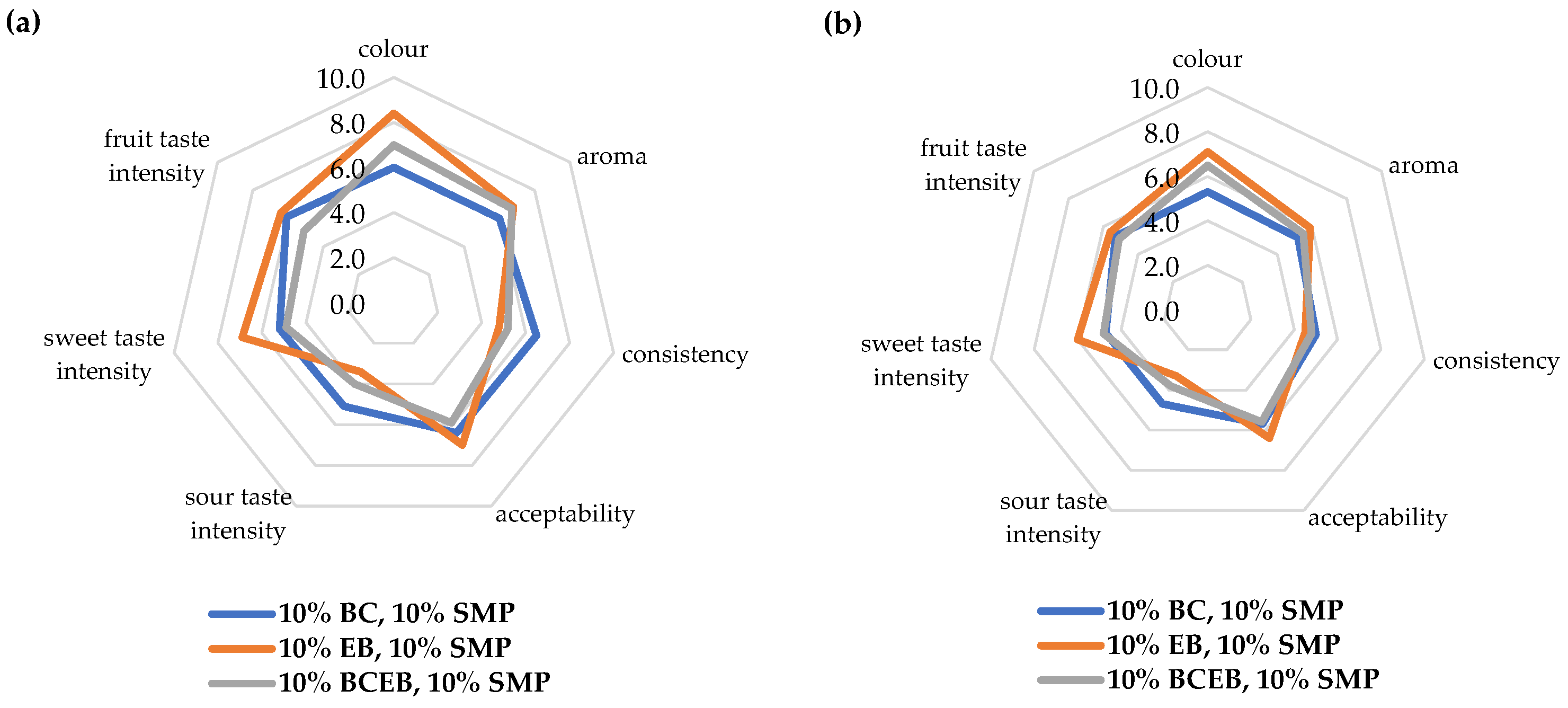
3.3. Properties of Fruit Yogurts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Givens, D.I. MILK Symposium review: The importance of milk and dairy foods in the diets of infants, adolescents, pregnant women, adults, and the elderly. J. Dairy Sci. 2020, 103, 9681–9699. [Google Scholar] [CrossRef] [PubMed]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial effects of yoghurts and probiotic fermented milks and their functional food potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: A review of influential factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Sarkar, S. Potentiality of probiotic yoghurt as a functional food—A review. Nutr. Food Sci. 2019, 49, 182–202. [Google Scholar] [CrossRef]
- Statista. Yogurt—Worldwide. 2023. Available online: https://www.statista.com/outlook/cmo/food/dairy-products-eggs/yogurt/worldwide (accessed on 13 December 2023).
- Rybowska, A.; Gromowska, K. The consumer versus innovative dairy products. Sci. J. Gdyn. Marit. Univ. 2022, 124/22, 81–94. [Google Scholar] [CrossRef]
- Sajdakowska, M.; Tekień, A. To raise or not to raise the level of ingredients in yoghurts: Polish consumer preferences regarding dairy products. Nutrients 2019, 11, 2526. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Well. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Gomaa, M.A.E.; Allam, M.G.; Haridi, A.; Eliwa, A.M.; Darwish, A.M.G. High-protein concentrated pro-yogurt (Pro-WPI) enriched with whey protein isolate improved athletic anemia and performance in a placebo-controlled study. Front. Nutr. 2021, 8, 788446. [Google Scholar] [CrossRef] [PubMed]
- Keršienė, M.; Jasutienė, I.; Eisinaitė, V.; Pukalskienė, M.; Venskutonis, P.R.; Damulevičienė, G.; Knašienė, J.; Lesauskaitė, V.; Leskauskaitė, D. Development of a high-protein yoghurt-type product enriched with bioactive compounds for the elderly. LWT-Food Sci. Technol. 2020, 131, 109820. [Google Scholar] [CrossRef]
- Jorgensen, C.E.; Abrahamsen, R.K.; Rukke, E.O.; Hoffmann, T.K.; Johansen, A.G.; Skeie, S.B. Processing of high-protein yoghurt—A review. Int. Dairy J. 2019, 88, 42–59. [Google Scholar] [CrossRef]
- Uduwerella, G.; Chandrapala, J.; Vasiljevic, T. Minimising generation of acid whey during Greek yoghurt manufacturing. J. Dairy Res. 2017, 84, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Saad, M.F.; Hafiz, N.M. Safety and quality aspects of whole and skimmed milk powders. Acta Sci. Polon.-Techn. 2021, 20, 165–177. [Google Scholar] [CrossRef]
- Damin, M.R.; Alcântara, M.R.; Nunes, A.P.; Oliveira, M.N. Effects of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yogurt. LWT-Food Sci.Technol. 2009, 42, 1744–1750. [Google Scholar] [CrossRef]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr. Clin. Pract. 2013, 28, 684–690. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E.P.; Memelink, R.G.; Verlaan, S.; Wolfe, R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.F.C.A.; do Amaral, C.R.S.; Bernardino, P.D.L.D. The addition of skim milk powder and dairy cream influences the physicochemical properties and the sensory acceptance of concentrated Greek-style yogurt. Int. J. Gastron. Food Sci. 2021, 24, 100349. [Google Scholar] [CrossRef]
- Wajs, J.; Brodziak, A.; Król, J. Shaping the physicochemical, functional, microbiological and sensory properties of yoghurts using plant additives. Foods 2023, 12, 1275. [Google Scholar] [CrossRef]
- Buchweitz, M.; Speth, M.; Kammerer, D.R.; Carle, R. Impact of pectin type on the storage stability of black currant (Ribes nigrum L.) anthocyanins in pectic model solutions. Food Chem. 2013, 139, 1168–1178. [Google Scholar] [CrossRef]
- Nemetz, N.J.; Schieber, A.; Weber, F. Application of crude pomace powder of chokeberry, bilberry, and elderberry as a coloring foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Raikos, V.; Ni, H.; Hayes, H.; Ranawana, V. Antioxidant properties of a yogurt beverage enriched with salal (Gaultheria shallon) berries and blackcurrant (Ribes nigrum) pomace during cold storage. Beverages 2019, 5, 2. [Google Scholar] [CrossRef]
- Sankowski, L.V.; Morales-Medina, R.; Arguello, C.F.; Reibner, A.M.; Struck, S.; Rohm, H.; Drusch, S.; Brückner-Gühmann, M. Thermal-mechanical treatment of blackcurrant pomace for enrichment in yoghurt. Food Hydrocoll. 2024, 146, 109296. [Google Scholar] [CrossRef]
- Cais-Sokolinska, D.; Walkowiak-Tomczak, D. Consumer-perception, nutritional, and functional studies of a yogurt with restructured elderberry juice. J. Dairy Sci. 2021, 104, 1318–1335. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagala, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef]
- ON 57 0534; Milk Fermentationability Determination. Standard of Milk Industry: Prague, Czech Republic, 1986. (In Czech)
- ČSN ISO 7889 (571420); Yogurt Enumeration of Characteristic Microorganisms—Colony-Count Technique at 37 °C. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2003. (In Czech)
- Koval, D.; Alishevich, K.; Sasínová, K.; Ramešová, A.; Marhons, Š.; Nešporová, T.; Čurda, L.; Kumherová, M.; Bárta, J.; Filip, V.; et al. Formation of dihydrophenolic acids and aroma-active volatile phenols by new strains of Limosilactobacillus fermentum. Eur. Food Res. Technol. 2022, 248, 599–611. [Google Scholar] [CrossRef]
- Begum, A.; Harikrishna, S. Evaluation of some tree species to absorb air pollutants in three industrial locations of South Bengaluru, India. E-J. Chem. 2010, 7, S151–S156. [Google Scholar] [CrossRef]
- Myrtilli fructus siccum, the content analysis. In Czech Pharmacopoeia; Grada Publishing: Praha, Czech Republic, 2017; p. 4115. (In Czech)
- Lachman, J.; Hosnedl, V.; Pivec, V. Polyphenols in cereals and their positive and negative role in human and animal nutrition. In Cereals for Human Health and Preventive Nutrition; Vaculová, K., Ehrenbergerová, J., Eds.; MZLU: Brno, Czech Republic, 1998; pp. 118–124. [Google Scholar]
- Šulc, M.; Lachman, J.; Hamouz, K.; Orsák, M.; Dvořák, P.; Horáčková, V. Selection and evaluation of methods for determination of antioxidant activity of purple- and red-fleshed potato varieties. Chem. Listy 2007, 101, 584–591. [Google Scholar]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agr. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- ČSN ISO 11035 (560061); Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2003. (In Czech)
- ČSN ISO 4121 (560052); Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2009. (In Czech)
- Silva, J.V.C.; O’Mahony, J.A. Flowability and wetting behaviour of milk protein ingredients as influenced by powder composition, particle size and microstructure. Int. J. Dairy Technol. 2017, 70, 277–286. [Google Scholar] [CrossRef]
- Marafon, A.P.; Sumi, A.; Granato, D.; Alcântara, M.R.; Tamime, A.Y.; de Oliveira, M.N. Effects of partially replacing skimmed milk powder with dairy ingredients on rheology, sensory profiling, and microstructure of probiotic stirred-type yogurt during cold storage. J. Dairy Sci. 2011, 94, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Bong, D.D.; Moraru, C.I. Use of micellar casein concentrate for Greek-style yogurt manufacturing: Effects on processing and product properties. J. Dairy Sci. 2014, 97, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt; Tamime, A.Y., Robinson, R.K., Eds.; Woodhead Publishing: Cambridge, UK, 2007; pp. 1–808. [Google Scholar]
- Tamime, A.Y.; Hickey, M.; Muir, D.D. Strained fermented milks—A review of existing legislative provisions, survey of nutritional labelling of commercial products in selected markets and terminology of products in some selected countries. Int. J. Dairy Technol. 2014, 67, 305–333. [Google Scholar] [CrossRef]
- Remeuf, F.; Mohammed, S.; Sodini, I.; Tissier, J.P. Preliminary observations on the effects of milk fortification and heating on microstructure and physical properties of stirred yogurt. Int. Dairy J. 2003, 13, 773–782. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What is the color of milk and dairy products and how is it measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef]
- Decree on Requirements for Milk and Dairy Products, Frozen Creams and Edible Fats and Oils. [In Czech]. 2016. Available online: https://faolex.fao.org/docs/pdf/cze174739.pdf (accessed on 30 June 2024).
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Sidira, M.; Santarmaki, V.; Kiourtzidis, M.; Argyri, A.A.; Papadopoulou, O.S.; Chorianopoulos, N.; Tassou, C.; Kaloutsas, S.; Galanis, A.; Kourkoutas, Y. Evaluation of immobilized Lactobacillus plantarum 2035 on whey protein as adjunct probiotic culture in yoghurt production. LWT-Food Sci. Technol. 2017, 75, 137–146. [Google Scholar] [CrossRef]
- Krastanov, A.; Yeboah, P.J.; Dulari Wijemanna, N.W.; Eddin, A.S.; Ayivi, R.D.; Ibrahim, S.A. Volatile Aromatic Flavor Compounds in Yogurt: A Review. In Current Issues and Advances in the Dairy Industry; Salam, A.I., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Amal, A.M.N.; Mahmoud, A.M.; Zidan, N.S. Fruit flavored yoghurt: Chemical, functional and rheological properties. Int. J. Envir. Agric. Res. 2016, 2, 57–66. [Google Scholar]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef]
- Diviš, P.; Pořízka, J.; Vespalcová, M.; Matějíček, A.; Kaplan, J. Elemental composition of fruits from different black elder (Sambucus nigra L.) cultivars grown in the Czech Republic. J. Elementol. 2015, 20, 549–557. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Heide, O.M.; Sønsteby, A.; Wold, A.B.; Remberg, S.F. Yield and fruit quality of black currant (Ribes nigrum L.) are favoured by precipitation and cool summer conditions. Acta Agr. Scand. B-Soil Plant Sci. 2015, 65, 702–712. [Google Scholar] [CrossRef]
- Viberg, U.; Ekström, G.; Fredlund, K.; Öste, R.E.; Sjöholm, I. A study of some important vitamins and antioxidants in a blackcurrant jam with low sugar content and without additives. Int. J. Food Sci. Nutr. 1997, 48, 57–66. [Google Scholar] [CrossRef]
- Baeza, R.; Busso, C.; Sanchez, V.; López, P.; Chirife, J. Physicochemical properties and anthocyanin content of commercially manufactured elderberry jams from Patagonia (Argentina). Int. J. Food Eng. 2024, 20, 17–26. [Google Scholar] [CrossRef]
- Kennas, A.; Amellal-Chibane, H.; Kessal, F.; Halladj, F. Effect of pomegranate peel and honey fortification on physicochemical, physical, microbiological and antioxidant properties of yoghurt powder. J. Saudi Soc. Agric. Sci. 2020, 19, 99–108. [Google Scholar] [CrossRef]
- Bajramova, A.; Spégel, P. A comparative study of the fatty acid profile of common fruits and fruits claimed to confer health benefits. J. Food Compos. Anal. 2022, 112, 104657. [Google Scholar] [CrossRef]
- del Castillo, M.L.R.; Dobson, G.; Brennan, R.; Gordon, S. Fatty acid content and juice characteristics in black currant (Ribes nigrum L.) genotypes. J. Agr. Food Chem. 2004, 52, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crop. Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, sweet, salty, sour and umami taste perception decreases with age: Sex-specific analysis, modulation by genetic variants and taste-preference associations in 18 to 80 year-old subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Noel, C.; Dando, R. The effect of emotional state on taste perception. Appetite 2015, 95, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Vennerod, F.F.F.; Nicklaus, S.; Lien, N.; Almli, V.L. The development of basic taste sensitivity and preferences in children. Appetite 2018, 127, 130–137. [Google Scholar] [CrossRef]
| Experiment | Type of Yogurt | SMP (%) | Fruit Jam (10%) | Purpose |
|---|---|---|---|---|
| 1 | natural | 0 | – | to evaluate the effect of the addition of SMP on the selected parameters (chemical composition, acidity, viscosity, viable yogurt bacteria (Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus salivarius subsp. thermophilus), color, volatile compounds) |
| natural | 5 | – | ||
| natural | 10 | – | ||
| 2 | fruit | 5 | black currant | to evaluate the effect of the addition of SMP, and the fruit jam on the selected parameters (chemical composition, acidity, color, organoleptic properties) |
| fruit | 5 | elderberry | ||
| fruit | 10 | black currant | ||
| fruit | 10 | elderberry | ||
| 3 | fruit | 10 | black currant | to evaluate the effect of the addition of SMP, and the fruit jam on the selected parameters (chemical composition, acidity, color, organoleptic properties) |
| fruit | 10 | elderberry | ||
| fruit | 10 | black currant + elderberry (1:1) |
| Parameters 1 | Raw Milk | Pasteurized Milk | p-Value | ||
|---|---|---|---|---|---|
| 0% SMP | 5% SMP | 10% SMP | |||
| Milk for preparation of natural yogurts (Experiment 1) | |||||
| Fat (g/100 g) | 3.43 ± 0.01 | 3.43 ± 0.13 | 3.27 ± 0.21 | 3.06 ± 0.22 | 0.2103 |
| Crude protein (g/100 g) | 3.28 a ± 0.06 | 3.30 a ± 0.06 | 4.99 b ± 0.03 | 6.68 c ± 0.00 | <0.001 |
| Lactose (g/100 g) | 4.95 a ± 0.03 | 5.00 a ± 0.03 | 7.69 b ± 0.03 | 10.39 c ± 0.09 | <0.001 |
| SNF (g/100 g) | 8.98 a ± 0.14 | 9.04 a ± 0.13 | 13.56 b ± 0.06 | 18.09 c ± 0.03 | <0.001 |
| Total solids (g/100 g) | 12.41 a ± 0.14 | 12.47 a ± 0.26 | 16.83 b ± 0.27 | 21.14 c ± 0.20 | <0.001 |
| SCC (×103 cells/mL) | 246 ± 81 | 256 ± 86 | 298 ± 102 | 341 ± 120 | 0.7658 |
| FPD (°C × −1000) | 526 a ± 1 | 526 a ± 2 | 796 b ± 7 | 1066 c ± 13 | <0.001 |
| Milk for preparation of fruit yogurts (Experiments 2 and 3) | |||||
| Fat (g/100 g) | 3.86 bc ± 0.06 | 3.92 c ± 0.13 | 3.56 ab ± 0.06 | 3.35 a ± 0.05 | 0.0069 |
| Crude protein (g/100 g) | 3.43 a ± 0.04 | 3.44 a ± 0.08 | 4.88 b ± 0.02 | 6.39 c ± 0.04 | <0.001 |
| Lactose (g/100 g) | 4.95 a ± 0.09 | 4.95 a ± 0.07 | 7.47 b ± 0.43 | 10.10 c ± 0.79 | 0.0009 |
| SNF (g/100 g) | 9.10 a ± 0.10 | 9.11 a ± 0.01 | 13.35 b ± 0.36 | 17.80 c ± 0.74 | 0.0001 |
| Total solids (g/100 g) | 13.00 a ± 0.10 | 13.06 a ± 0.16 | 16.33 ab ± 1.12 | 19.94 b ± 2.40 | 0.0173 |
| SCC (×103 cells/mL) | 320 ± 107 | 296 ± 141 | 341 ± 203 | 431 ± 115 | 0.8114 |
| FPD (°C × −1000) | 532 a ± 13 | 537 a ± 1 | 807 b ± 18 | 1090 c ± 57 | 0.0001 |
| Experiment | Panelist | n | Gender (%) | Age (Years) | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | |||
| 2 | experienced | 10 | 50 | 50 | 42.7 ± 10.8 | 39.0 ± 10.9 | 46.4 ± 10.5 |
| 3 | experienced | 12 | 50 | 50 | 36.7 ± 9.5 | 38.3 ± 11.9 | 35.0 ± 7.2 |
| trained | 69 | 49 | 51 | 27.1 ± 8.8 | 26.2 ± 9.2 | 27.9 ± 8.5 | |
| Parameters 1 | Natural Yogurt Samples | p-Value | ||
|---|---|---|---|---|
| 0% SMP | 5% SMP | 10% SMP | ||
| Active acidity (pH) | 4.25 a ± 0.07 | 4.34 ab ± 0.07 | 4.42 b ± 0.07 | 0.0225 |
| Titratable acidity (SH) | 40.7 a ± 4.7 | 56.1 b ± 4.9 | 71.5 c ± 5.1 | <0.001 |
| Titratable acidity (mmol/L) | 101.7 a ± 11.7 | 140.3 b ± 12.2 | 178.8 c ± 12.7 | <0.001 |
| Fat (g/100 g) | 3.58 c ± 0.02 | 3.24 b ± 0.11 | 2.89 a ± 0.19 | 0.0001 |
| Protein (g/100 g) | 3.50 a ± 0.05 | 4.93 b ± 0.23 | 6.36 c ± 0.43 | <0.001 |
| Total solids (g/100 g) | 12.12 a ± 1.29 | 17.48 b ± 3.30 | 19.15 b ± 1.91 | 0.0052 |
| Viscosity (mPa·s) | 1295 a ± 194 | 2789 b ± 214 | 4106 c ± 196 | <0.001 |
| Color (L*) | 80.06 ± 1.39 | 81.33 ± 1.02 | 81.55 ± 0.70 | 0.1627 |
| Color (a*) | −1.53 ± 0.04 | −1.50 ± 0.06 | −1.52 ± 0.04 | 0.5566 |
| Color (b*) | 5.43 a ± 0.55 | 5.90 a ± 0.10 | 6.56 b ± 0.06 | 0.0028 |
| ST (log CFU/g) | 8.59 ± 0.30 | 8.68 ± 0.13 | 8.93 ± 0.08 | 0.0798 |
| LB (log CFU/g) | 8.47 ± 0.47 | 8.71 ± 0.04 | 8.63 ± 0.07 | 0.4831 |
| Acetone (mg/kg) | 1.92 ± 0.54 | 1.50 ± 0.30 | 1.57 ± 0.57 | 0.4768 |
| Diacetyl (mg/kg) | 6.27 b ± 0.18 | 5.36 ab ± 0.56 | 4.30 a ± 0.28 | 0.0315 |
| Acetoin (mg/kg) | 144.90 a ± 7.27 | 180.05 a ± 23.31 | 268.56 b ± 17.72 | 0.0122 |
| Heptanone (mg/kg) | 4.69 a ± 0.19 | 6.58 ab ± 0.15 | 9.22 b ± 1.40 | 0.0262 |
| Parameters 1 | Fruit | p-Value | Jam | p-Value | |||
|---|---|---|---|---|---|---|---|
| BC | EB | BC | EB | BCEB | |||
| TPC (mg GAE/100 g FW) | 430 ± 62.3 | 575 ± 90.9 | 0.0390 | 319 a ± 53.3 | 559 c ± 40.5 | 407 b ± 27.2 | 0.0001 |
| Vitamin C (mg/100 g FW) | 138 ± 0.72 | 12.7 ± 0.49 | <0.001 | 59.5 c ± 0.19 | 6.07 a ± 0.21 | 31.8 b ± 0.67 | <0.001 |
| Anthocyanins (mg/100 g FW) | 543 ± 16.1 | 1005 ± 87.6 | <0.001 | 205 a ± 7.15 | 410 c ± 19.7 | 305 b ± 16.2 | <0.001 |
| ABTS (mg AAE/100 g FW) | 949 ± 39.4 | 1071 ± 16 | 0.0012 | 656 b ± 6.67 | 665 b ± 17.4 | 618 a ± 24.8 | 0.0105 |
| DPPH (mg AAE/100 g FW) | 413 ± 32.9 | 474 ± 19.1 | 0.0193 | 336 b ± 25.9 | 300 a ± 15.3 | 287 a ± 3.24 | 0.0085 |
| FRAP (mg AAE/100 g FW) | 479 ± 19 | 830 ± 29.8 | <0.001 | 390 a ± 1.9 | 1016 c ± 29.9 | 954 b ± 16.1 | <0.001 |
| Parameters | Fruit Yogurt Samples (10% Jam) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 5% SMP | 10% SMP | ||||||
| BC | EB | BC | EB | SMP | Fruit | SMP * Fruit | |
| Active acidity (pH) | 4.12 a ± 0.02 | 4.21 b ± 0.03 | 4.16 ab ± 0.03 | 4.30 c ± 0.03 | 0.0010 | <0.001 | 0.1071 |
| Fat (g/100 g) | 3.91 ab ± 0.21 | 3.39 ab ± 0.27 | 3.63 ab ± 0.29 | 3.22 a ± 0.39 | 0.1588 | 0.0091 | 0.7168 |
| Protein (g/100 g) | 4.79 a ± 0.35 | 4.22 a ± 0.21 | 6.21 c ± 0.53 | 5.80 bc ± 0.20 | 0.0001 | 0.0832 | 0.7630 |
| Total solids (g/100 g) | 21.31 a ± 0.34 | 21.96 a ± 0.30 | 25.22 c ± 0.36 | 25.38 b ± 0.32 | <0.001 | 0.0306 | 0.1625 |
| Total saccharides (g/100 g) | 12.77 a ± 0.17 | 13.73 ab ± 0.75 | 15.88 c ± 1.49 | 15.25 bc ± 0.97 | 0.0004 | 0.7388 | 0.1250 |
| Color (L*) | 63.54 c ± 0.74 | 55.74 a ± 2.47 | 64.98 c ± 0.13 | 59.83 b ± 0.60 | 0.0013 | <0.001 | 0.0698 |
| Color (a*) | 7.74 b ± 0.56 | 14.66 d ± 0.65 | 6.61 a ± 0.41 | 11.62 c ± 0.21 | <0.001 | <0.001 | 0.0020 |
| Color (b*) | −1.65 a ± 0.19 | −1.14 b ± 0.19 | −1.51 a ± 0.02 | −0.79 c ± 0.08 | 0.0044 | <0.001 | 0.1657 |
| Parameters | Fruit Yogurt Samples (10% Jam) | p-Value | ||
|---|---|---|---|---|
| 10% SMP | ||||
| BC | EB | BCEB | ||
| Acidity (pH) | 4.17 a ± 0.04 | 4.29 b ± 0.03 | 4.21 a ± 0.01 | 0.0003 |
| Fat (g/100 g) | 3.50 ± 0.16 | 3.57 ± 0.07 | 3.54 ± 0.22 | 0.8516 |
| Protein (g/100 g) | 5.46 ± 0.20 | 5.66 ± 0.07 | 5.71 ± 0.12 | 0.0691 |
| Total solids (g/100 g) | 25.44 ± 0.43 | 25.24 ± 0.35 | 25.28 ± 0.21 | 0.7089 |
| Total saccharides (g/100 g) | 14.86 ± 0.44 | 14.37 ± 0.23 | 14.60 ± 0.21 | 0.1337 |
| Color (L*) | 62.02 c ± 0.30 | 57.83 b ± 0.56 | 59.63 a ± 0.44 | <0.001 |
| Color (a*) | 10.69 a ± 0.15 | 15.57 b ± 0.40 | 13.92 c ± 0.18 | <0.001 |
| Color (b*) | −4.40 a ± 0.02 | −2.04 b ± 0.08 | −2.94 c ± 0.08 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janoušek Honesová, S.; Samková, E.; Dadáková, E.; Hasoňová, L.; Jarošová, M.; Reindl, K.; Bárta, J. Effect of Skimmed Milk Powder and Fruit Jams Addition on the Physicochemical Characteristics of Yogurt. Fermentation 2024, 10, 462. https://doi.org/10.3390/fermentation10090462
Janoušek Honesová S, Samková E, Dadáková E, Hasoňová L, Jarošová M, Reindl K, Bárta J. Effect of Skimmed Milk Powder and Fruit Jams Addition on the Physicochemical Characteristics of Yogurt. Fermentation. 2024; 10(9):462. https://doi.org/10.3390/fermentation10090462
Chicago/Turabian StyleJanoušek Honesová, Simona, Eva Samková, Eva Dadáková, Lucie Hasoňová, Markéta Jarošová, Karolína Reindl, and Jan Bárta. 2024. "Effect of Skimmed Milk Powder and Fruit Jams Addition on the Physicochemical Characteristics of Yogurt" Fermentation 10, no. 9: 462. https://doi.org/10.3390/fermentation10090462
APA StyleJanoušek Honesová, S., Samková, E., Dadáková, E., Hasoňová, L., Jarošová, M., Reindl, K., & Bárta, J. (2024). Effect of Skimmed Milk Powder and Fruit Jams Addition on the Physicochemical Characteristics of Yogurt. Fermentation, 10(9), 462. https://doi.org/10.3390/fermentation10090462






