Optimization of Polyphenols Release from Highland Barley Bran by Solid-State Fermentation and Antioxidant Activity Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Methods
2.3.1. Sample Preparation
2.3.2. Single-Factor and Response Surface Experimental Design
2.3.3. Polyphenol Content Determination
2.3.4. Phenolic Acid Content Determination
2.3.5. Determination of Antioxidant Activity
2.3.6. Data Analyses and Processing
3. Results and Discussion
3.1. Results of One-Factor Test on Fermentation of Highland Barley Bran
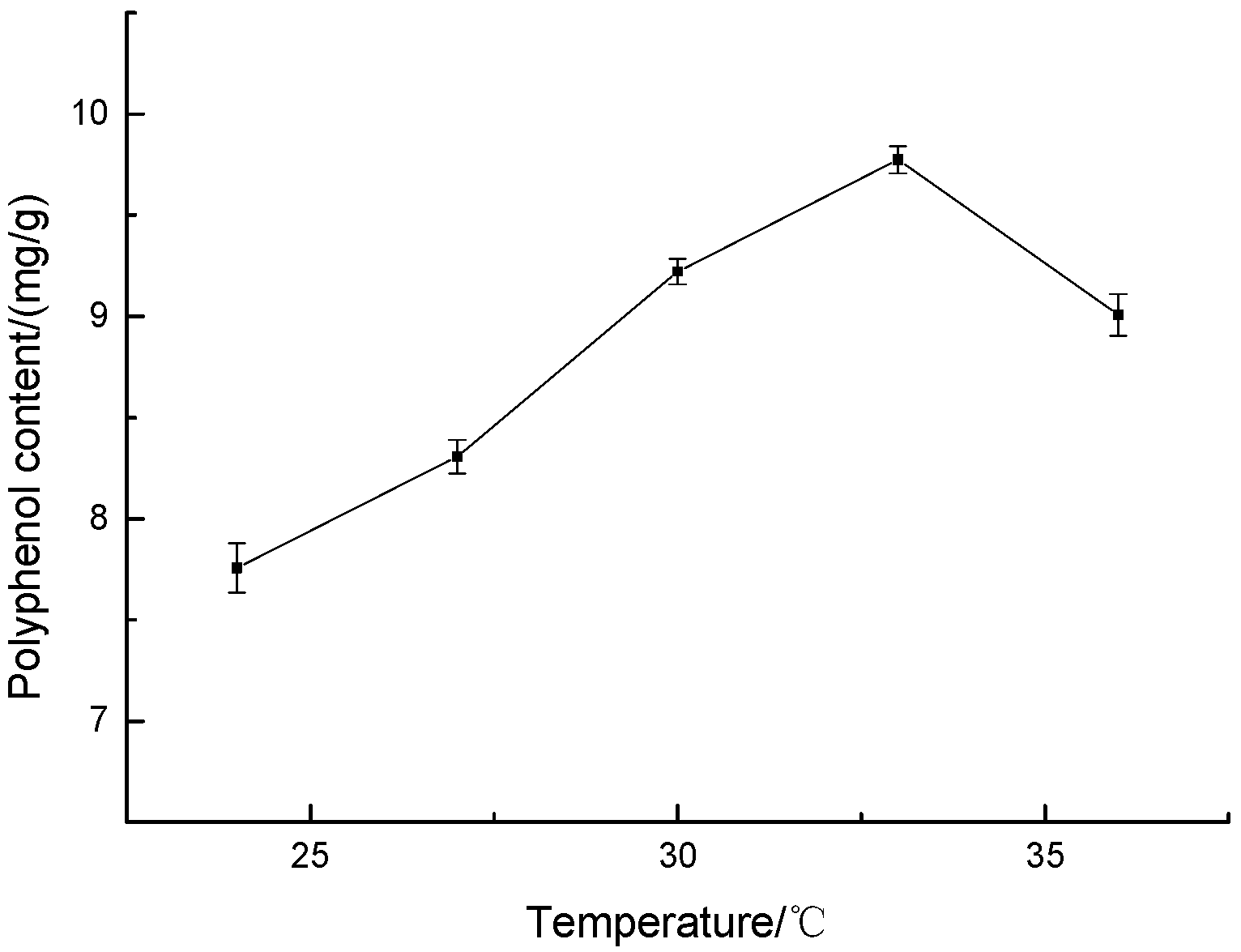
3.1.1. Effect of Fermentation Temperature on Polyphenol Content of Highland Barley Bran
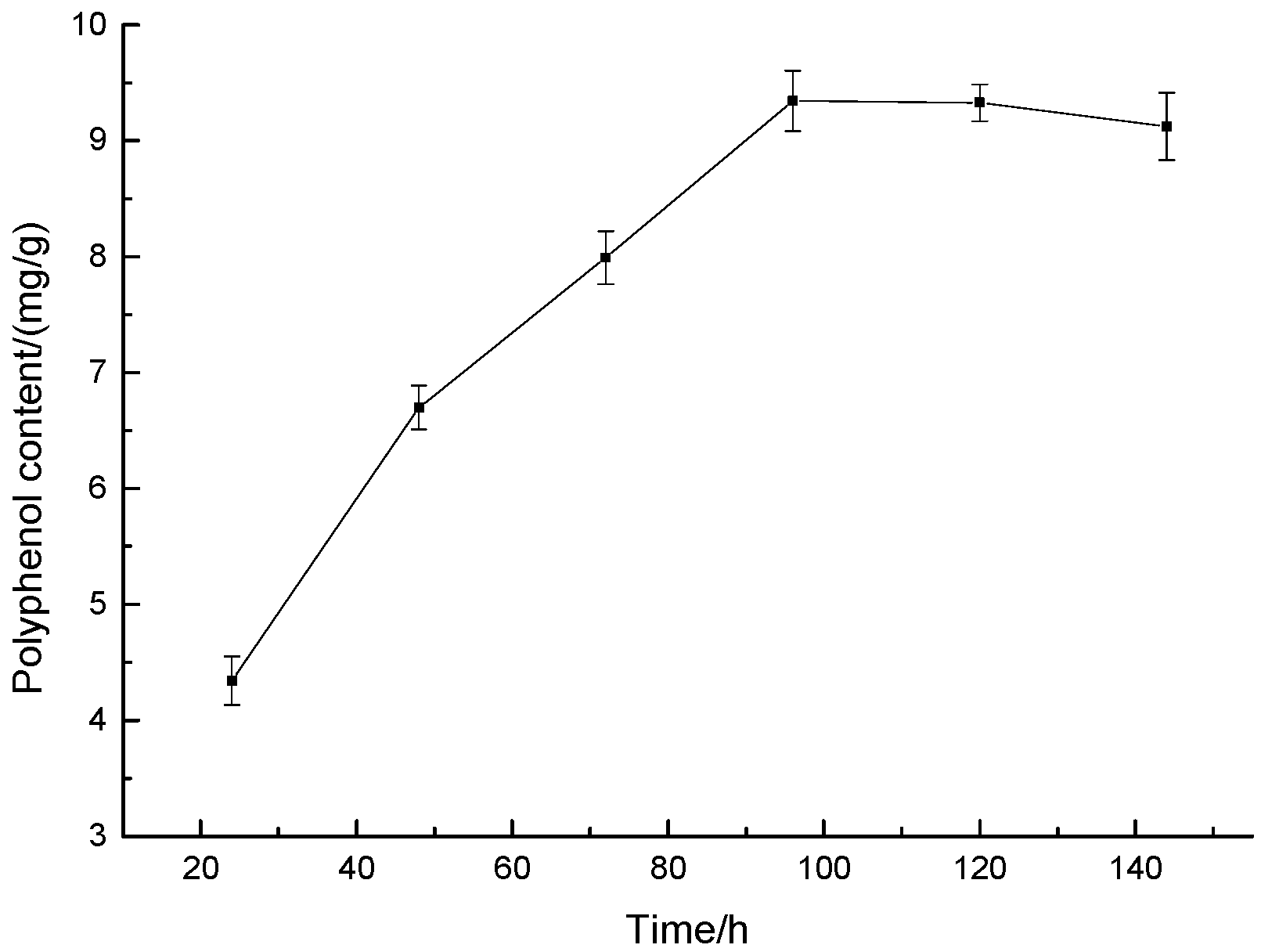
3.1.2. Effect of Fermentation Time on Polyphenol Content of Highland Barley Bran
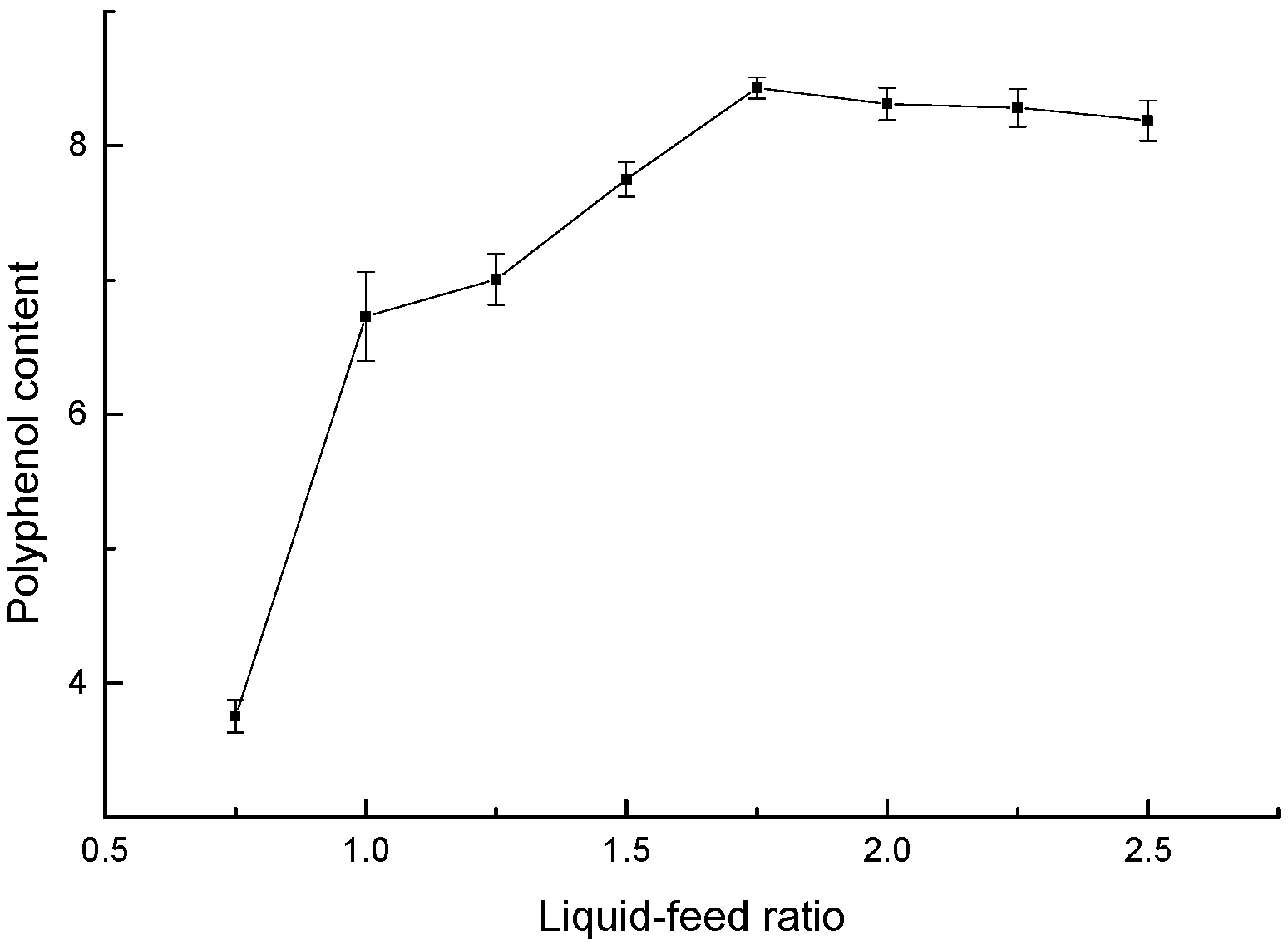
3.1.3. Effect of Liquid–Feed Ratio on the Polyphenol Content of Highland Barley Bran
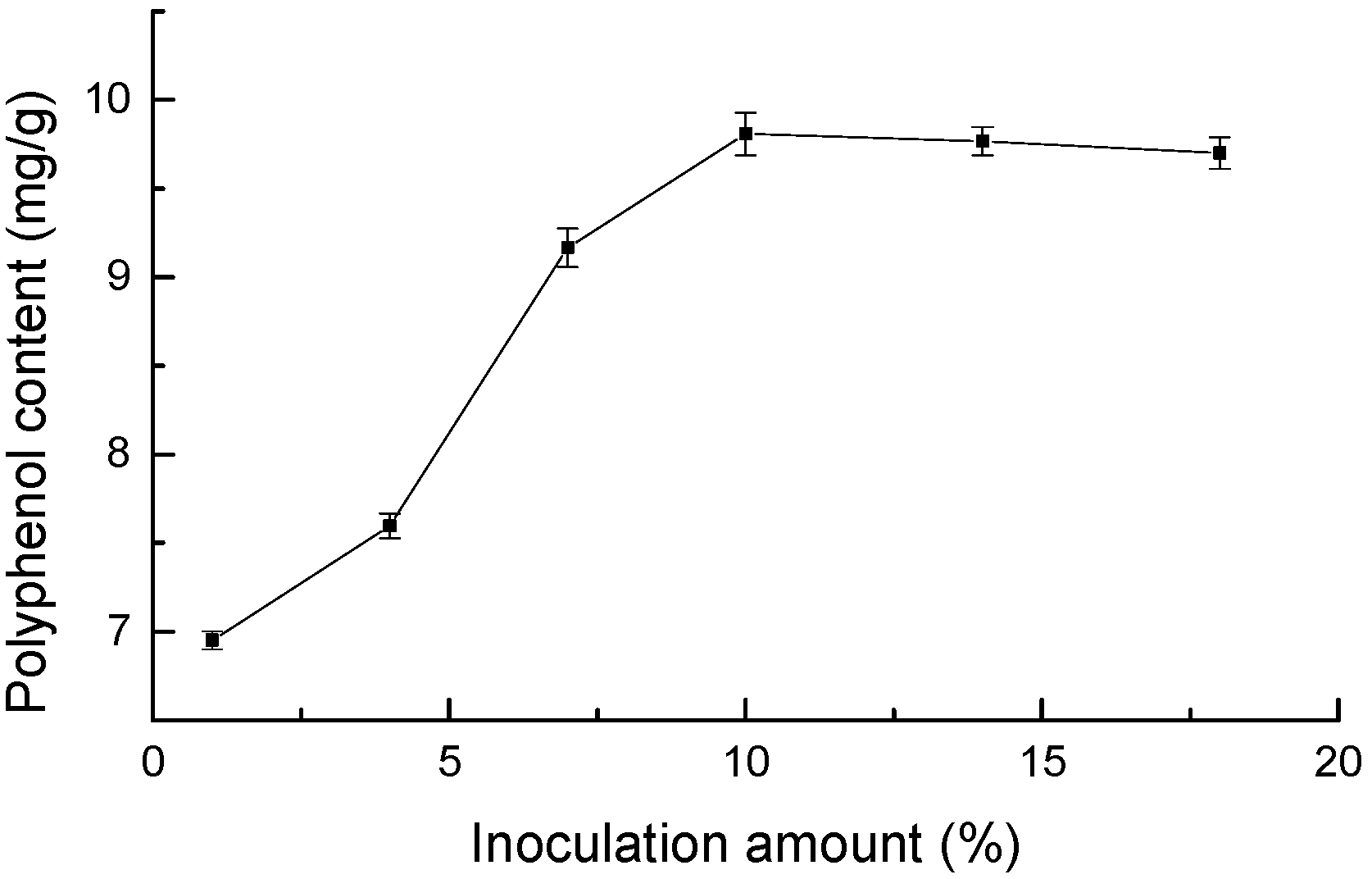
3.1.4. Effect of Inoculum Amount on Polyphenol Content of Highland Barley Bran
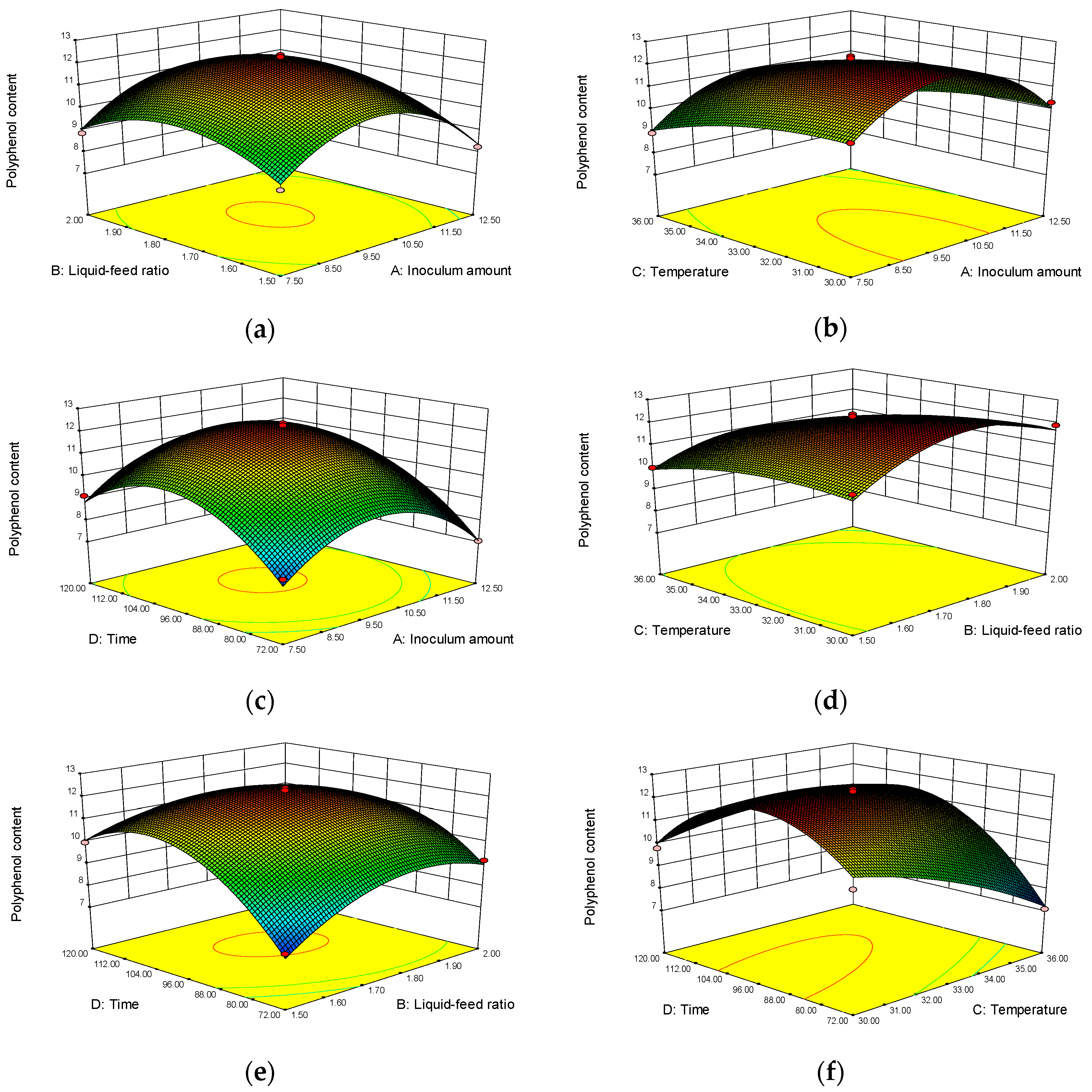
3.2. Optimization of Highland Barley Bran Fermentation Process by Response Surface Methodology
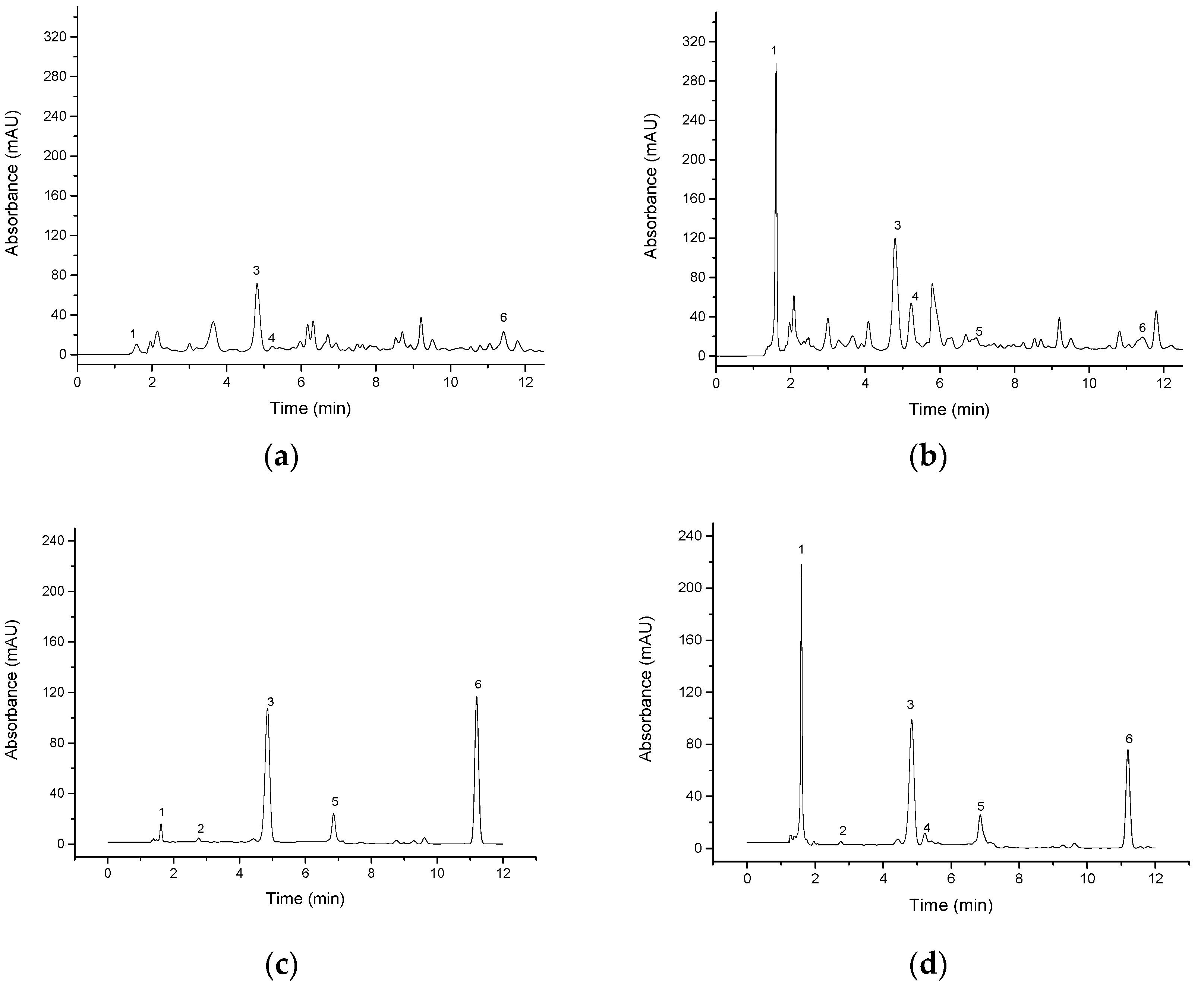
3.3. Determination of Phenolic Acid Content of Highland Barley Bran before and after Fermentation
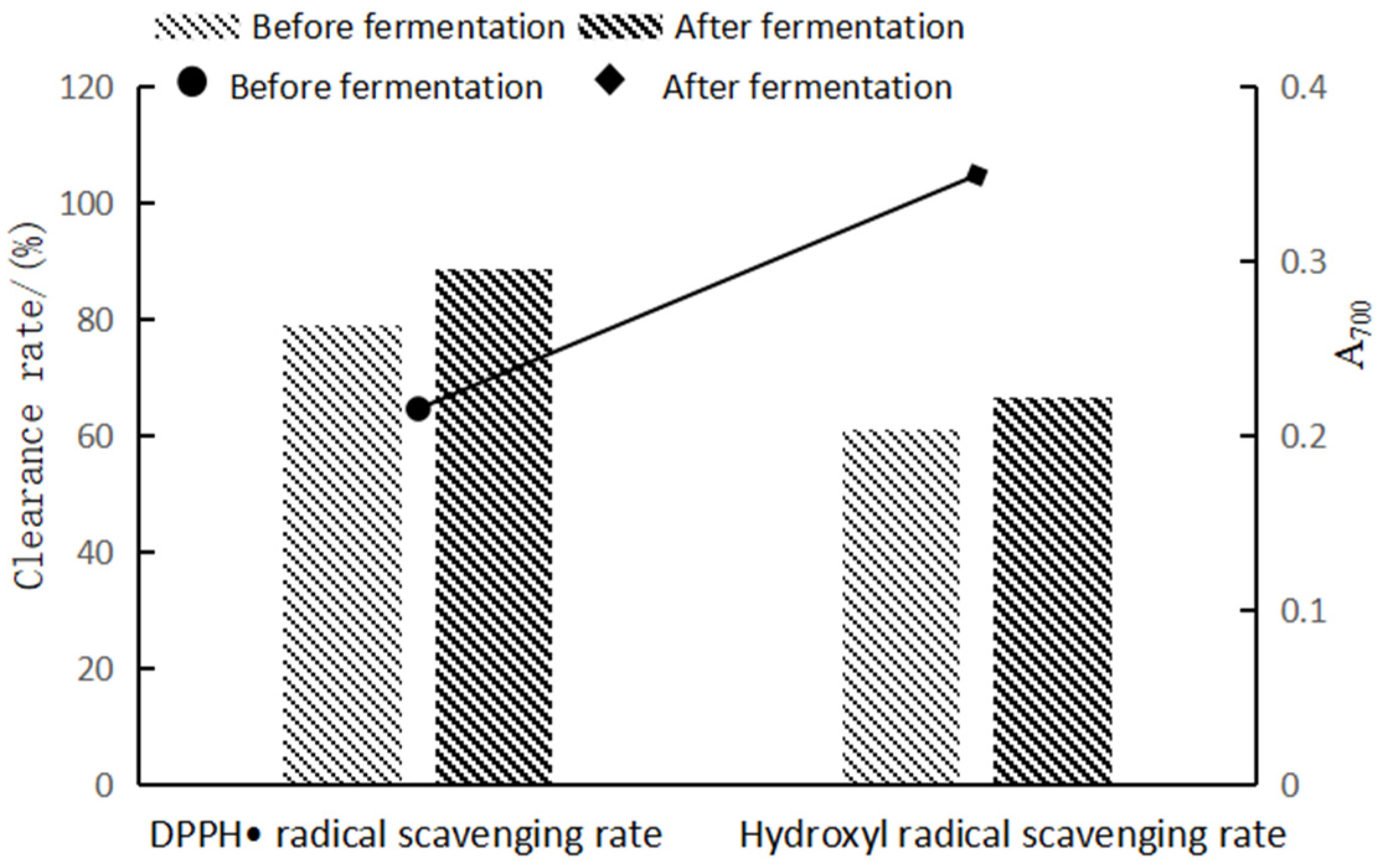
3.4. Analysis of the Antioxidant Activity of Highland Barley Bran before and after Fermentation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (qingke): A review. Trends Food Sci. Technol 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Li, J.; Zhao, S.; Zhao, Y. Study on the origin traceability of tibet highland barley (Hordeum vulgare L.) based on its nutrients and mineral elements. Food Chem 2020, 346, 128928. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, C.; Zhang, H.; Jiang, H. Characteristics of three typical chinese highland barley varieties: Phenolic compounds and antioxidant activities. J. Food Biochem. 2017, 42, e12488. [Google Scholar] [CrossRef]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Sajid, B.; Mahdi, A.A.; Al-Maqtari, Q.A.; Wang, L. Molecular structure, morphological, and physicochemical properties of highlands barley starch as affected by natural fermentation. Food Chem. 2021, 356, 129665. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Assessment of the phenolic profiles, hypoglycemic activity, and molecular mechanism of different highland barley (Hordeum vulgare L.) varieties. Int. J. Mol. Sci. 2020, 21, 1175. [Google Scholar] [CrossRef] [PubMed]
- Imi, G.; Horvat, D.; Lali, A.; Komleni, D.K.; Zduni, Z. Distribution of β-glucan, phenolic acids, and proteins as functional phytonutrients of hull-less barley grain. Foods 2019, 8, 680. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Zhang, D.; Wang, J.; Wang, S.; Sun, B. Effects of highland barley bran extract rich in phenolic acids on the formation of n-epsilon-carboxymethyllysine in a biscuit model. J. Agric. Food Chem. 2018, 66, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; You, M.; Liu, H.; Liu, X. Comparison of distribution and physicochemical properties of β-glucan extracted from different fractions of highland barley grains. Int. J. Biol. Macromol. 2021, 189, 91–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2020, 339, 127849. [Google Scholar] [CrossRef]
- y Postigo, L.O.C.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Amezquita, L.E.G.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2020, 35, 1153–1169. [Google Scholar] [CrossRef]
- Hlker, U.; Lenz, J. Solid-state fermentation–are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef]
- Ooijkaas, L.P.; Weber, F.J.; Buitelaar, R.M.; Tramper, J.; Rinzema, A. Defined media and inert supports: Their potential as solid-state fermentation production systems. Cheminform 2010, 31, 356–360. [Google Scholar] [CrossRef]
- Oiza, N.; Moral-Vico, J.; Sánchez, A.; Oviedo, E.R.; Gea, T. Solid-state fermentation from organic wastes: A new generation of bioproducts. Processes 2022, 10, 2675. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Ren, X.; Qi, J.; Liu, N.; Wang, R.; Wang, Y.; An, X. Effect of microbial Fermentation on content, composition and antioxidant activity of water-soluble polyphenols in wheat bran. Sci. Technol. Food Ind. 2020, 3, 104–109. [Google Scholar]
- Pei, F.; Du, Y.F.; Sun, L.; Li, W.; Fang, Y.; Hu, Q.H. Effects of solid-state fermentation on antioxidant properties of wheat bran and sensory quality of whole wheat bread. Food Sci. 2022, 43, 212–220. [Google Scholar] [CrossRef]
- Liu, B.; Cui, C.; Chen, Z. Effects of Lactobacillus Fermentation on the Content and Composition of Black Rice Polyphenols. Sci. Technol. Food Ind. 2023, 44, 114–120. [Google Scholar] [CrossRef]
- Chen, S.K.; Lin, H.F.; Wang, X.; Yuan, Y.; Yin, J.Y.; Song, X.X. Comprehensive analysis in the nutritional composition, phenolic species and in vitro antioxidant activities of different pea cultivars. Food Chem. 2023, 17, 100599. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Pan, X.; Lao, F.; Liao, X.; Shi, Y.; Wu, J. Improvement of antioxidant properties of jujube puree by biotransformation of polyphenols via. Food Chem. 2022, 13, 100214. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with hplc. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- Ofili, S.G.N.O. Hypocholesterolemic effects of crude extract of leaf of moringa oleifera lam in high-fat diet fed wistar rats. J. Ethnopharmacol. 2020, 69, 21–25. [Google Scholar] [CrossRef]
- Hong-Fei, D.; Mao-Run, F.U.; Qing-Li, Q.U. Effects of superfine grinding on physicochemical characteristics and antioxidant activity of lotus plumule. J. Food Saf. Qual. 2015, 6, 787–793. [Google Scholar]
- Musa, K.H. Determination of DPPH free radical scavenging activity: Application of artificial neural networks. Food Chem. 2015, 194, 705–711. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed himanthalia elongata from western coast of ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Gu, H.; Bughio, A.A.; Liu, J. Optimization of Fermentation Conditions for 2,3,5-Trimethylpyrazine Produced by Bacillus amyloliquefaciens from Daqu. Fermentation 2024, 10, 112. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, M.; Huang, Y.; Li, C.; Pan, X.; Zhu, W.; Huang, Y. Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum-fermented loose tea. LWT. 2017, 82, 248–254. [Google Scholar] [CrossRef]
- Lu, Y. Biotransformation of Functional Components in Oats by Solid-State Fermentation of Monascus spp. Master’s Thesis, South China University of Technology, Guangzhou, China, 2016. [Google Scholar]
- Li, W.; Su, Y.; Cheng, F.; Shi, H.; Han, J.C.; Yu, Y.H. Optimization of mixed solid-state fermentation of soybean meal by lactobacillus species and clostridium butyricum. Pol. J. Microbiol. 2018, 67, 297–305. [Google Scholar] [CrossRef]
- Akbari, M.; Urios, C.; Razavi, S.; Khodaiyan, F.; Blesa, J.; Esteve, M.J. Optimization of solid-state fermentation conditions to improve phenolic content in corn bran, followed by extraction of bioactive compounds using natural deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2024, 93, 103621. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Wu, Z. Impact of fermentation degree on phenolic compositions and bioactivities during the fermentation of guava leaves with Monascus anka and Bacillus sp. J. Funct. Foods 2018, 41, 183–190. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Xu, H.; Sun, Y.; Zhang, Y.; Wang, Y. Improvement of the nutritional, antioxidant and bioavailability properties of corn gluten-wheat bran mixture fermented with lactic acid bacteria and acid protease. LWT 2021, 144, 111161. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Wang, X.; Xu, H.; Hou, Y.; Chen, X. Effect of Rhizopus Oryzae Fermentation on Nutritional Components and Antioxidant Activity of Defatted Adlay Bran. J. Chin. Cereals Oils Assoc. 2021, 36, 16–22. [Google Scholar]
- Nie, P.; Ding, X.; Wu, Y.; Song, L. Effects of Lactobacillus plantarum Fermentation on Enrichment of Polyphenols in Whole Grain Black Barley. J. Chin. Inst. Food Sci. Technol. 2023, 23, 186–196. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhang, Y.Z.; Dong, L.Z.; Li, Y.H.; Liu, Y.; Liu, Y.; Liu, L.Y. Fermentation of Lactobacillus fermentum NB02 with feruloyl esterase production increases the phenolic compounds content and antioxidant properties of oat bran. Food Chem 2024, 437, 137834. [Google Scholar] [CrossRef]
- Singh, A.K.; Jagbir, R.; Amarjeet, K.; Gagan, J. Enhancement of attributes of cereals by germination and fermentation: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.L.; Liu, C.L.; Yin, T.T.; Yan, X.T.; Wu, C.L.; He, H.L.; Yi, C.P. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem 2020, 330, 127156. [Google Scholar] [CrossRef]
| Fermentation Conditions | Process Parameters | |||||
|---|---|---|---|---|---|---|
| Fermentation temperature/(°C) | 24 | 27 | 30 | 33 | 36 | |
| Fermentation time/(h) | 24 | 48 | 72 | 96 | 120 | 144 |
| Liquid–feed ratio | 0.75 | 1 | 1.25 | 1.5 | 1.75 | 2 |
| Vaccination load/(%) | 2.5 | 5 | 7.5 | 10 | 12.5 | 15 |
| Fermentation temperature/(°C) | 24 | 27 | 30 | 33 | 36 | |
| Level | Factors | |||
|---|---|---|---|---|
| Fermentation Temperature/(°C) | Fermentation Temperature Time/(h) | Liquid–Feed Ratio | Vaccination Load/(%) | |
| −1 | 30 | 72 | 1.5:1 | 7.5 |
| 0 | 33 | 96 | 1.75:1 | 10 |
| 1 | 36 | 120 | 2:1 | 12.5 |
| Test No. | A Inoculum Amount (%) | B Liquid- Feed Ratio | C Fermentation Temperature (°C) | D Fermentation Time (h) | Y Polyphenol Content (mg/g) |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 1 | 1 | 10.86 ± 0.21 |
| 2 | −1 | 0 | −1 | 0 | 10.60 ± 0.17 |
| 3 | 0 | 0 | 0 | 0 | 12.02 ± 0.05 |
| 4 | 0 | 1 | 0 | 1 | 8.95 ± 0.09 |
| 5 | 0 | 0 | 1 | −1 | 7.06 ± 0.05 |
| 6 | 1 | 0 | 0 | −1 | 7.03 ± 0.11 |
| 7 | 1 | 1 | 0 | 0 | 8.81 ± 0.20 |
| 8 | 0 | 0 | 0 | 0 | 12.27 ± 0.27 |
| 9 | 0 | 0 | 0 | 0 | 12.14 ± 0.21 |
| 10 | 0 | 0 | 0 | 0 | 12.02 ± 0.21 |
| 11 | 0 | −1 | 1 | 0 | 9.99 ± 0.18 |
| 12 | 0 | 0 | −1 | 1 | 9.81 ± 0.09 |
| 13 | 0 | 0 | 0 | 0 | 12.36 ± 0.29 |
| 14 | 0 | −1 | 0 | 1 | 9.97 ± 0.22 |
| 15 | 1 | −1 | 0 | 0 | 8.23 ± 0.21 |
| 16 | −1 | 0 | 0 | −1 | 7.77 ± 0.10 |
| 17 | 0 | 1 | −1 | 0 | 11.88 ± 0.19 |
| 18 | 0 | −1 | 0 | −1 | 7.39 ± 0.11 |
| 19 | 1 | 0 | −1 | 0 | 10.32 ± 0.09 |
| 20 | −1 | 1 | 0 | 0 | 8.86 ± 0.18 |
| 21 | 1 | 0 | 1 | 0 | 8.86 ± 0.23 |
| 22 | −1 | 0 | −1 | 1 | 9.12 ± 0.19 |
| 23 | −1 | 0 | 1 | 0 | 8.92 ± 0.07 |
| 24 | 0 | 0 | −1 | −1 | 10.16 ± 0.08 |
| 25 | 1 | 0 | 0 | 1 | 8.62 ± 0.21 |
| 26 | 0 | 1 | 0 | −1 | 9.16 ± 0.25 |
| 27 | 0 | −1 | −1 | 0 | 10.86 ± 0.21 |
| 28 | 0 | 1 | 1 | 0 | 9.62 ± 0.09 |
| 29 | −1 | −1 | 0 | 0 | 8.64 ± 0.16 |
| Source | Square Sum | Degrees of Freedom | Mean Square | Value of F | Value of p | Significance |
|---|---|---|---|---|---|---|
| Model | 70.05 | 14 | 5.00 | 73.27 | <0.0001 | ** |
| A | 0.35 | 1 | 0.35 | 5.08 | 0.0408 | * |
| B | 0.40 | 1 | 0.40 | 5.91 | 0.0291 | * |
| C | 5.77 | 1 | 5.77 | 84.47 | <0.0001 | ** |
| D | 6.39 | 1 | 6.39 | 93.64 | <0.0001 | ** |
| AB | 0.032 | 1 | 0.032 | 0.47 | 0.5022 | |
| AC | 0.012 | 1 | 0.012 | 0.18 | 0.6802 | |
| AD | 0.014 | 1 | 0.014 | 0.21 | 0.6531 | |
| BC | 0.48 | 1 | 0.48 | 7.07 | 0.0187 | * |
| BD | 1.95 | 1 | 1.95 | 28.50 | 0.0001 | ** |
| CD | 4.31 | 1 | 4.31 | 63.05 | <0.0001 | ** |
| A2 | 28.26 | 1 | 28.26 | 413.81 | <0.0001 | ** |
| B2 | 10.38 | 1 | 10.38 | 151.94 | <0.0001 | ** |
| C2 | 1.27 | 1 | 1.27 | 18.58 | 0.0007 | ** |
| D2 | 27.85 | 1 | 27.85 | 407.88 | <0.0001 | ** |
| Residuals | 0.96 | 14 | 0.068 | |||
| Lost proposal | 0.86 | 10 | 0.086 | 3.77 | 0.1063 | insignificant |
| Pure error | 0.092 | 4 | 0.023 | |||
| Sum | 71.00 | 28 |
| Items/(mg/kg) | Free Phenol Pre-Fermentation | Free Phenol after Fermentation | Bound Phenol Pre-Fermentation | Bound Phenol after Fermentation |
|---|---|---|---|---|
| gallic acid | 0.36 ± 0.07 | 205.12 ± 0.15 | 5.93 ± 0.09 | 68.19 ± 0.22 |
| coumaric acid | ND | ND | 1.57 ± 0.09 | 1.29 ± 0.11 |
| catechin | 64.42 ± 0.16 | 122.22 ± 0.33 | 266.39 ± 0.13 | 194.05 ± 0.24 |
| catechol | 0.93 ± 0.06 | 17.82 ± 0.08 | ND | 6.07 ± 0.19 |
| caffeic acid | ND | 0.07 ± 0.01 | 1.92 ± 0.03 | 2.28 ± 0.03 |
| ferulic acid | 2.83 ± 0.07 | 0.90 ± 0.03 | 187.78 ± 0.11 | 158.32 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, M.; Cheng, Z.; Meng, J.; Li, Y. Optimization of Polyphenols Release from Highland Barley Bran by Solid-State Fermentation and Antioxidant Activity Characterization. Fermentation 2024, 10, 438. https://doi.org/10.3390/fermentation10080438
Zhang Q, Li M, Cheng Z, Meng J, Li Y. Optimization of Polyphenols Release from Highland Barley Bran by Solid-State Fermentation and Antioxidant Activity Characterization. Fermentation. 2024; 10(8):438. https://doi.org/10.3390/fermentation10080438
Chicago/Turabian StyleZhang, Qianfang, Min Li, Zhe Cheng, Jingyan Meng, and Yunlong Li. 2024. "Optimization of Polyphenols Release from Highland Barley Bran by Solid-State Fermentation and Antioxidant Activity Characterization" Fermentation 10, no. 8: 438. https://doi.org/10.3390/fermentation10080438
APA StyleZhang, Q., Li, M., Cheng, Z., Meng, J., & Li, Y. (2024). Optimization of Polyphenols Release from Highland Barley Bran by Solid-State Fermentation and Antioxidant Activity Characterization. Fermentation, 10(8), 438. https://doi.org/10.3390/fermentation10080438






