Abstract
Red clover (Trifolium pratense) is a fodder plant grown in many regions of the world. It is also known as a medicinal plant. Red clover contains large amounts of isoflavones, which are, due to their similarity to estrogen, called phytoestrogen; it is believed that they can increase the concentration of estrogen in women. Some studies have refuted this information, but this topic is still being researched. The aim of the work was to produce beer to which red clover is added during the boiling phase and to monitor the transfer of isoflavones from red clover to beer. Red clover was not added to the control sample during boiling. During production and fermentation, the basic physical-chemical properties of wort and beer were monitored, as well as acceptability among potential consumers, which was determined by sensory analysis. The results show that phytoestrogens do end up in beer. The analyzed beer contained biochanin A, formononetin, genistein, and daidzein, in a total concentration of 12.42 µg/mL. The control sample contained none of the aforementioned compounds. Sensory analysis gave promising results, and the tested consumers all approved the taste, smell, and aroma of the produced beer. The most notable aroma that was singled out by consumers was “hay-like”.
1. Introduction
Red clover (Trifolium pratense) is commonly used as fodder plant and can be grown in many parts of the world. It is rich in proteins, vitamins, and minerals; thus, it is used as animal feed. It can be dried and conserved, but it can be used fresh in the animal diet, as well [1,2]. Apart from being highly important as animal feed, it has a positive influence on ecological systems [3]. Red clover is rich in secondary metabolites and has been known to be utilized in traditional and modern medicine and the pharmaceutical industry [4,5]. Thus, it is regarded in the literature as a medicinal herb [6,7]. Due to this property, red clover has become a hot topic in the last few decades. It contains significant amounts of four estrogenic isoflavones: formononetin, biochanin A, daidzein, and genistein [7]. Red clover preparations can be sold on the market as dietary supplements and are meant for long-term use as a phytoestrogen source due to their “natural” form of hormone replacement therapy [6]. Red clover (or some other species such as Trifolium amabile and Trifolium ciliatum) is not commonly used as a source of food for humans, but there are certain populations where different parts of clover (flower, leaves, stems) are used in small amounts as food flavoring [8,9,10,11]. The consumption of red clover sprouts is gaining popularity as an addition to salads or as a garnish. Leaves and flowers are also edible and have a tender and sweet taste when consumed raw [12].
There are many research papers regarding the use of red clover for alleviation of menopausal alterations in women’s bodies, and some refer to red clover extract [13,14,15,16,17,18]. There are papers that combine and compare red clover with hops (Humulus lupulus), a plant characteristic for use in brewing. Namely, hops also contains isoflavones, which can be of use for menopause-related problems such as hot flushes and night sweats. Hops contains 8-prenylnaringenin (8-PN), an isomerized form of the precursor chalcone [19,20]; 8-PN appears to be the most potent estrogen in hops, according to Overk et al. [21]. Various research studies refer to red clover as estrogenic and analyzed the effect of red clover extract on menopausal women [14,22]. However, Ghazanfarpour et al. [16] suggest that deliberate consumption of isoflavone-rich foods in the placebo group during study could affect the results. According to Voon et al. [23], extraction of isoflavones with decoction can result in the highest content of total glycosides. Decoction is continuous boiling of plant material in water for a period of time. Malca-Garcia et al. [24] tested different methods of extraction of isoflavones and their stability over time. They concluded that decoction gives the best results for biochanin A and formononetin (0.27% and 0.51% w/w). Tinctures (alcoholic extract) show the highest stability over time, meaning such products could be used over a longer period [24]. Brewing combines both of these processes: decoction during boiling (usually of hop compounds, producing aroma or bitterness) and fermentation where alcohol is produced, which can be interpreted as a basic for tincture. Thus, the combination of red clover and brewing could result in a useful product.
Beer is made from mash/wort obtained by extracting sugars from crushed malt during mashing with water (infusion) and then boiled with hops (decoction) to extract the bitter acids and flavors from hops; it is considered to be an extract. This acted as a background for this research—the possibility to extract isoflavones from red clover to wort during boiling, which would end up in beer. Also, since research shows controversial results regarding the effects of red clover on inflammatory markers and coagulation factors, it has been noted that the key variable is the bioavailability in the body [22,25,26]. Novel research indicates that fermentation processes can affect the therapeutic potential of red clover isoflavones by converting isoflavone glycosides to aglycones [22]. This can increase the efficiency of the therapeutic intention of such products [17]. This was the background for this research: the fact that red clover contains isoflavones and could be used as an extract, as isoflavones withstand boiling temperatures (red clover is often used as tea) [24].
The premise of this research was that adding red clover (whole plant in full bloom) into the boil, together with hops, and then subjecting it to fermentation can result in beer with valuable compounds such as isoflavones. This kind of beer could be utilized as a menopausal supplement. This would be a great way to replace the synthetic hormone therapy for women in menopause, since such therapy can induce various unfavorable symptoms or conditions (various types of cancer, breast tenderness, uterine bleeding, etc.) [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. In other words, we aimed to assess the transfer of analyzed isoflavones from red clover plant material (whole plant in full bloom) to beer and to check if fermentation obstructs the concentrations of these compounds in the final beer. Another important result is the beer acceptability to the consumers, sensory-wise. Physical-chemical properties were also determined in the final beer.
2. Materials and Methods
2.1. Beer Production
For this purpose, pale ale beer was brewed. A total of 20 L of wort was prepared containing pale ale malt (5 kg). The pale ale was Simpsons Malt (Northumberland, UK). Malt was purchased from the local brewing and malting equipment store. Reverse osmosis water was adjusted for pale ale production by adding 6.1 g calcium chloride, 4.5 g Epsom salt, 1.8 g gypsum, 3 g chalk, 3 g baking soda, and 2 mL of 80% (v/v) lactic acid. Mashing was done at 65 °C for 60 min, and mash out was done at 75 °C for 10 min. Boiling was done at 100 °C for 60 min. A total of 200 g of shredded red clover was added during boiling time (60 min), alongside hops, with 60 g of Magnum. The red clover sample was collected in the period of full bloom. Cooling was conducted using a coil chilled with tap water such that the temperature was reduced to 25 °C. Then, 20 L of wort was separated into two fermentation vessels, 10 L each, marked as “RC”. Inoculation was done with Safale US-05 yeast (Fermentis, Marcq en Baroeul, France). One bag (11.5 g) of dried yeast was used for 20 L of wort. Upon inoculation, vessels were stored at 21 °C (in a cooling chamber). At the end of fermentation, beer was transferred to kegs, carbonated, and stored in a cool place (4 °C).
The control beer was produced in the same way, with the same ingredients, but without the addition of red clover during boiling.
2.2. Physical-Chemical Analysis
Before the inoculation of wort, the samples were analyzed using an Anton Paar Beer Analyser (Anton Paar GmbH, Graz, Austria). Fermentation was followed via portable EasyDens Anton Paar (Anton Paar GmbH, Graz, Austria). Polyphenol, color, and bitterness analyses were conducted according to [44], and final beers were analyzed using an Anton Paar Beer Analyser (Anton Paar GmbH, Graz, Austria). All analyses were done in duplicate for both fermentation vessels.
2.3. Isoflavone Profile
2.3.1. Chemicals
The chemical materials used in this study were of analytical or HPLC grade. Purified water from a Milli-Q Element A10 System (Millipore, Milford, MA, USA) was used for the sample, reagent solutions, and mobile phase preparation. According to previously reported data for Trifolium pretense L., the following components of isoflavones, purchased from Sigma-Aldrich (Saint Louis, MO, USA) were analyzed: daidzein, genistein, formononetin, biochanin A. Standard stock solutions (1.0 mg/mL) of isoflavones were prepared by dissolving them in methanol.
2.3.2. Determination and Quantification of Isoflavones by HPLC
Individual isoflavones in beer extract were analyzed using a Series 200 HPLC system (Perkin Elmer, Waltham, MA, USA) coupled with a Kinetex Core-Shell RP-C18 column (150 × 4.6 mm, 100 Å, 5 µm) and a diode array detector (DAD). Prior to HPLC analysis, a sample of beer extract was degassed in an ultrasonic bath (Bandelin, Sonorex, Germany) for 15 min, without heating, followed by centrifugation (Universal 320R, Hettich, Tuttlingen, Germany) for 10 min at 10 000 rpm, and filtered through a 0.22 µm nylon filter (Ahlstrom GmbH, Helsinki, Finland) before HPLC analysis. The mobile phase for analysis included solvent A (Millipore water acidified with 1% trifluoroacetic acid (v/v)) and solvent B (acetonitrile acidified with 1% trifluoroacetic acid (v/v)). Elution was performed using linear gradients from 35–50% B for 15 min, isocratic 25% of B for 3 min, and column equilibration for 2 min. At a column temperature of 30 °C and a flow rate of 1.0 mL/min, peaks were detected at 260 nm. Isoflavones were identified by comparison of UV absorption spectra and retention times with those of standards, while their quantification was completed using a six-point external calibration curve.
2.4. Sensory Evaluation
2.4.1. Evaluation of Beer Characteristics
Sensory evaluation was conducted by 20 untrained consumers (females). The panel consisted of different age-group beer consumers (aged 23–57 years old). The materials used in ranking tests for intensity and sensory descriptors were adjusted from the general evaluation sheet for beer [45]. The intensity scales producing the best discrimination between samples and the most reproducible results were chosen. The scoring of each sensory attribute was conducted using a five-point intensity scale, where 1 point means ‘fault’ and 5 points means ‘excellent’. Descriptors were used to anchor/explain the points of the scale (for 7 sensory attributes, descriptors included grainy, honey-like, caramel-like, smoky, grassy, hay-like, yeasty, fruity, flowery, spicy, hoppy). Foam, color, bitterness, carbonation, mouthfeel, astringency and smell were also evaluated. The highest possible score was 100. Tasting tests were performed in an appropriate room, and beer samples were kept at room temperature for 10 min before the test. Samples were poured into a clean glass and covered with a watch glass to prevent volatile compounds from escaping the glass. All beer samples were numbered, and every sample was tested in triplicate. Evaluators were offered flat mineral water between the samples, together with plane white bread. Both beers, including the control sample, were served as draught beer. No personal data were collected from the panelists. The evaluation was done again after 1 month of storage.
2.4.2. Drinkability Test
After the sensory evaluation, the drinkability test was performed. The drinkability test included the produced pale ale beers, with one being a control sample. The same panelists who participated in sensory evaluation were called again (20 consumers, aged 23–57). Panelists were initially offered both beers (100 mL in a glass marked with numbers) in a relaxed atmosphere (a bar-like environment with a person waiting on the participants). After the initial tasting of all beers, panelists were left to choose on their own between the samples. Testing lasted for 2 h and was conducted in the afternoon (6–8 PM). The consumers were engaged in a conversation and offered snacks (beer pretzels and peanuts), and they only had to note in the report the number of beer glasses consumed for each beer. Each panelist was always given 300 mL of beer in the glass. Leftover volumes were subtracted from the reports for each panelist. The initial 100 mL were not included in the result. The final result was obtained by summing up the number of fully consumed glasses for each beer. All beers, including the control sample, were served as draught beer.
2.5. Statistical Analysis
Analysis of variance (ANOVA) and Fisher’s least significant difference test (LSD) were conducted, with the least statistical significance set to p < 0.05. Statistica 13.1. (TIBCO Software Inc., Palo Alto, CA, USA) was the software of choice for this data set. PCA (principal component analysis) was also done using Statistica 13.1. (TIBCO Software Inc., Palo Alto, CA, USA).
3. Results and Discussion
Beer is a loved beverage for both genders. Even though beer is mostly consumed by men, women find a share in consumption as well. According to Horowiec [46], 37% of women in the USA consume craft beer. Similar numbers are found in Netherlands, where women make up 32% of beer consumers [47]. This is a significant market, where many moderations can be introduced in order to increase the beer consumption among women. Moderations can be achieved in many ways, adding value to beer, and a specific or functional ingredient that is of interest for women could be one of them. Since many researchers have investigated the influence of red clover isoflavones on women in perimenopause and menopause, or even post-menopause, and because women make up approx. 30% of beer market, it would be useful to produce beer that could supplement or replace a significant share of isoflavones in women’s diets. This paper aimed to evaluate the possibility of the production of such beer and whether it would be sensorially acceptable for women.
The produced beers were subjected to physical chemical analysis, and the results are presented in Table 1.

Table 1.
Basic physical-chemical indicators of produced beers.
The results indicate that significant differences among samples can be detected, especially for certain physical-chemical indicators, such as bitterness, polyphenol content, and alcohol content. Since two batches of beer were produced, there were slight differences between the samples regarding specific gravity as well as original, real, and apparent extracts. However, alcohol content showed a significant difference between the samples. The control sample showed higher alcohol content, at 5.19% in regards to beer with the addition of red clover, which contained 4.28% alcohol. This could be due to higher protein share, since red clover contains a high amount of crude and insoluble proteins [48].
Polyphenol content showed significant difference (p < 0.05) between samples, where the red clover beer contained 600 mg/L, while the control sample had 292 mg/L. The amount of polyphenols in the control sample is in accordance with previous research reporting the average value of polyphenols in light beers [49]. The amount of polyphenols in the sample with red clover, however, is twofold higher. This indicates that the addition of red clover significantly increases the level of polyphenols in beer, a valuable ingredient for menopausal women [50]. This is understandable, since red clover contains approx. 50 mg GAE/g of DM [51]. This is interesting information, since there is research that supports the fact that increased content of polyphenols can aid in alleviating the hormonal-related changes that burden women during pre-menopause, menopause, and post-menopause. It has been reported that a polyphenol-rich diet helps in certain symptoms of menopause [51,52,53] such as osteoporosis and in reducing vasomotor symptoms. However, some authors dispute this due to their effect on estrogen levels. Namely, as Poschner et al. [54] described in an excellent review, they increase estrogen levels, which can be problematic and induce or support growth of (breast) cancer cells. This determines beer as an excellent natural source of polyphenolic compounds, which can aid menopausal women, and with the addition of red clover, an acknowledged natural remedy for menopausal problems, moderate beer consumption could be helpful in relieving menopausal problems [55]. Most polyphenols in beer originate from malt (70–80%) and then hops (approx. 15%) [56,57]; however, it has been proven that boiling, fermentation, and hop amount can influence the polyphenolic content in beer [13,58,59,60].
The addition of red clover slightly, but statistically significantly, increased bitterness as well. The bitterness of beer with red clover was 25 IBU, while the control beer had 18 IBU. This is due to the increased content of polyphenols in beer with the addition of red clover [61]. However, according to [62], flavonoids can also affect bitterness. Beers were intentionally made less bitter, being lager-like in bitterness (the same amount of hops was added to each batch), so that the possible influence coming from red clover could be noted. Sensory analysis revealed that there are notable changes between the samples as well.
pH values (4–4.2) can cause astringency, which is often, by untrained consumers, mistaken for bitterness. Astringency is the feel of bitterness that remans for more than 60 s, while bitterness passes in 60 s. pH values for both beers showed significant differences, and the effect on bitterness and astringency can be evaluated via sensory analysis.
Beer color was also slightly, but statistically significantly, higher in samples with red clover addition. This is expected, since red clover contains additional chlorophyll and other components, such as additional proteins, which can affect color.
In order to check if the transfer of isoflavones occurred, the four most significant isoflavones were determined in beer. The results are presented in Table 2.

Table 2.
Four significant isoflavones determined in beer.
As can be seen in Table 2, the most significant isoflavones determined in the final beer were in significantly higher concentrations that in the control sample. Namely, the control sample contained no detectable concentrations of isoflavones. The most significant levels were determined for formononetin, amounting to 5.336 µg/mL. This is by far the most valuable isoflavone, as many researches refer to it as promising in alleviating menopausal changes [63,64,65].
The next most significant isoflavone was genistein, with 2.997 µg/mL, followed by biochanin A (2.470 µg/mL). Genistein is reportedly the compound with the highest estrogenic activity on both estrogen receptors [66]. The lowest levels were determined for daidzein, at 1.411 µg/mL. The total amount of isoflavones in produced beer amounted to 12.214 µg/mL. Chromatograms of analyzed beers can be seen in Figure 1 and Figure 2.
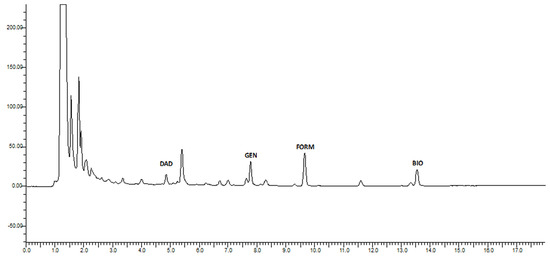
Figure 1.
Chromatogram of beer with red clover addition.
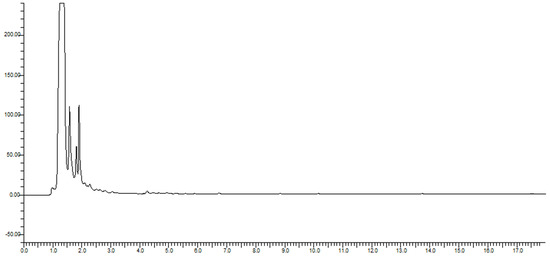
Figure 2.
Chromatogram of control beer.
Various research reports give different amounts of isoflavones for women in perimenopause, menopause, or post-menopause, but they generally vary between 40 and 120 mg daily [15]. The total isoflavones in red clover used for the production of beer was high, at 39,148.2 µg/L. This means that about 30% of isoflavones ended up in beer, which is a solid percentage of transferred isoflavones. Considering that the recommended daily amount of beer for women is 0.5 L [67], consumption of one recommended serving of beer per day would not satisfy the daily needs for isoflavones in women; however, with optimization of the production process, this could be achieved. However, we can consider that hops contain prenylflavonoids and that in commercial beers the concentration of 8-PN reaches 1–240 µg/mL, while other prenylflavonoids can be significantly higher [68]. This implies that perhaps consumption of one serving of beer per day could completely satisfy the need for estrogen-like compounds. However, further research is necessary to obtain more information about this matter. The red clover used in this research was picked in full bloom, in the phase when it contains the most isoflavones.
One of the concerns when producing this beer was the sensory evaluation and consumer acceptance. Even though the sample for evaluation was rather small, at 20 women, it gave us insight into the overall acceptance in comparison to the control sample. The results are presented in Table 3.

Table 3.
Results of sensory evaluation.
It is notable that the red clover sample received a significantly lower score (80/100) than the control sample (90/100). Evaluators mostly noted the hay-like, grassy, and grainy aroma in the sample with red clover. However, after 1 month of storage, this beer received a better score (90/100), and the hay-like, grassy, and grainy components were no longer as pronounced. The consumers all agreed that aging made this beer more enjoyable. A similar situation occurred with the control sample, where it received a higher score after 1 month of storage.
Foam was rated very high for both samples and in both evaluations. It was even more stable in the sample with the addition of red clover.
Even though the color was a bit more notable in the sample with red clover, the consumers did not notice this.
Higher bitterness was noted in sample with red clover, but it was described as pleasant and was not acknowledged as astringency. Since the pH value was higher in beer with red clover, this probably affected the sensation of bitterness, but it also reduced the feeling of astringency, even though the polyphenolic content was higher in beer with red clover.
Perhaps the most notable was the drinkability test, where the evaluators got to choose the beer they liked better. The drinkability test quantifies the consumer’s wish to have another glass of tested beer. From the physiological point of view, drinkability can be described as the beer’s characteristic that urges the consumer to continue taking in liquid and initiates thirst despite the satisfactory level of water in the body [69]. This test has many versions, and it is continuously being upgraded and developed by scholars [69,70,71,72,73]. The drinkability test is a significant indicator of beer’s acceptance among consumers [68]. The importance of drinkability can be seen since before 2006, when a scientific congress was dedicated to drinkability. A trained sensory panel can be influenced by the professional familiarity with the brewing process, while the intention of the whole beer industry is to sell beer to consumers, people who know little about the production process but subconsciously choose a certain style or brand of beer. This is why this test is conducted in a relaxed, bar-like atmosphere. This kind of scenery can act in such a way as to activate the subconscious of the consumer, who chooses the more appealing beer without even realizing they are in a state of testing. A more appealing beer or a beer with a higher drinkability will be consumed faster and in higher volumes [69]. This kind of test is useful and common when introducing novel raw materials in brewing (hops, barley variety intended for brewing, yeast) because it gives realistic feedback for the brewing process and marketing direction. The results of the drinkability test applied for the two produced beers are shown in Table 4.

Table 4.
Results of drinkability test.
The results indicate that control sample was consumed in a bigger volume, meaning that it probably was more enjoyable to consumers. However, the results are very close and indicate a significant liking of the red clover beer. Further work could be pointed toward the improvement of sensory characteristics. Perhaps combinations with different malts (caramel or darker, black malt) could provide an even more appealing beverage. Hops could also affect the sensory properties of the final product. Namely, aromatic hop varieties would probably positively influence the acceptance of such beer. Dry hopping, such as in IPAs (Indian Pale Ales), could also affect the sensory characteristics. Another possible variation is the change of yeast used for fermentation, as it could result in more appealing sensory characteristics in terms of aroma and taste.
Also, the addition of different red clover varieties and different amounts of clover should be examined in order to increase the level of isoflavones in beer. The optimal time of the addition of red clover during mashing or boiling should be tested as well. However, the addition of red clover extract would also be a direction to follow, as it would allow a pure combination of isoflavones to enter the beer. It could also be added independently of the boiling process, at the beginning of fermentation or even at the end of it, depending which would give a better yield of isoflavones in the final beer.
However, as any other estrogen-replacement therapy, the addition of red clover can have negative implications, especially in long-term usage, due to estrogen levels, which can have an adverse impact on women who are susceptible to cancer, as higher levels of estrogen can increase the possibility of certain types of cancer or some other conditions [6,74]. The current propaganda where plant food is gaining popularity can bring serious consequences, since many plant commodities naturally contain phytoestrogens. They can be found in beans, cabbage, flax seed, rye, berries, grains, and soya products (including soya milk, tofu, and miso). Thus, products containing phytoestrogens should be should be regulated on the European Union market as they are in the USA [6,75].
4. Conclusions
The addition of red clover in beer results in a potential functional product. The results of this research showed that this beer can hold satisfactory quality regarding physical-chemical properties. Sensory evaluation showed good results, with the consumers inclined toward very positive grades. The most interesting data involved the isoflavone content in finished beers. Namely, isoflavones were found in beer produced with red clover addition, while the control beer showed none of the isoflavones. About 30% of the isoflavones transferred to the beer, which is a satisfactory yield. However, this could probably be higher if some modifications were to be applied. In conclusion, this aspect of utilization of red clover in brewing to obtain an isoflavone-rich beer has great potential and needs to be further investigated. The application of different amounts of red clover, the red clover variety, and the addition of red clover during mashing instead of the boiling phase could result in higher extraction of isoflavones into beer. This could be a potential functional product for pre-menopausal, menopausal, and post-menopausal women. Nevertheless, this kind of replacement product should not be used by women who are susceptible to cancer, as higher levels of estrogen can increase the possibility of certain types of cancer.
Author Contributions
Conceptualization, K.H. and M.K.B.; methodology, M.K.B.; software, D.H. and M.A.; validation, K.M. and N.K.; formal analysis, M.K.B.; investigation, L.P.; writing—original draft preparation, K.H.; writing—review and editing, V.K.; visualization, K.H.; supervision, V.K. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petrović, M.P.; Stanković, M.S.; Anđelković, B.S.; Babić, S.Ž.; Zornić, V.G.; Vasiljević, S.L.; DajićStevanović, Z.P. Quality parameters and antioxidant activity of three clover species in relation to the livestock diet. Not. Bot. Horti Agrobot. 2016, 44, 201–208. [Google Scholar] [CrossRef]
- Phelan, P.; Moloney, A.P.; McGeough, E.J.; Humphreys, J.; Bertilsson, J.; O’Riordan, E.G.; O’Kiely, P. Forage legumes for grazing and conserving in ruminant production systems. Crit. Rev. Plant Sci. 2015, 34, 281–326. [Google Scholar] [CrossRef]
- Tucak, M.; Popović, S.; Horvat, D.; Čupić, T.; Krizmanić, G.; Viljevac Vuletić, M.; Ravlić, M. The characterization of isoflavone content in the croatian red clover collection. Poljoprivreda 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Kumar, M.A.; Sravanthi Pammi, S.S.; Sukanya, M.S.; Giri, A. Enhanced production of pharmaceutically important isoflavones from hairy root rhizoclones of Trifolium pratense L. In Vitro. Cell Dev. Biol. Plant 2018, 54, 94–103. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreno, M.M.; Pérez-Martin, L.; Rodriguez-Gonzalo, E.; DominguezAlvarez, J. Analysis of isoflavones in foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Fugh-Berman, A.; Kronenberg, F. Red clover (Trifolium pratense) for menopausal women: Current state of knowledge. Menopause 2001, 8, 333–337. [Google Scholar] [CrossRef]
- Foster, S.; Tyler, V.E. Tyler’s Honest Herbal, 4th ed.; Haworth Press: New York, NY, USA, 1999; pp. 315–317. [Google Scholar]
- Duke, J.A. CRC Handbook of Edible Weeds, 1st ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Facciola, S. Cornucopia—A Source Book of Edible Plants, 1st ed.; Kampong Publications: Vista, CA, USA, 1990. [Google Scholar]
- Tanaka, T. Tanaka’s Cyclopedia of Edible Plants of the World; Keigaku Publishing Company: Tokyo, Japan, 1976. [Google Scholar]
- Uphof, J.C.T. Dictionary of Economic Plants, 2nd ed.; Stechert-Hatner Service Agency: New York, NY, USA, 1968. [Google Scholar]
- Mikulić, M.; Atanacković Krstonošić, M.; Kladar, N.; Vasiljević, S.; Katanski, S.; Mamlić, Z.; Rakić, D.; Cvejić, J. Phytochemical Composition of Different Red Clover Genotypes Based on Plant Part and Genetic Traits. Foods 2024, 13, 103. [Google Scholar] [CrossRef]
- Gartoulla, P.; Han, M.M. Red clover extract for alleviating hot flushes in postmenopausal women: A meta-analysis. Maturitas 2014, 79, 58–64. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thorup, A.C.; Hansen, E.S.S.; Jeppesen, P.B. Combined Red Clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS ONE 2017, 12, e0176590. [Google Scholar] [CrossRef]
- Kanadys, W.; Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Kanecki, K.; Malm, M. Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1258. [Google Scholar] [CrossRef]
- Ghazanfarpour, M.; Sadeghi, R.; Latifnejad Roudsari, R.; Mirzaii Najmabadi, K.; Mousavi Bazaz, M.; Abdolahian, S.; Khadivzadeh, T. Effects of red clover on hot flash and circulating hormone concentrations in menopausal women: A systematic review and meta-analysis. Avicenna J. Phytomed. 2015, 5, 498–511. [Google Scholar]
- Wickham, K.A.; Nørregaard, L.B.; Oxfeldt, M.; Cheung, S.S.; Gliemann, L.; Hansen, M.; Hellsten, Y. Short-Term Supplementation with Fermented Red Clover Extract Reduces Vascular Inflammation in Early Post-menopausal Women. Front. Cardiovasc. Med. 2022, 9, 826959. [Google Scholar] [CrossRef]
- Lipovac, M.; Chedraui, P.; Gruenhut, C.; Gocan, A.; Kurz, C.; Neuber, B.; Imhof, M. Effect of Red Clover Isoflavones over Skin, Appendages, and Mucosal Status in Postmenopausal Women. Obstet. Gynecol. Int. 2011, 2011, 949302. [Google Scholar] [CrossRef] [PubMed]
- Hänsel, R.V.; Schulz, J. Desmethylxanthohumol: Isolierung aus Hopfen und Cyclisierung zu Flavanonen. Arch Pharm. Weinheim 1988, 321, 37–40. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Nikolic, D.; Burdette, J.E.; Overk, C.R.; Bolton, J.L.; van Breemen, R.B.; Froehlich, R.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Estrogens and Congeners from Spent Hops (Humulus lupulus L.). J. Nat. Prod. 2004, 67, 2024–2032. [Google Scholar] [CrossRef]
- Overk, C.R.; Yao, P.; Chadwick, L.R.; Nikolic, D.; Sun, Y.; Cuendet, M.A.; Deng, Y.; Hedayat, A.S.; Pauli, G.F.; Farnsworth, N.R.; et al. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J. Agric. Food Chem. 2005, 53, 6246–6253. [Google Scholar] [CrossRef][Green Version]
- Thorup, A.C.; Lambert, M.N.; Kahr, H.S.; Bjerre, M.; Jeppesen, P.B. Intake of novel red clover supplementation improves bone status and estrogen metabolism in for 12 Weeks improves bone status in healthy menopausal women. Evid. Based Complement. Altern. Med. 2015, 6, 689138. [Google Scholar]
- Voon, H.C.; Bhat, R.; Rusul, G. Flower Extracts and Their Essential Oils as Potential Antimicrobial Agents for Food Uses and Pharmaceutical Applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 34–55. [Google Scholar] [CrossRef]
- Malca-Garcia, G.R.; Zagal, D.; Graham, J.; Nikolić, D.; Friesen, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Dynamics of the isoflavone metabolome of traditional preparations of Trifolium pratense L. J. Ethnopharmacol. 2019, 238, 111865. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, H.J.; Yu, S.H.; Lee, B.S.; Jeon, S.Y.; Lee, J.J.; Lee, Y.C. Combination of red clover and hops extract improved menopause symptoms in an ovariectomized rat model. Evid. Based Complement. Altern. Med. 2020, 2020, 7941391. [Google Scholar] [CrossRef]
- Simoncini, T.; Garibaldi, S.; Fu, X.D.; Pisaneschi, S.; Begliuomini, S.; Baldacci, C.; Lenzi, E.; Goglia, L.; Giretti, M.S.; Genazzani, A.R. Effects of phytoestrogens derived from red clover on atherogenic adhesion molecules in human endothelial cells. Menopause 2008, 15, 542–550. [Google Scholar] [CrossRef]
- Mainini, G.; Torella, M.; Di Donna, M.C.; Esposito, E.; Ercolano, S.; Correa, R.; Cucinella, G.; Stradella, L.; Luisi, A.; Basso, A.; et al. Nonhormonal management of postmenopausal women: Effects of a red clover based isoflavones supplementation on climacteric syndrome and cardiovascular risk serum profile. Clin. Exp. Obstet. Gynecol. 2013, 40, 337–341. [Google Scholar]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Naftolin, F.; Taylor, H.S.; Karas, R.; Brinton, E.; Newman, I.; Clarkson, T.B.; Mendelsohn, M.; Lobo, R.A.; Judelson, D.R.; Nachtigall, L.E.; et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil. Steril. 2004, 81, 1498–1501. [Google Scholar] [CrossRef]
- Miller, V.M.; Black, D.M.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Hodis, H.N.; Lobo, R.A.; Manson, J.E.; Merriam, G.R.; Naftolin, F.; et al. Using basic science to design a clinical trial: Baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J. Cardiovasc. Transl. Res. 2009, 2, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; Henderson, V.W.; Shoupe, D.; Budoff, M.J.; Hwang-Levine, J.; Li, Y.; Feng, M.; Dustin, L.; Kono, N.P.H.; et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 2016, 374, 1221–1231. [Google Scholar] [CrossRef]
- Palacios, S. Current perspectives on the benefits of HRT in menopausal women. Maturitas 1999, 33, S1–S13. [Google Scholar] [CrossRef]
- Rymer, J.; Wilson, R.; Ballard, K. Making decisions about hormone replacement therapy. Brit. Med. J. 2003, 326, 322–326. [Google Scholar] [CrossRef] [PubMed]
- de Lignieres, B.; de Vathaire, F.; Fournier, S.; Urbinelli, R.; Allaert, F.; Le, M.G.; Kuttenn, F. Combined hormone replacement therapy and risk of breast cancer in a French cohort study of 3175 women. Climacteric 2002, 5, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Eden, J. Progestins and breast cancer. Am. J. Obstet. Gynecol. 2003, 188, 1123–1131. [Google Scholar] [CrossRef][Green Version]
- Fernandez, E.; Gallus, S.; Bosetti, C.; Franceschi, S.; Negri, E.; La Vecchia, C. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case–control studies. Int. J. Cancer 2003, 105, 408–412. [Google Scholar] [CrossRef]
- Gambacciani, M.; Monteleone, P.; Sacco, A.; Genazzani, A.R. Hormone replacement therapy and endometrial, ovarian and colorectal cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 139–147. [Google Scholar] [CrossRef]
- Humphries, K.H.; Gill, S. Risks and benefits of hormone replacement therapy: The evidence speaks. CMAJ 2003, 168, 1001–1010. [Google Scholar]
- Kenemans, P.; Bosman, A. Breast cancer and post-menopausal hormone therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Marsden, J. The menopause, hormone replacement therapy and breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 83, 123–132. [Google Scholar] [CrossRef]
- Olsson, H.L.; Ingvar, C.; Bladstrom, A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer 2003, 97, 1387–1392. [Google Scholar] [CrossRef]
- Shah, S.H.; Alexander, K.P. Hormone replacement therapy for primary and secondary prevention of heart disease. Curr. Treat. Options Cardiovasc. Med. 2003, 5, 25–33. [Google Scholar] [CrossRef]
- Teede, H.J. The menopause and HRT. Hormone replacement therapy, cardiovascular and cerebrovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Middle European Brewing Analysis Commission (MEBAK); Band II.n Brautechnische Middle European Brewing Analysis Commission (MEBAK). Band II.n Brautechnische Analysenmethoden, 3rd ed.; Selbstverlag der MEBAK: Freising-Weihenstephan, Germany, 1997. [Google Scholar]
- McGreger, C.; McGreger, N. The Beer Brewing Guide. EBC Quality Handbook for Small Breweries, 1st ed.; Lanoo: Tielt, Belgium, 2021; p. 308. [Google Scholar]
- Horowiec, J. Beer Preferences of Women: Looking at Gender Stereotypes through the Consumption of Craft Beer. Master’s Thesis, Johnson & Wales University, Providence, RI, USA, 2 May 2022. [Google Scholar]
- Statista. Available online: https://www.statista.com/statistics/585091/share-of-individuals-regularly-drinking-beer-in-the-netherlands-by-gender/ (accessed on 15 June 2024).
- Horvat, D.; Tucak, M.; Viljevac Vuletić, M.; Čupić, T.; Krizmanić, G.; Kovačević Babić, M. Phenolic content and antioxidant activity of the Croatian red clover germplasm collection. Poljoprivreda 2020, 26, 3–10. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.-J.; Coxam, V. Twelve-month consumption of apolyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging. 2015, 19, 77e86. [Google Scholar] [CrossRef] [PubMed]
- Annekathrin, K.; Oliver, Z.; Georg, K. Hop extracts and hop substances in treatment of menopausal complaints. Planta Medica 2013, 79, 576–579. [Google Scholar]
- Fatemeh, A.; Hamid, M.; Nasibeh, R. Hops for menopausal vasomotor symptoms: Mechanisms of action. J. Menopausal. Med. 2016, 22, 62–64. [Google Scholar]
- Pedrera-Zamorano, J.; Lavado-Garcia, J.; Roncero-Martin, R.; Calderon-Garcia, J.; Rodriguez-Dominguez, T.; CanalMacias, M. Effect of beer drinking on ultrasound bone mass in women. Nutrition 2009, 25, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Poschner, S.; Maier-Salamon, A.; Thalhammer, T.; Jäger, W. Resveratrol and Other Dietary Polyphenols Are Inhibitors of Estrogen Metabolism in Human Breast Cancer Cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 11–18. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Lamuela-Raventós, R.M.; Estruch, R.; Sasot, G.; Doménech, M.; Tresserra-Rimbau, A. Beer Polyphenols and Menopause: Effects and Mechanisms-A Review of Current Knowledge. Oxidative Med. Cell. Longev. 2017, 2017, 4749131. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2014, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W.; Barofsky, E.; Kennedy, J.A.; Deinzer, M.L. Hop (Humulus lupulus L.) proanthocyanidins characterized by mass spectrometry, acid catalysis, and gel permeation chromatography. J. Agric. Food Chem. 2013, 51, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Intelmann, D.; Haseleu, G.; Hofmann, T. LC-MS/MS quantitation of hop-derived bitter compounds in beer using the ECHO technique. J. Agric. Food Chem. 2009, 57, 1172–1182. [Google Scholar] [CrossRef]
- Lipovac, M.; Chedraui, P.; Gruenhut, C.; Gocan, A.; Kurz, C.; Neuber, B.; Imhof, M. The effect of red clover isoflavone supplementation over vasomotor and menopausal symptoms in postmenopausal women. Gynecol. Endocrinol. 2012, 28, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Ismail, R.; Taylor-Swanson, L.; Cray, L.; Schnall, J.G.; Mitchell, E.S.; Woods, N.F. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: A systematic review. Maturitas 2014, 78, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, Y.; Ollennu-Chuasam, P.; Kortesniemi, M.; Selander, K. Compositional characteristics of red clover (Trifolium pratense) seeds and supercritical CO2 extracted seed oil as potential sources of bioactive compounds. Food Innov. Adv. 2024, 3, 11–19. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Lemeziene, N.; Padarauskas, A.; Butkute, B.; Ceseviciene, J.; Taujenis, L.; Norkeviciene, E.; Mikaliūnienė, J. The concentration of isoflavones in red clover (Trifolium pratense L.) at flowering stage. Zemdirb. Agric. 2015, 102, 443–448. [Google Scholar] [CrossRef]
- Liu, J.; Burdette, E.; Xu, H.; Gu, C.; van Breemen, R.B.; Bhat, K.P.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H.; et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Burdette, J.E.; Liu, J.; Lantvit, D.; Lim, E.; Booth, N.; Bhat, K.P.L.; Hedayat, S.; Van Breemen, R.B.; Constantinou, A.I.; Pezzuto, J.M.; et al. Trifolium pratense (Red Clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J. Nutr. 2002, 132, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Beck, V.; Rohr, U.; Jungbauer, A. Phytoestrogens Derived from Red Clover: An Alternative to Estrogen Replacement Therapy? J. Steroid Biochem. Mol. Biol. 2005, 94, 499–518. [Google Scholar] [CrossRef]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant activity and the most abundant phenolics in commercial dark beers. Int. J. Food Prop. 2017, 20 (Suppl. 1), S595–S609. [Google Scholar] [CrossRef]
- Tronina, T.; Popłoński, J.; Bartmańska, A. Flavonoids as Phytoestrogenic Components of Hops and Beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef]
- Cejka, P.; Dvořák, J.; Kellner, V.; Čulík, J.; Olšovská, J. Drinkability of beers and the methods applied for its assessment. Kvas. Prum. 2011, 57, 406–412. [Google Scholar] [CrossRef]
- Beer Quality Evaluation—A Sensory Aspect. Available online: https://www.researchgate.net/publication/358974823_Beer_Quality_Evaluation-A_Sensory_Aspect (accessed on 17 June 2024).
- Cuřín, J. Posuzování pitnosti piva. Kvas. Prum. 1978, 24, 169–172. [Google Scholar] [CrossRef]
- French, S.J.; Read, N.W.; Booth, D.A. Satisfaction of hunger and thirst by foods and drinks. Brit. Food. J. 1993, 95, 19–26. [Google Scholar] [CrossRef]
- Guinard, J.-X.; Souchard, A.; Picot, M.; Rogeax, M.; Sieffermann, J.-M. Sensory determinant of the thirst-quenching character of beer. Apetite 1998, 31, 101–115. [Google Scholar] [CrossRef]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in postmenopause:the state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef]
- EU Invests in Plant Food. Available online: https://cordis.europa.eu/article/id/22440-eu-invests-in-plant-food (accessed on 15 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).