Initial Medium Optimization of Nigrospora oryzae JL-4 and Its Biocontrol Potential on Solanum rostratum
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.1.1. Tested Strains
2.1.2. Tested Plants
2.1.3. Instruments and Reagents
2.2. Initial Medium Optimization for N. oryzae
2.2.1. Preparation of Fermentation Filtrate by N. oryzae
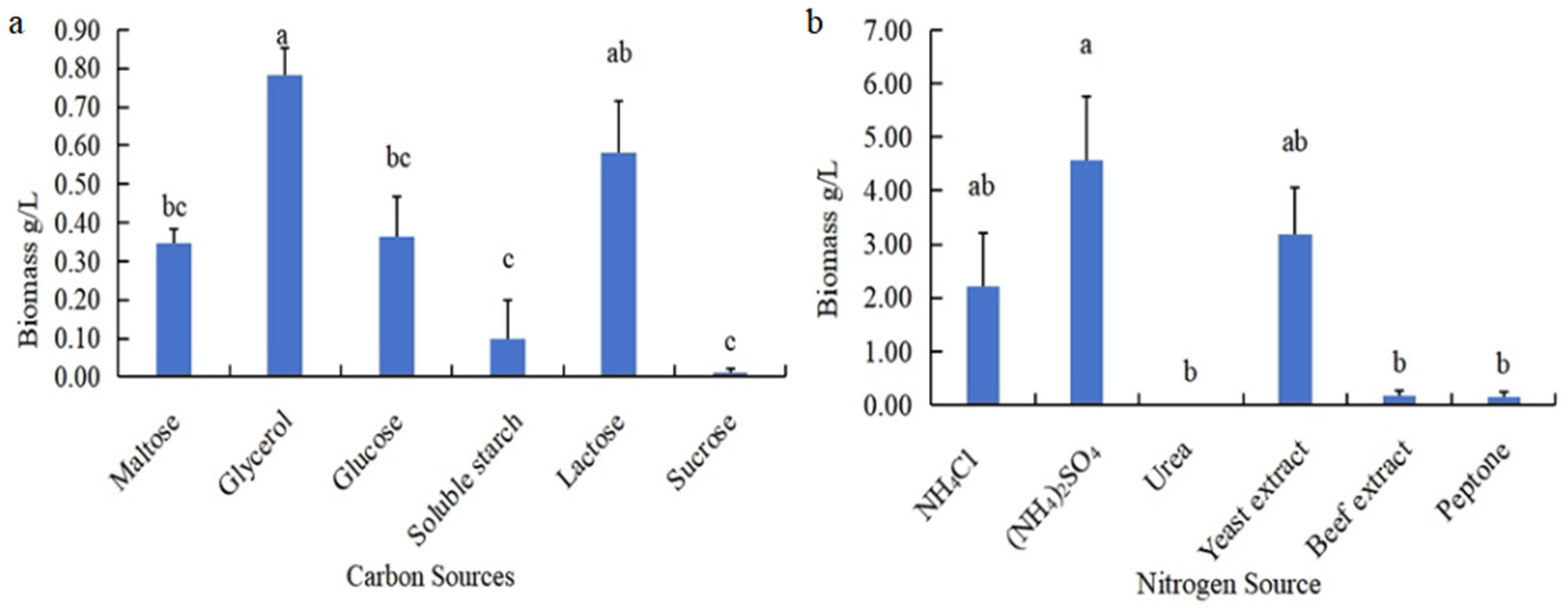
2.2.2. Screening Significant Carbon Source (C) Components for N. oryzae
2.2.3. Screening Significant Nitrogen Source (N) Components for N. oryzae
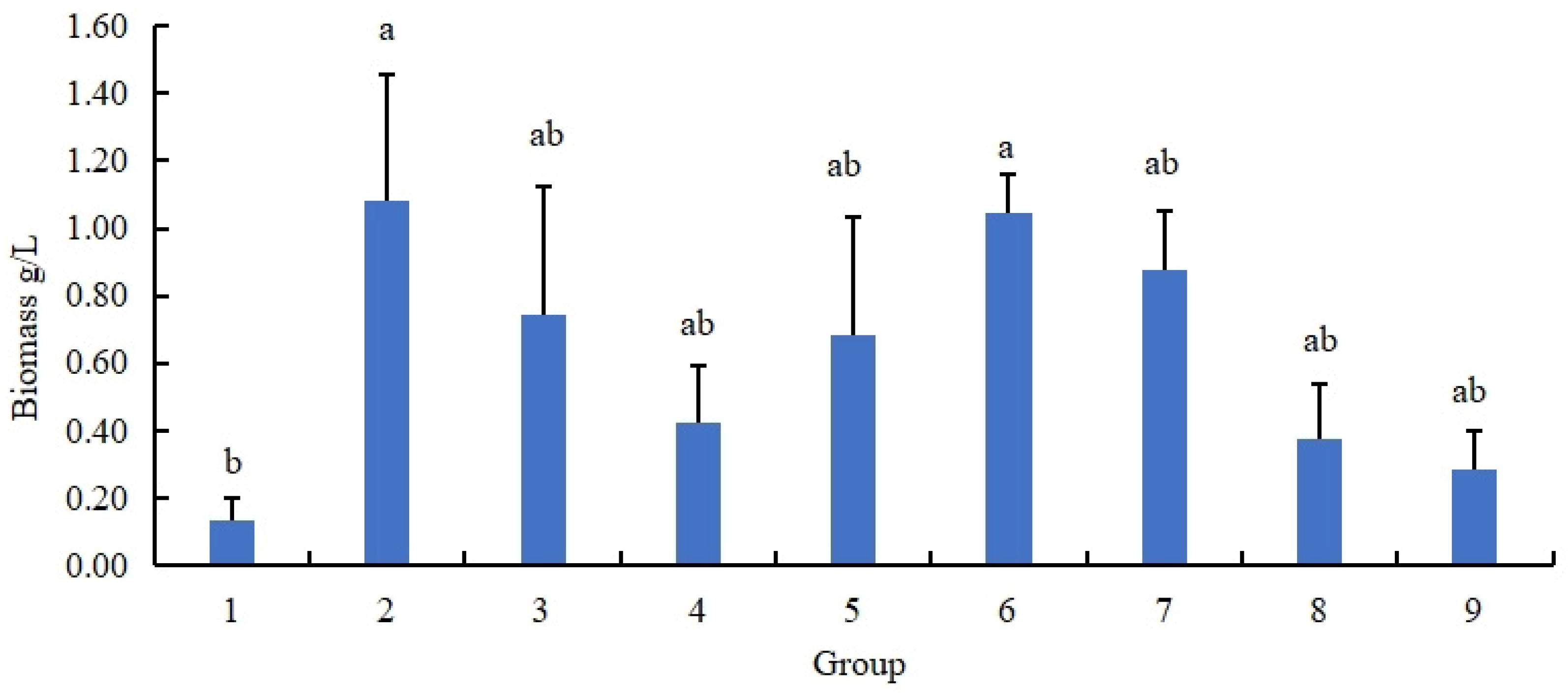
2.2.4. Screening Significant Carbon: Nitrogen Ratio Components for N. oryzae
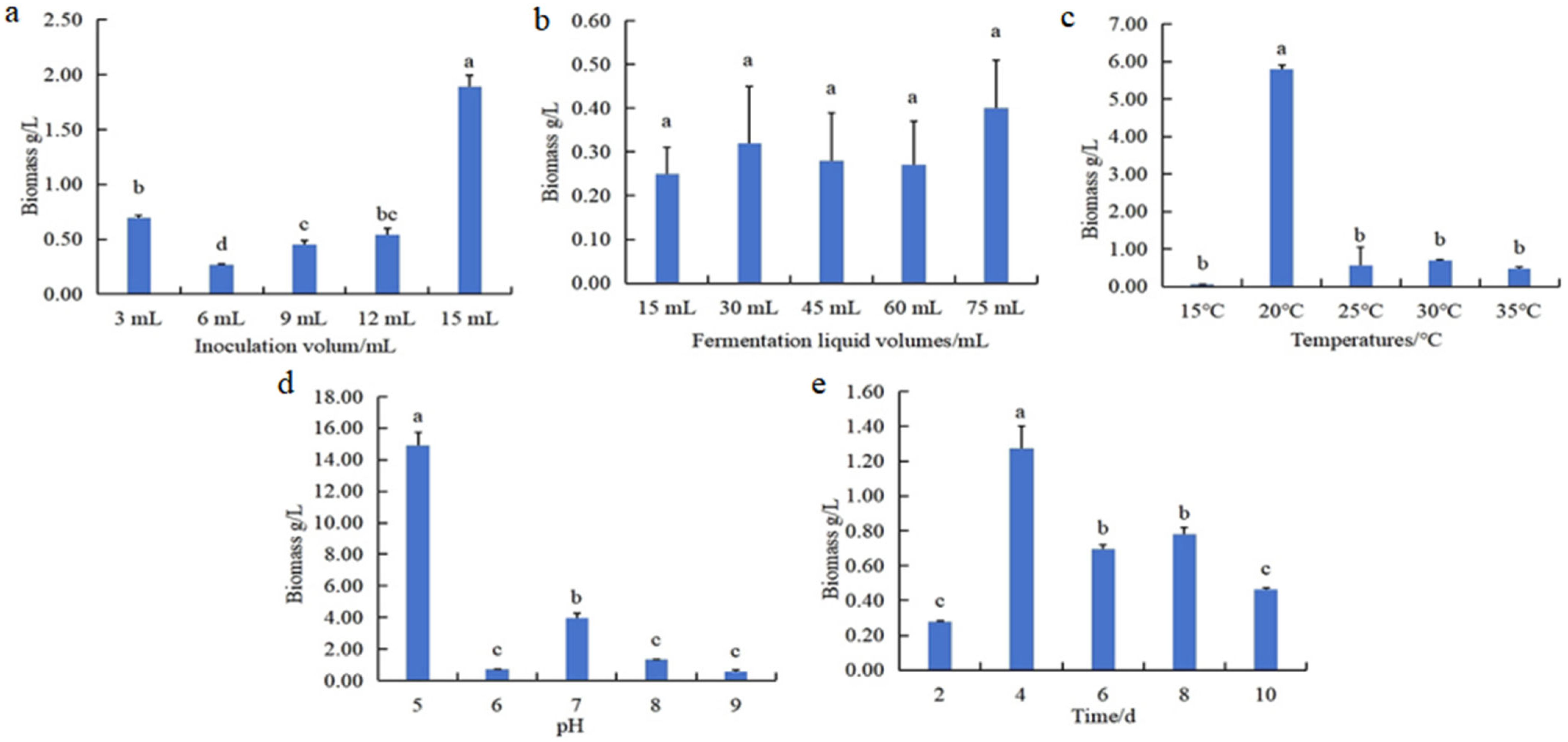
2.2.5. Screening Significant Fermentation Conditions for N. oryzae
2.3. Pathogenicity Test of Fermentation Broth on S. rostratum
2.4. Evaluation of the Fermentation Broth for Seven Crops
2.5. Statistical Analysis
3. Results
3.1. Optimization Results of the Fermentation Medium Components of N. oryzae
3.1.1. The Biomass of N. oryzae Was Affected by Different Carbon and Nitrogen Sources
3.1.2. The Biomass of N. oryzae Was Affected by Different Carbon: Nitrogen Ratios
3.1.3. Optimization of Fermentation Conditions in N. oryzae
3.2. Pathogenicity Test Results of Fermentation Broth on S. rostratum
3.3. The Safe Evaluation Result of the Fermentation Broth on Seven Crops
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, K.; Shao, H.; Han, C.X.; Zokir, T. Diversity of the Rhizosphere Soil Fungi of the Invasive Plant (Solanum rostratum Dunal) and the Allelopathic Potential of Their Secondary Metabolites. Chin. J. Soil Sci. 2022, 53, 548–557. [Google Scholar]
- Yan, W.F.; Wang, J.; Zheng, Y.N. Advances in Hazard status and Control technology of Solanum rostratum. Terr. Ecosyst. Conserv. 2022, 2, 73–79. [Google Scholar]
- Zhou, S.X.; Zhu, X.Z.; Shi, K.; Han, C.X.; Nigora Zhang, C.; Shao, H. Chemical composition and allelopathic potential of the invasive plant Solanum rostratum Dunal essential oil. Flora 2021, 274, 151730. [Google Scholar]
- Zhou, Q.L.; Cao, W.; Zhang, Y.; Jin, Y.H.; Wang, Y.C.; Ma, Y.P.; Chen, H.; Cui, X. Invasion characteristics of the alien invasive plant Solanum rostratum and its control strategies. J. Biosaf. 2023, 32, 314–322. [Google Scholar]
- Gao, F.; Xu, C.; Zhou, Y.L. The evaluation of potential fatalness for a kind of exotic species Solanum rostratum strategies for its control. J. Beijing Norm. Univ. (Nat. Sci.) 2005, 41, 420–424. [Google Scholar]
- Bassett, I.; Munro, D. The biology of Canadian weeds.78 Solanum carolinense L. and Solanum rostratum Dunal. Can. J. Plant Sci. 1986, 66, 977–991. [Google Scholar] [CrossRef]
- Guo, J.W.; Han, C.X.; Zhang, Y.G.; Lu, Y.X.; Wang, H.Y.; Wang, Y.; Baldwin, T.C.; Li, C.-Y.; Li, W.-J.; Shao, H. First report of Alternaria black spot disease caused by Alternaria alternata on the invasive weed Solanum rostratum in Xinjiang, China. Plant Dis. 2019, 103, 1022. [Google Scholar] [CrossRef]
- Thomas, P.E.; Hassan, S. First report of twenty-two new hosts of potato leafroll virus. Plant Dis. 2007, 86, 561. [Google Scholar] [CrossRef]
- Matzrafi, M.; Abu-Nassar, J.; Klap, C.; Shtarkman, M.; Smith, E.; Dombrovsky, A. Solanum elaeagnifolium and S. rostratum Potential Hosts Tomato Brown Rugose Fruit Virus. PLoS ONE 2023, 18, e0282441. [Google Scholar] [CrossRef]
- Xu, W.N.; Li, H.; Sivasithamparam, K.; Tran, D.T.; Jones, M.G.K.; Chen, X.; Wylie, S.J. Spillover of a Tobamovirus from the Australian Indigenous Flora to Invasive Weeds. Viruses 2022, 14, 1676. [Google Scholar] [CrossRef]
- Bszkowski, J. The Occurrence of Septoria nodorum Berk. and Associated Mycoflora in Seeds of Wheat Cultivated in the Szczecin voivodeship. Acta Oncol. 1994, 29, 43–52. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Pan, H.; Chen, M.Y.; Zhang, S.J.; Zhong, C.H. First Report of Nigrospora oryzae Causing Brown/Black Spot Disease of Kiwifruit in China. Plant Dis. 2017, 102, 243. [Google Scholar] [CrossRef]
- Eken, C.; Spanbayev, A.; Tulegenova, Z.; Yechshzhanov, T. First Report of Nigrospora oryzae on Wheat in Kazakhstan. Plant Dis. 2016, 100, 861. [Google Scholar] [CrossRef]
- Zheng, L.; Shi, F.; Kelly, D.; Hsiang, T. First report of leaf spot of Kentucky Bluegrass (Poa pratensis) caused by Nigrospora oryzae in Ontario. Plant Dis. 2012, 96, 909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Huang, L.L.; Liu, Y.J.; Ai, Y.; Peng, D.H. First Report of Leaf Spot of Lotus (Nelumbo nucifera) Caused by Nigrospora oryzae in China. Plant Dis. 2018, 102, 1038. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, S.S.; Tan, G.J.; Shen, J.T.; He, T. First report of Nigrospora oryzae causing leaf spot of cotton in China. Plant Dis. 2012, 96, 1379. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Liu, S.; Pu, Z.; Lv, J.; Lu, L. Virulence determination of Isaria javanica MSC-F1 against panonychus citri and optimization of the liquid fermentation process. Chin. J. Biol. Control 2024, 3, 1–15. [Google Scholar]
- Dai, Y.; Wang, Y.H.; Li, M.; Zhu, M.L.; Wen, T.Y.; Wu, X.Q. Medium optimization to analyze the protein composition of Bacillus pumilus HR10 antagonizing Sphaeropsis sapinea. AMB Express 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.D.; Yang, Q. Optimization of solid-state fermentation conditions for Trichoderma harzianum using an orthogonal test. Genet. Mol. Res. 2015, 14, 1771–1781. [Google Scholar] [CrossRef]
- Li, B.; Peng, B.; Li, D.H.; Li, H.Y. Screening of weed inhibition activity of plant pathogenic fungi and active metabolites of target spot of Bipolaris sorghicola. Agrochemicals 2023, 62, 542–546. [Google Scholar]
- Yu, S.T. Study on Black Spot of Hibiscus mutabilis and Biocontrol Effect of Its Endogenous Bacteria. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2022. [Google Scholar]
- Zehra, A.; Dubey, M.K.; Meena, M.; Upadhyay, R.S. Effect of different environmental conditions on growth and sporulation of some Trichoderma species. J. Environ. Biol. 2017, 38, 197–203. [Google Scholar] [CrossRef]
- Cheng, J.L.; Zheng, M.; Lou, J.Q. Comparison of several common optimal experimental design methods. Res. Explor. Lab. 2012, 31, 7–11. [Google Scholar]
- Sun, H.; Yang, L.R.; Quan, X.; Xue, B.Q.; Zhu, Z.X.; Wu, X. Research Advances on Mechanism of Biological Control and Application about Trichoderma spp. Chin. Agric. Sci. Bull. 2011, 27, 242–246. [Google Scholar]
- Zhu, H.X.; Ma, Y.Q. Study on fermentation optimization of herbicidal activity strain Alternaria tenuissima. J. Gansu Agric. Univ. 2020, 55, 63–71. [Google Scholar]
- Zhang, L.F.; Cao, Y.; Zhang, L. Fermentation process optimization and suspension concentrate preparation of Trichode harzianum T-102. J. Peanut Sci. 2023, 53, 35–41. [Google Scholar]
- Chen, Z.Y.; Wang, P.; Wang, W.; Jin, G.H.; Tai, L.M.; Guo, Y.X.; Sun, D.M.; Jin, X.H. Screening of antagonistic bacteria against Streptomyces scabies and identification of BKS104. Microbiol. China 2021, 48, 4145–4155. [Google Scholar]
- Sun, Y.; Bai, Q.R. Pathogen Identification and Biological Characteristics of Rice Sheath Blight Caused by Nigrospora oryzae in Jilin Province and Susceptibility to Fungicides. Agrochemicals 2018, 57, 757–761+767. [Google Scholar]
- Li, W.Q.; Fu, H.F.; Li, H.B. Biological Characteristics and Fungicides Screening of Nigrospora oryzae. J. Chang. Veg. 2016, 06, 80–84. [Google Scholar]
- Hu, S.P.; Yu, J.; Wei, K.F.; Lan, B.; Li, M.Q.; Xie, B.Y.; Zhu, Y.C.; Yang, Y.N.; Zhu, J.M.; Zhang, L.; et al. Studies on the Biological Characteristics and Pathogenic Identification of Rice Spikelet Rot Disease in Jiangxi Province. Acta Agric. Univ. Jiangxiensis 2019, 41, 234–242. [Google Scholar]
- Li, J.; Ding, Z.Y.; Gu, Z.H.; Zhang, L.; Shi, G.Y. Effects of Mixed Carbon Sources on Production and Antitumor Activity of Ganoderma lucidum Exopolysaccharides by Submerged Culture. J. Food Sci. Biotechnol. 2017, 36, 129–135. [Google Scholar]
- Lu, W.J.; Wang, Y.Q.; Sun, D.W.; Yin, G.F.; Wang, L.H. Identification and biological characteristics of pathogen causing brown spot of buckwheat in Yunnan province. Southwest China J. Agric. Sci. 2022, 35, 98–104. [Google Scholar]
- Tebeest, D.O. Microbial Control of Weeds; Chapman Hall: New York, NY, USA, 1990; pp. 1–284. [Google Scholar]
- Watson, A.K. Current advances in bioherbicide research. In Brighton Crop Protection Conference—Weeds; British Crop Protection Council: Brighton, UK, 1989; pp. 987–996. [Google Scholar]
- Liang, D.D. Study on the Optimization of Liquid Fermentation Technology and Water Emulsions of Phoma herbarum SYAU-06 Strain. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Camargo, A.F.; Bonatto, C.; Scapini, T.; Klanovicz, N.; Tadioto, V.; Cadamuro, R.D.; Bazoti, S.F.; Kubeneck, S.; Michelon, W.; Júnior, F.W.R.; et al. Fungus-based bioherbicides on circular economy. Bioprocess Biosyst. Eng. 2023, 46, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.F.; Zheng, W.T.; Zheng, Y.N. Isolation, Pathogenicity and Safety Evaluation of Pathogen from Solanum rostratum in China. Liaoning University: Shenyang, China, 2024; to be submitted. [Google Scholar]

| Levels | Factors | |||
|---|---|---|---|---|
| A Yeast Extract/g | B (NH4)2SO4/g | C Lactose/g | D Glycerol/mL | |
| 1 | 0.6 | 0.6 | 0.6 | 0.6 |
| 2 | 0.6 | 1.2 | 1.2 | 1.2 |
| 3 | 0.6 | 1.8 | 1.8 | 1.8 |
| 4 | 1.2 | 0.6 | 1.2 | 1.8 |
| 5 | 1.2 | 1.2 | 1.8 | 0.6 |
| 6 | 1.2 | 1.8 | 0.6 | 1.2 |
| 7 | 1.8 | 0.6 | 1.8 | 1.2 |
| 8 | 1.8 | 1.2 | 0.6 | 1.8 |
| 9 | 1.8 | 1.8 | 1.2 | 0.6 |
| Spray Days | Incidence Area Rate % | Disease Index | Pathogenicity |
|---|---|---|---|
| 3 d | 0.08 ±0.04 b | 20.00 ± 0.00 c | Weak |
| 7 d | 4.54 ± 1.58 a | 30.00 ± 5.77 bc | Moderate |
| 11 d | 22.60 ± 6.40 a | 45.00 ± 5.00 ab | Moderate |
| 14 d | 30.81 ± 6.93 a | 60.00 ± 8.16 a | Strong |
| Crops | Incidence Area Rate % | Disease Index | Disease Severity |
|---|---|---|---|
| Z. mays | 1.67 ± 0.07 b | 4.44 ± 0.07 b | NS |
| T. aestivum | 2.11 ± 0.40 b | 11.11 ± 0.13 a | MS |
| S. lycopersicum | 6.00 ± 2.52 a | 17.78 ± 0.07 a | MS |
| S. melongena | 0.00 ± 0.00 b | 0.00 ± 0.00 b | NS |
| F. arundinacea | 0.22 ± 0.22 b | 2.22 ± 0.07 b | NS |
| B. inermis | 0.00 ± 0.00 b | 0.00 ± 0.00 b | NS |
| M. sativa | 0.00 ± 0.00 b | 0.00 ± 0.00 b | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Yan, W.; Ding, N.; Zheng, Y. Initial Medium Optimization of Nigrospora oryzae JL-4 and Its Biocontrol Potential on Solanum rostratum. Fermentation 2024, 10, 424. https://doi.org/10.3390/fermentation10080424
Zheng W, Yan W, Ding N, Zheng Y. Initial Medium Optimization of Nigrospora oryzae JL-4 and Its Biocontrol Potential on Solanum rostratum. Fermentation. 2024; 10(8):424. https://doi.org/10.3390/fermentation10080424
Chicago/Turabian StyleZheng, Wanting, Wenfeng Yan, Ning Ding, and Yanan Zheng. 2024. "Initial Medium Optimization of Nigrospora oryzae JL-4 and Its Biocontrol Potential on Solanum rostratum" Fermentation 10, no. 8: 424. https://doi.org/10.3390/fermentation10080424
APA StyleZheng, W., Yan, W., Ding, N., & Zheng, Y. (2024). Initial Medium Optimization of Nigrospora oryzae JL-4 and Its Biocontrol Potential on Solanum rostratum. Fermentation, 10(8), 424. https://doi.org/10.3390/fermentation10080424






