Bioprocess Optimization by Taguchi Design and Response Surface Analysis for Obtaining Active Yeast Used in Vinification
Abstract
1. Introduction
2. Materials and Methods
2.1. Biotechnological Investigation
2.1.1. Obtaining Solid Culture (Preinoculum)
2.1.2. Obtaining the Liquid Inoculum
2.1.3. Micropilot Fermentation
2.1.4. Downstream Process
2.2. Experimental Design to Optimize Yeast Production
2.3. Winemaking Process for Tămâioasă Românească and Busuioacă de Bohotin
2.4. Analytical Investigation
2.4.1. Determination of Dry Matter Content
2.4.2. Determination of Protein Content
2.4.3. Ethanol Content
2.4.4. Yeast Biomass Viability
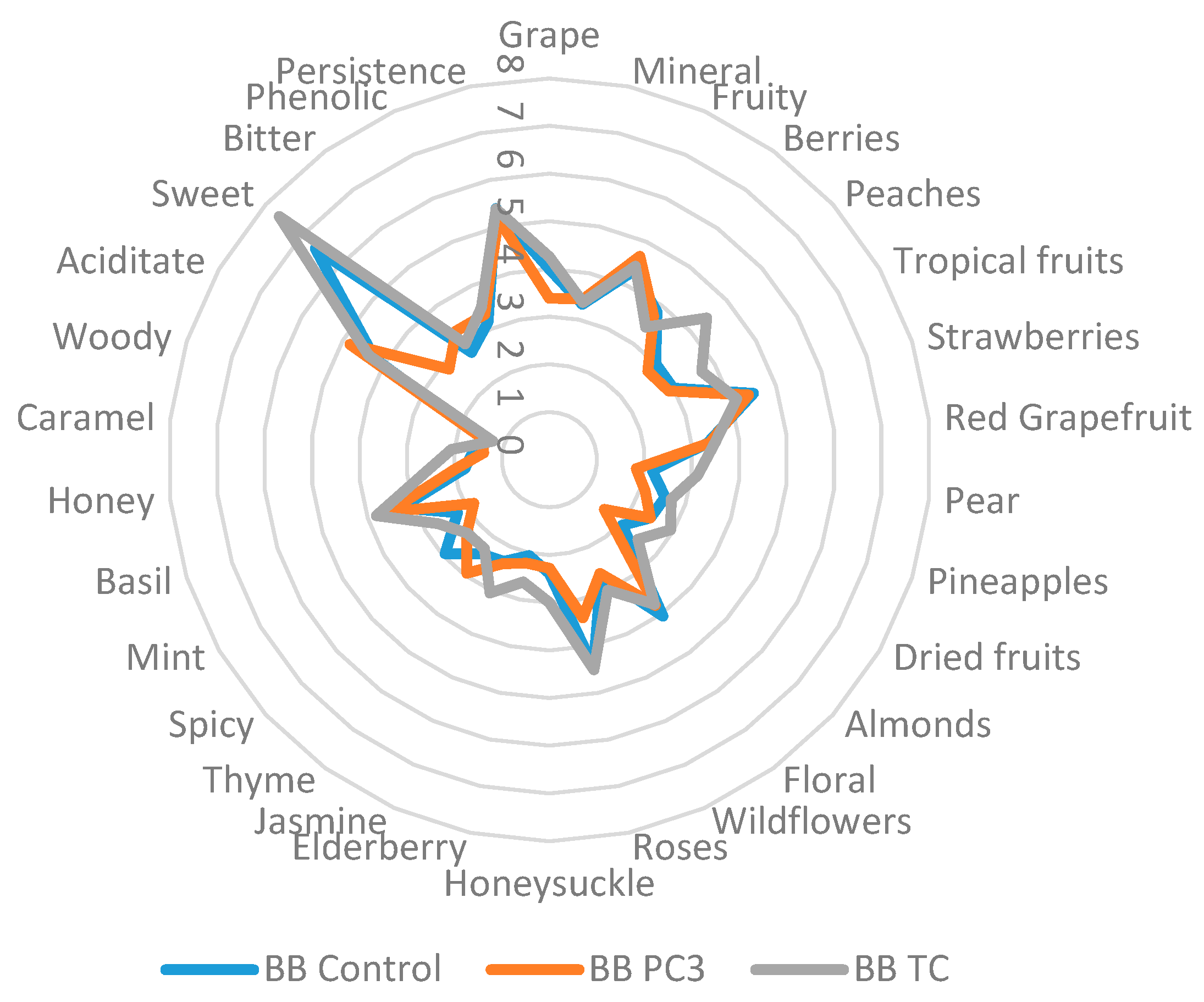
2.5. Sensory Characterization
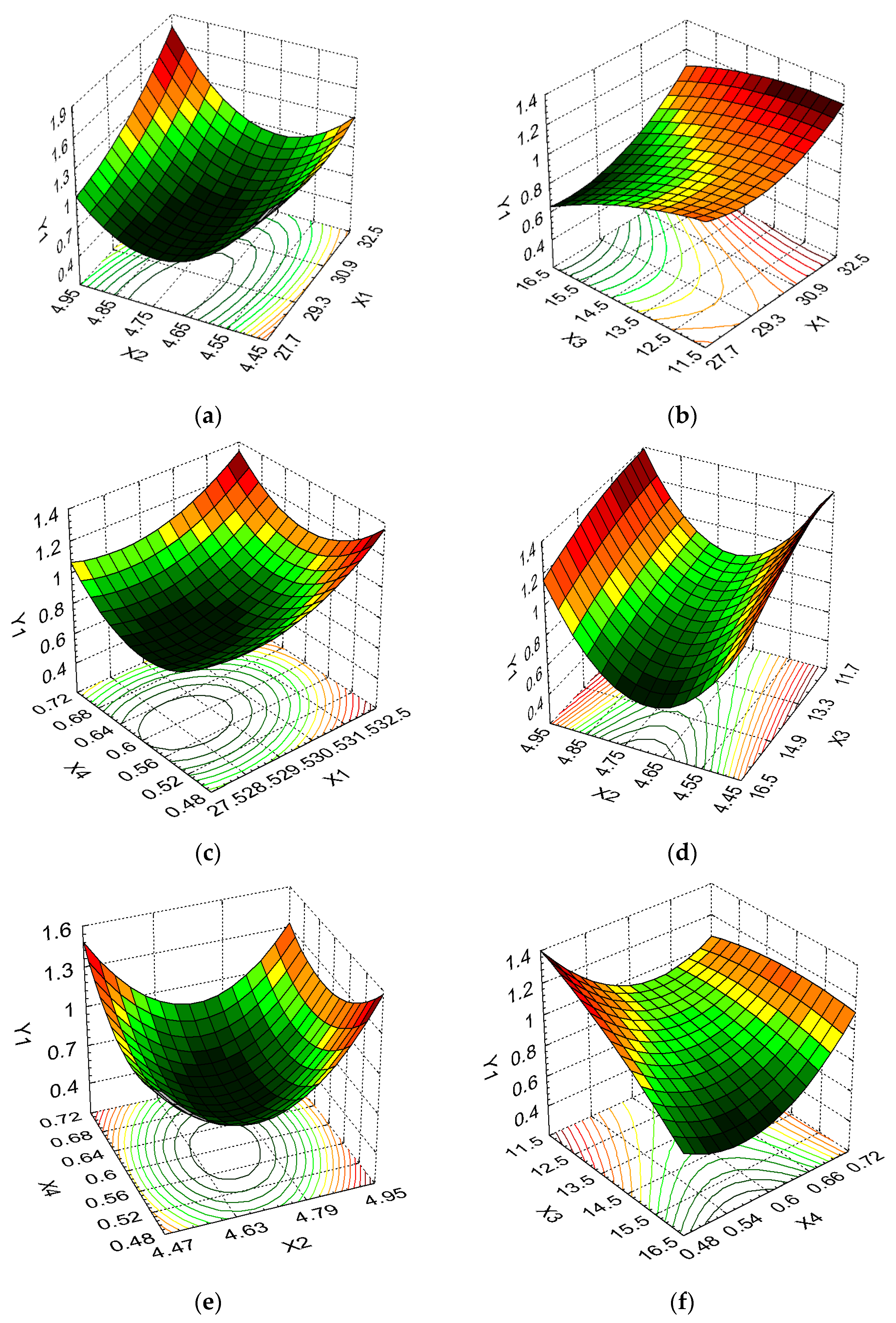
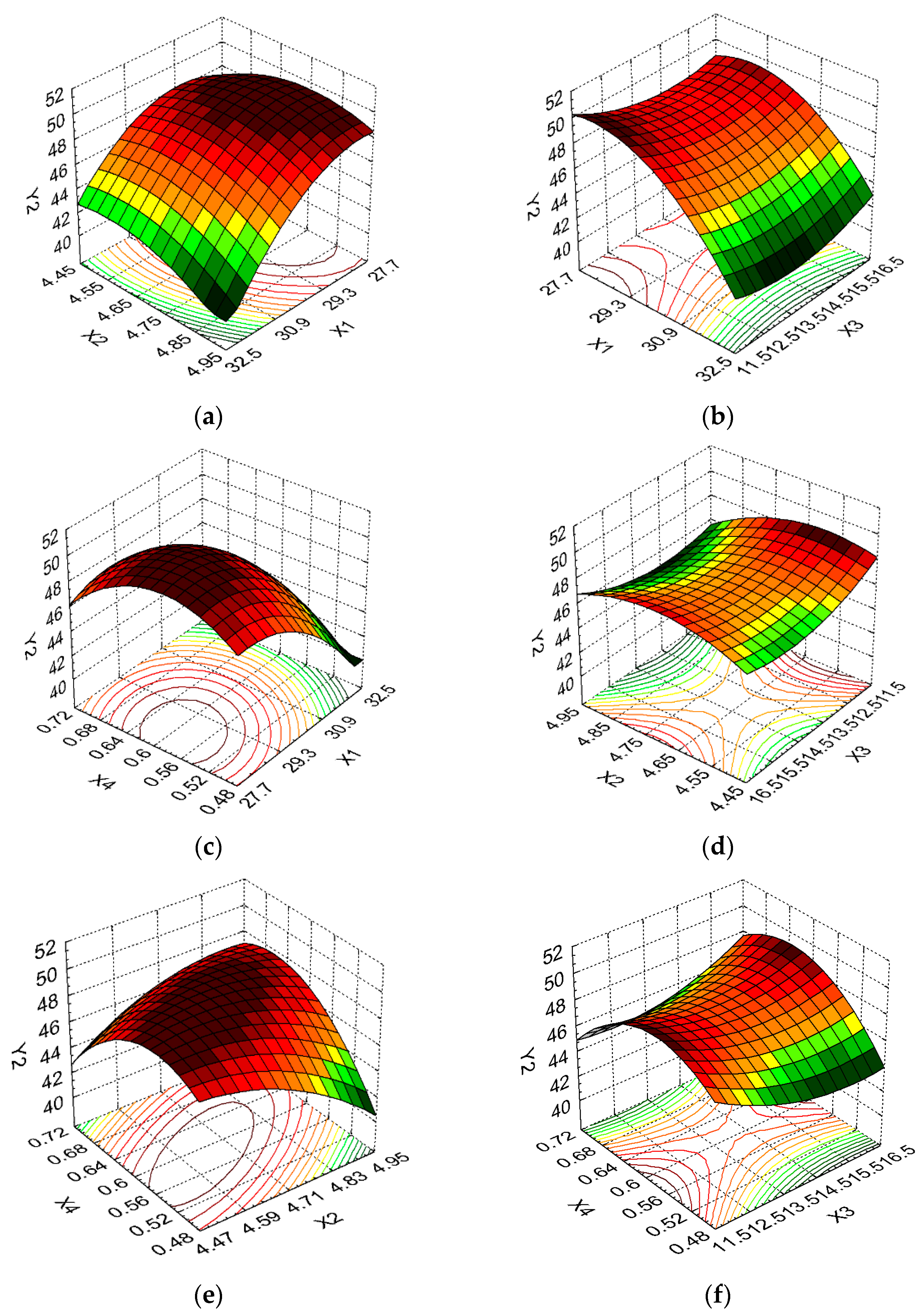
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2023 Wine Production—OIV First Estimates. Available online: https://www.oiv.int/sites/default/files/documents/PPT_OIV_2023_World_Wine_Production_Outlook.pdf (accessed on 21 April 2024).
- 2022 Wine Production—OIV First Estimates. Available online: https://www.oiv.int/sites/default/files/documents/PPTWorld%20Wine%20Production%20Outlook_2022_Press%20Conf.pdf (accessed on 4 November 2023).
- Colibaba, L.C.; Cotea, V.V.; Lacureanu, F.G.; Tudose-Sandu-Ville, S.; Rotaru, L.; Niculaua, M.; Luchian, C.E. Studies of Phenolic and Aromatic Profile of Busuioacă de Bohotin Wines. BIO Web Conf. 2015, 5, 02008. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Picca Nicolino, M.; Voordeckers, K.; Verstrepen, K.J. Improving Industrial Yeast Strains: Exploiting Natural and Artificial Diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma Profile of Montepulciano d’Abruzzo Wine Fermented by Single and Co-Culture Starters of Autochthonous Saccharomyces and Non-Saccharomyces Yeasts. Front. Microbiol. 2016, 7, 610. [Google Scholar] [CrossRef]
- Blanco, P.; Vázquez-Alén, M.; Garde-Cerdán, T.; Vilanova, M. Application of Autochthonous Yeast Saccharomyces cerevisiae XG3 in Treixadura Wines from D.O. Ribeiro (NW Spain): Effect on Wine Aroma. Fermentation 2021, 7, 31. [Google Scholar] [CrossRef]
- Mouret, J.-R.; Aguera, E.; Perez, M.; Farines, V.; Sablayrolles, J.-M. Study of Oenological Fermentation: Which Strategy and Which Tools? Fermentation 2021, 7, 155. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Truth in Wine Yeast. Microb. Biotechnol. 2022, 15, 1339–1356. [Google Scholar] [CrossRef]
- Guindal, A.M.; Gonzalez, R.; Tronchoni, J.; Roodink, J.S.; Morales, P. Directed Evolution of Saccharomyces cerevisiae for Low Volatile Acidity during Winemaking under Aerobic Conditions. Food Microbiol. 2023, 114, 104282. [Google Scholar] [CrossRef]
- Guerrini, S.; Barbato, D.; Guerrini, L.; Mari, E.; Buscioni, G.; Mangani, S.; Romboli, Y.; Galli, V.; Parenti, A.; Granchi, L. Selection of Indigenous Saccharomyces cerevisiae Strains and Exploitation of a Pilot-Plant to Produce Fresh Yeast Starter Cultures in a Winery. Fermentation 2021, 7, 99. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Yeasts for the Future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Pepe, V.; Comitini, F.; Comi, G.; Ciani, M. Enological and Genetic Traits of Isolated from Former and Modern Wineries. FEMS Yeast Res. 2004, 5, 237–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sablayrolles, J.-M. Kinetics and Control of Alcoholic Fermentation during Wine Production. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 283–313. ISBN 978-1-4939-9780-0. [Google Scholar]
- Ciani, M.; Comitini, F. Use of Non-Saccharomyces Yeasts in Red Winemaking. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 51–68. ISBN 978-0-12-814399-5. [Google Scholar]
- Rosini, G. Wine-Making by Cell-Recycle-Batch Fermentation Process. Biotechnology 1986, 24, 140–143. [Google Scholar] [CrossRef]
- Ocón, E.; Gutiérrez, A.R.; Garijo, P.; López, R.; Santamaría, P. Presence of Non-Saccharomyces Yeasts in Cellar Equipment and Grape Juice during Harvest Time. Food Microbiol. 2010, 27, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Siesto, G.; Capece, A.; Pietrafesa, R.; Lanciotti, R.; Patrignani, F.; Granchi, L.; Galli, V.; Bevilacqua, A.; Campaniello, D.; et al. Validation of a Standard Protocol to Assess the Fermentative and Chemical Properties of Saccharomyces cerevisiae Wine Strains. Front. Microbiol. 2022, 13, 830277. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Raspor, P.; Milek, D.M.; Polanc, J.; Smole Možina, S.; Čadež, N. Yeasts Isolated from Three Varieties of Grapes Cultivated in Different Locations of the Dolenjska Vine-Growing Region, Slovenia. Int. J. Food Microbiol. 2006, 109, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.; Laurie, V.F.; Mas, A.; Romero, J. Microbial Terroir in Chilean Valleys: Diversity of Non-Conventional Yeast. Front. Microbiol. 2016, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-Conventional Yeast Species for Lowering Ethanol Content of Wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast Interactions in Inoculated Wine Fermentation. Front. Microbiol. 2016, 7, 555. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with Non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Valero, E.; Cambon, B.; Schuller, D.; Casal, M.; Dequin, S. Biodiversity of Saccharomyces Yeast Strains from Grape Berries of Wine-Producing Areas Using Starter Commercial Yeasts. FEMS Yeast Res. 2007, 7, 317–329. [Google Scholar] [CrossRef]
- Manzano, M.; Medrala, D.; Giusto, C.; Bartolomeoli, I.; Urso, R.; Comi, G. Classical and Molecular Analyses to Characterize Commercial Dry Yeasts Used in Wine Fermentations. J. Appl. Microbiol. 2006, 100, 599–607. [Google Scholar] [CrossRef][Green Version]
- Úbeda, J.; Barrajón, N.; Briones, A. Optimizing Small-Scale Production of Fresh Wine Yeast Biomass. J. Food Process Eng. 2013, 36, 686–693. [Google Scholar] [CrossRef]
- Fleet, G.H. (Ed.) Wine Microbiology and Biotechnology; Harwood Academic Publishers: Chur, Switzerland; Philadelphia, PA, USA, 1993; ISBN 978-3-7186-5132-0. [Google Scholar]
- Mannazzu, I.; Clementi, F.; Ciani, M. Strategies and Criteria for the Isolation and Selection of Autochthonous Starters in Biodiversity and Biotechnology of Wine Yeasts; Research Signpost: Trivandrum, India, 2002; pp. 19–35. [Google Scholar]
- Maqueda, M.; Pérez-Nevado, F.; Regodón, J.A.; Zamora, E.; Alvarez, M.L.; Rebollo, J.E.; Ramírez, M. A Low-Cost Procedure for Production of Fresh Autochthonous Wine Yeast. J. Ind. Microbiol. Biotechnol. 2011, 38, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi Methodology as a Statistical Tool for Biotechnological Applications: A Critical Appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef]
- José Moreira Ferreira, D.; Noble, J. Yeast Strain Optimization for Enological Applications. In Advances in Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-612-6. [Google Scholar]
- Tronchoni, J.; Gonzalez, R.; Guindal, A.M.; Calleja, E.; Morales, P. Exploring the Suitability of Saccharomyces cerevisiae Strains for Winemaking under Aerobic Conditions. Food Microbiol. 2022, 101, 103893. [Google Scholar] [CrossRef]
- Ivanov, A.; Ivanova, K.; Kostov, G. Modeling and Optimization of Processes for Craft Beer Production: Malt Mixture Modeling and Mashing Optimization for Lager Beer Production. Appl. Sci. 2023, 13, 11554. [Google Scholar] [CrossRef]
- Bhonsale, S.; Mores, W.; Van Impe, J. Dynamic Optimisation of Beer Fermentation under Parametric Uncertainty. Fermentation 2021, 7, 285. [Google Scholar] [CrossRef]
- Saelee, N.; Cheong, L.-Z.; Chaijan, M. Optimized Acetic Acid Production by Mixed Culture of Saccharomyces cerevisiae TISTR 5279 and Gluconobacter Oxydans TBRC 4013 for Mangosteen Vinegar Fermentation Using Taguchi Design and Its Physicochemical Properties. Foods 2023, 12, 3256. [Google Scholar] [CrossRef]
- Dubencovs, K.; Liepins, J.; Suleiko, A.; Suleiko, A.; Vangravs, R.; Kassaliete, J.; Scerbaka, R.; Grigs, O. Optimization of Synthetic Media Composition for Kluyveromyces Marxianus Fed-Batch Cultivation. Fermentation 2021, 7, 62. [Google Scholar] [CrossRef]
- Schnierda, T.; Bauer, F.; Divol, B.; Van Rensburg, E.; Görgens, J. Optimization of Carbon and Nitrogen Medium Components for Biomass Production Using Non- Saccharomyces Wine Yeasts. Lett. Appl. Microbiol. 2014, 58, 478–485. [Google Scholar] [CrossRef]
- Navidad-Murrieta, M.S.; Pérez-Larios, A.; Sanchéz-Burgos, J.A.; Ragazzo-Sánchez, J.A.; Luna-Bárcenas, G.; Sáyago-Ayerdi, S.G. Use of a Taguchi Design in Hibiscus Sabdariffa Extracts Encapsulated by Spray-Drying. Foods 2020, 9, 128. [Google Scholar] [CrossRef]
- Bărbulescu, I.D.; Ghica, M.V.; Begea, M.; Albu Kaya, M.G.; Teodorescu, R.I.; Popa, L.; Mărculescu, S.I.; Cîrîc, A.I.; Dumitrache, C.; Lupuliasa, D.; et al. Optimization of the Fermentation Conditions for Brewing Yeast Biomass Production Using the Response Surface Methodology and Taguchi Technique. Agriculture 2021, 11, 1237. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12, 658. [Google Scholar] [CrossRef]
- Singh, S.; Deepa, N.; Rastogi, D.; Chaturvedi, S.; Syed, N.; Singh, A.; Nannaware, A.D.; Rout, P.K. Biotransformation of Ricinoleic Acid to γ-Decalactone Using Novel Yeast Strains and Process Optimization by Applying Taguchi Model. J. Biotechnol. 2023, 377, 34–42. [Google Scholar] [CrossRef]
- Dutta, D.; Das, M.D. Optimization and Partial Characterization of Intracellular Anticandidal Protein from Aspergillus Giganteus MTCC 8408 Using Taguchi DOE. Bioengineered 2017, 8, 536–548. [Google Scholar] [CrossRef][Green Version]
- Shukla, S.; Goyal, A. Development of Efficient Fermentation Process at Bioreactor Level by Taguchi’s Orthogonal Array Methodology for Enhanced Dextransucrase Production from Weissella confusa Cab3. AiM 2012, 02, 277–283. [Google Scholar] [CrossRef]
- Malhotra, G.; Chapadgaonkar, S.S. Taguchi Optimization and Scale up of Xylanase from Bacillus Licheniformis Isolated from Hot Water Geyser. J. Genet. Eng. Biotechnol. 2020, 18, 65. [Google Scholar] [CrossRef]
- Dar, M.A.; Kaushik, G. Optimization of Process Parameters for Biodegradation of Malathion by Micrococcus Aloeverae MAGK3 Using Taguchi Methodology and Metabolic Pathway Analysis. Biocatal. Agric. Biotechnol. 2022, 42, 102362. [Google Scholar] [CrossRef]
- Barbulescu, I.D.; Teodorescu, R.I.; Dumitrache, C.; Begea, M.; Diguta, C.F.; Frincu, M.; Banita, D.C.; Marculescu, S.I.; Ciric, A.I.; Tudor, V.; et al. Obtaining Active Dry Yeasts Biomass for the Production of Pietroasa Wines. Agrolife 2023, 12, 18–24. [Google Scholar] [CrossRef]
- Dumitrache, C.; Frîncu, M.; Alexandru, T.; Diana, I.; Tudor, V.; Ionuţ, R. Identification by PCR ITS-RFLP Technique of New Yeast Isolates from Pietroasa Vineyard. Sci. Pap. Ser. B Hortic. 2020, 64, 287–293. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E. Selection of Optimal Operating Conditions for Extraction of Myrtus communis L. Essential Oil by the Steam Distillation Method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.M.; Albu Kaya, M.G.; Iovu, H.; Stavarache, C.E.; Chelaru, C.; Constantinescu, R.R.; Dinu-Pîrvu, C.-E.; Ghica, M.V. Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis. Coatings 2020, 10, 990. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response Surface Methodology and Taguchi Approach to Assess the Combined Effect of Formulation Factors on Minocycline Delivery from Collagen Sponges. Pharmazie 2013, 68, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Popa, M.; Leca, M.; Lupuliasa, D.; Moisescu, T. Optimization of the Pharmaceutical Products and Process Design Applying Taguchi Quality Engineering Principles. Farmacia 2011, 59, 321–328. [Google Scholar]
- Moț, A.; Ion, V.A.; Madjar, R.M.; Bădulescu, L. Dynamic PREGL-DUMAS Technique Applied in Nitrogen Determination from Inputs Used in Organic Agriculture. Sci. Pap. Ser. A Agron. 2022, 65, 105–110. [Google Scholar]
- OIV. OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions; OIV: Dijon, France, 2021. [Google Scholar]
- ISO 8589:2007; Sensory Analysis–General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- ISO 3591:1977; Sensory Analysis–Apparatus–Wine–Tasting Glass. ISO: Geneva, Switzerland, 1977.
- Ghica, M.; Albu Kaya, M.; Dinu-Pîrvu, C.-E.; Lupuleasa, D.; Udeanu, D. Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Albu, M.G.; Leca, M.; Popa, L.; Moisescu, S. Design and Optimization of Some Collagen-Minocycline Based Hydrogels Potentially Applicable for the Treatment of Cutaneous Wound Infections. Pharmazie 2011, 66, 853–861. [Google Scholar] [CrossRef]
- Dumitrache, C.; Matei, F.; Barbulescu, D.I.; Frincu, M.; Tudor, V.; Hirjoaba, L.N.; Teodorescu, R.I. Protein Sources for Animal Feed: Yeast Biomass of Beer and/or Wine—Review. Earth Obs. 2019, 8, 175–182. [Google Scholar]





| 1. Freezing | 2. Sample Loading | 3. Freezing | 4. Main Freeze-Drying | 5. Main Freeze-Drying | 6. Main Freeze-Drying | 7. Main Freeze-Drying | 8. Main Freeze-Drying | 9. Final Freeze-Drying | 10. Final Freeze-Drying | 11. Final Freeze-Drying | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | −40 | −40 | −40 | −40 | −40 | 10 | 20 | 35 | 30 | 30 | 35 |
| Pressure (mbar) | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.01 | 0.01 |
| Time (minutes) | 90 | 15 | 210 | 15 | 285 | 600 | 480 | 720 | 15 | 45 | 420 |
| Independent Variables | Coded Symbol | Levels of Variation in Coded and Physical Form | ||
|---|---|---|---|---|
| Low (1) | Middle (2) | High (3) | ||
| Temperature, T (°C) | X1 | 28 | 30 | 32 |
| pH | X2 | 4.5 | 4.7 | 4.9 |
| Carbon source, SC (%) | X3 | 12 | 14 | 16 |
| Yeast extract, ED (%) | X4 | 0.5 | 0.6 | 0.7 |
| Dependent variables | Coded Symbol | Constraints | ||
| Dry biomass, DCW (g/100 mL) | Y1 | Maximize | ||
| Protein content, PC (%) | Y2 | Maximize | ||
| Exp. No. | Independent Variables (Coded Level/Real Level) | System Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 T (°C) | X2 PH | X3 SC (g/100 mL) | X4 YE (g/100 mL) | Y1-DCW (g/100 mL) | Y2-Protein (%) * | |||
| Obs. | Pred. | Obs. | Pred. | |||||
| 1 | 1 (28) | 1 (4.5) | 1 (12) | 1 (0.5) | 1.16 | 1.18 | 48.47 | 48.43 |
| 2 | 1 (28) | 2 (4.7) | 2 (14) | 2 (0.6) | 0.51 | 0.49 | 50.47 | 50.21 |
| 3 | 1 (28) | 3 (4.9) | 3 (16) | 3 (0.7) | 0.93 | 0.95 | 47.82 | 47.80 |
| 4 | 2 (30) | 1 (4.5) | 2 (14) | 3 (0.7) | 1.11 | 1.06 | 47.23 | 47.61 |
| 5 | 2 (30) | 2 (4.7) | 3 (16) | 1 (0.5) | 0.56 | 0.57 | 47.98 | 48.14 |
| 6 | 2 (30) | 3 (4.9) | 1 (12) | 2 (0.6) | 1.01 | 1.02 | 49.21 | 49.27 |
| 7 | 3 (32) | 1 (4.5) | 3 (16) | 2 (0.6) | 0.98 | 1.01 | 45.18 | 44.92 |
| 8 | 3 (32) | 2 (4.7) | 1 (12) | 3 (0.7) | 1.05 | 1.07 | 44.15 | 44.01 |
| 9 | 3 (32) | 3 (4.9) | 2 (14) | 1 (0.5) | 1.33 | 1.29 | 41.24 | 41.35 |
| Responses | Sources of Variation | Sum of Squares | df | Mean of Squares | F | p |
|---|---|---|---|---|---|---|
| Y1 | Regression Residual Total | 0.567 0.007 0.574 | 6 2 8 | 0.094 0.003 | 28.260 | <0.05 |
| Y2 | Regression Residual Total | 64.837 0.345 65.181 | 6 2 8 | 10.806 0.172 | 62.63 | <0.05 |
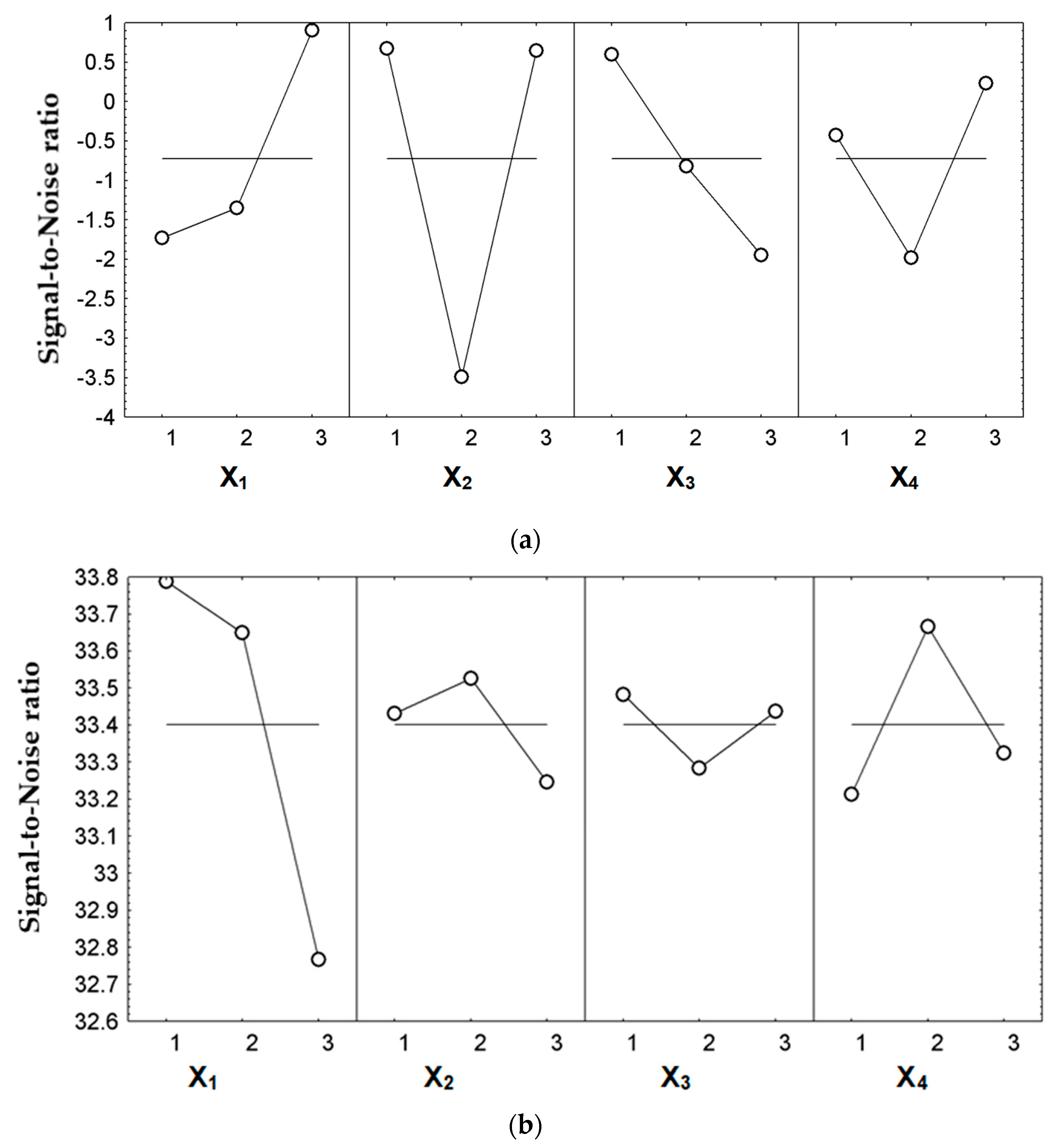
| Control Factors (Input Variables) | Y1 | Y2 | ||
|---|---|---|---|---|
| “Larger-the-Better” | Effect Size | “Larger-the-Better” | Effect Size | |
| X1 | 3 | 1.632 | 1 | 0.386 |
| X2 | 1 | 1.396 | 2 | 0.125 |
| X3 | 1 | 1.323 | 1 | 0.082 |
| X4 | 3 | 0.956 | 2 | 0.265 |
| S/N ratio expected (dB) | 4.584 | 34.259 | ||
| No. of Sample | Experimental Variants | CFU/mL |
|---|---|---|
| 1 | F01 | 0.8 × 1011 |
| 2 | F02 | 0.5 × 1011 |
| 3 | F03 | 0.4 × 1011 |
| 4 | F04 | 0.5 × 1011 |
| 5 | F05 | 0.8 × 1011 |
| 6 | F06 | 0.7 × 1011 |
| 7 | F07 | 0.7 × 1011 |
| 8 | F08 | 0.6 × 1011 |
| 9 | F09 | 0.5 × 1011 |
| 10 | FV1A | 1.3 × 1011 |
| 11 | FV1B | 1.2 × 1011 |
| Variety | Coding | Alcohol Content (% v/v) |
|---|---|---|
| Busuioacă de Bohotin rosé wine | BB PC3 | 14.6 |
| BB Control | 13.1 | |
| BB TC | 11.1 | |
| Tămâioasă românească white wine | TR PC3 | 14.6 |
| TR Control | 13.7 | |
| TR TC | 13.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrache, C.; Ghica, M.V.; Frîncu, M.; Bărbulescu, I.D.; Begea, M.; Diguță, C.F.; Baniță, C.; Cotea, V.V.; Israel-Roming, F.; Teodorescu, R.I. Bioprocess Optimization by Taguchi Design and Response Surface Analysis for Obtaining Active Yeast Used in Vinification. Fermentation 2024, 10, 413. https://doi.org/10.3390/fermentation10080413
Dumitrache C, Ghica MV, Frîncu M, Bărbulescu ID, Begea M, Diguță CF, Baniță C, Cotea VV, Israel-Roming F, Teodorescu RI. Bioprocess Optimization by Taguchi Design and Response Surface Analysis for Obtaining Active Yeast Used in Vinification. Fermentation. 2024; 10(8):413. https://doi.org/10.3390/fermentation10080413
Chicago/Turabian StyleDumitrache, Corina, Mihaela Violeta Ghica, Mihai Frîncu, Iuliana Diana Bărbulescu, Mihaela Begea, Camelia Filofteia Diguță, Cornel Baniță, Valeriu V. Cotea, Florentina Israel-Roming, and Răzvan Ionuț Teodorescu. 2024. "Bioprocess Optimization by Taguchi Design and Response Surface Analysis for Obtaining Active Yeast Used in Vinification" Fermentation 10, no. 8: 413. https://doi.org/10.3390/fermentation10080413
APA StyleDumitrache, C., Ghica, M. V., Frîncu, M., Bărbulescu, I. D., Begea, M., Diguță, C. F., Baniță, C., Cotea, V. V., Israel-Roming, F., & Teodorescu, R. I. (2024). Bioprocess Optimization by Taguchi Design and Response Surface Analysis for Obtaining Active Yeast Used in Vinification. Fermentation, 10(8), 413. https://doi.org/10.3390/fermentation10080413









