Abstract
Simvastatin, a semisynthetic drug widely used to lower cholesterol, is among the most prescribed statins worldwide. This study focuses on the direct production of a simvastatin-like biomolecule using alternative substrates by Aspergillus spp. strains. Two species, A. terreus UCP 1276 and A. flavus UCP 0316, were initially evaluated in synthetic media as control. Subsequently, the carbon and nitrogen sources were replaced by agro-industrial substrates, resulting in five modified media. Cultures were maintained at 28 °C, pH 6.5, at 180 rpm for 21 days. Fungal growth kinetics were evaluated and a 23 full-factorial design (FFD) was used to investigate the influence of substrate concentration on statin yield. Presence of inhibitors was confirmed by bioassay, UV–visible spectrophotometry, and thin-layer chromatography (TLC). According to the results, A. flavus UCP yielded 0.24 mg/g of statin in condition 2 of FFD (medium containing 4.5% soluble starch and saline base), suggesting it as a promising candidate for direct production of the biomolecule. Statistical analysis showed the significant effect of soluble starch on inhibitor production, making it a viable and profitable alternative substrate. Moreover, the isolated statin exhibited broad-spectrum antimicrobial activity, including efficacy against Gram-negative and Gram-positive bacteria and yeasts, indicating therapeutic potential against antimicrobial resistance.
1. Introduction
The increasing demand for HMG-CoA reductase inhibitors, statins, has driven the search for more efficient and sustainable production methods. Traditionally, the biosynthesis of these molecules occurs through chemical processes or using conventional substrates []. However, the use of alternative substrates, particularly those derived from agro-industrial residues, emerges as a promising strategy for the biotechnological production of statins. This approach not only promotes sustainability by reducing the environmental impact of waste but also can enhance the economic viability of the production process [].
Species of the genus Aspergillus are known for their ability to produce a wide variety of secondary metabolites, including HMG-CoA reductase inhibitors. These filamentous fungi have been extensively studied due to their efficiency in bioconversion processes, especially using low-cost and widely available substrates. Furthermore, several Aspergillus species exhibit antimicrobial properties, which can add value to the final product, providing a dual benefit in pharmaceutical and medical contexts [].
The use of agro-industrial residues, such as fruit peels, sugarcane bagasse, and cereal residues, as alternative substrates has proven effective in inducing the production of statins by Aspergillus spp. The bioconversion of these materials not only adds value to the residues but also contributes to the circular economy by promoting the reuse and recycling of natural resources [,].
HMG-CoA reductase inhibitors play a crucial role in controlling cholesterol levels in the body and are widely used in the prevention and treatment of cardiovascular diseases. Therefore, the efficient and sustainable production of these compounds is of paramount importance for public health. The antimicrobial potential of Aspergillus species adds an extra dimension to this research, as it could lead to the development of new combined therapies that simultaneously combat infections and regulate cholesterol levels [,].
In this context, the objective of this work was to explore the ability of different Aspergillus species to metabolize alternative substrates for the production of statins. This approach aims to provide a sustainable and economically viable strategy for the production of HMG-CoA reductase inhibitors, contributing to significant advances in biotechnology and global health.
2. Materials and Methods
2.1. Microorganisms
The filamentous fungi A. terreus UCP 1276 and A. flavus UCP 0316 were isolated, respectively, from soils of the Caatinga Biome and the Igarassu River in the state of Pernambuco. They belong to the Culture Collection of the Multi-User Center for Analysis and Characterization in Biomolecules and Surface Materials (CEMACBIOS), at the Catholic University of Pernambuco, registered with the World Federation for Culture Collection-WFCC.
2.2. Residues and Alternative Substrate (Soluble Starch)
The residues used to produce HMG-CoA reductase inhibitors were cassava wastewater (CW) and corn steep liquor (CSL), kindly provided an industry located in Cabo de Santo Agostinho (PE). Commercial soluble starch (SS) derived from corn, obtained in a commercial establishment located in Recife (PE), was also used as an alternative substrate.
2.3. Preparation of the Standard Solution of HMG-CoA Reductase Inhibitors (Sinvastatin)
The standard solution of simvastatin was prepared from simvastatin tablets purchased from a local pharmacy (PE). Ten (10) simvastatin tablets containing 10 mg (equivalent to a final concentration of 100 mg) were dissolved in 30 mL of acetonitrile according to the methodology described by [], with modifications.
2.4. Detection by Bioassay Test
The detection of HMG-CoA reductase inhibitors production was carried out by the bioassay test according to the methodology of [,], using Candida albicans as an indicator microorganism. For this, C. albicans UCP 0993 was grown on potato dextrose agar (PDA) medium at 28 °C for 24 h. Then, young cells were transferred to sterile water to obtain a suspension at 102 spores/mL and inoculated into MGYP medium (0.3% malt extract, 1% glucose, 0.3% yeast extract, 0.5% peptone, and 1.8% agar). Subsequently, wells were perforated in the malt glucose yeast extract (MGYP) medium, and 50 µL aliquots of the extract containing simvastatin extracted from the metabolic liquid (solubilized in acetonitrile) were added. The solution containing the standard of HMG-CoA reductase inhibitor (simvastatin) was solubilized in acetonitrile and used as a positive control for production, while acetonitrile alone was used as a negative control for production. The plates were incubated for 24 h. The presence of an inhibition halo around the well indicates the potential of the microorganism to produce simvastatin. The diameter of the inhibition zone is proportional to the concentration of simvastatin in the sample and was calculated as follows:
Diameter of the inhibition zone (mm) = 0.195 × Statin dose (µg)
2.5. Production of HMG-CoA Reductase Inhibitors by A. terreus UCP 1276 and A. flavus UCP 0316
The filamentous fungi A. terreus and A. flavus were cultured on Petri dishes containing PDA medium and maintained at 28 °C for 7 days until sporulation occurred. To obtain the inoculum, young spores were collected from the PDA medium and transferred to sterile water to obtain a suspension of 108 spores/mL. Ten percent of this inoculum was used in the production medium.
The production of HMG-CoA reductase inhibitors was carried out in 250 mL Erlenmeyer flasks containing 100 mL of synthetic medium as a control consisting of salt base. Glucose and monosodium glutamate, as described by [], were modified with the following concentrations: glucose (45 g/L), monosodium glutamate (15 g/L), KH2PO4, (4.0 g/L), K2HPO4 (5.0 g/L), FeSO4.7H2O (0.2 g/L), MnSO4.4H2O (0.1 g/L), MgSO4.7H2O (0.1 g/L), ZnSO4.7H2O (0.2 g/L), CaCl2.2H2O (20 mg/L) e (NH4)2SO4 (5.0 mg/L). Subsequently, alternative media were formulated using renewable sources composed of agro-industrial residues and classified as Modified Medium I (CW only), Modified Medium II (CW + SS), Modified Medium III (SS only), Modified Medium IV (CW + CSL), and Modified Medium V (SS + CSL), according to Table 1, in order to define the inducing source of HMG-CoA reductase inhibitors. The concentrations used in the modified media were based on the control medium described by []. In all media, the saline base was maintained. The pH was adjusted to 6.5, and the flasks were maintained under orbital agitation at 180 rpm, at 28 °C, for 21 days.

Table 1.
Alternative culture media for inducing the production of HMG-CoA reductase inhibitors compared to synthetic medium.
2.6. Growth Profile of A. terreus s UCP 1276 and A. flavus UCP 0316
Fungal growth and pH were monitored at 3th, 6th, 9th, 12th, 15th, 18th, and 21th days. The biomass obtained after filtration and centrifugation was washed, lyophilized, and kept in a desiccator until constant weight. Biomass was quantified by dry weight, and the yield was expressed in g/L, while pH was determined by potentiometry using the Electron Orion pH meter (model 310).
2.7. Determination of Glucose Consumption of A. terreus s UCP 1276 and A. flavusUCP 0316
Glucose consumption was determined using the enzymatic colorimetric method (Lab-Test), with a standard curve prepared using glucose solutions (0.1–5.0 g/L). Readings were taken using a digital spectrophotometer.
2.8. Determination of Biomass, Growth Kinetics, and Productivity of HMG-CoA Reductase Inhibitors
The mycelial mass was removed by vacuum filtration, washed with cold distilled water, then frozen and lyophilized. The biomass was kept in a desiccator until a constant weight was achieved, and it was estimated by gravimetry. The growth kinetics of A. terreus UCP 1276 and A. flavus UCP 0316 were established through the maximum growth rate µMax (h−1), generation time (GT), maximum production (P), and biomass yield from the consumed substrate (Yx/s), to evaluate the performance of A. terreus UCP 1276 and A. flavus UCP 0316 in the production of HMG-CoA reductase inhibitors.
2.9. Production of HMG-CoA Reductase Inhibitors in a Selected Medium Containing Concentration Established by Full-Factorial Design
To analyze the influence of SS, CW and CSL concentrations, as well as the interactions between them, on the production of HMG-CoA reductase inhibitors by A. terreus UCP 1276 and A. flavus UCP 0316, a 23 full-factorial design (FFD) was conducted, using the yield of HMG-CoA reductase inhibitors as the response variable. A set of eight experiments was performed with four replications at the central points (Table 2). Statistical analysis of the data obtained in the experiments was carried out using STATISTICA software version 8.0 (StatSoft Inc., Tulsa, OK, USA), and the significance of the results was tested at p ≤ 0.05. Under all conditions of the design, the pH was adjusted to 6.5, and the flasks were maintained under orbital agitation at 180 rpm, at 28 °C, for 7 days.

Table 2.
Matrix of the 23 factorial design to produce HMG-CoA reductase inhibitors.
2.10. Extraction and Purification of HMG-CoA Reductase Inhibitor
The extraction of the HMG-CoA reductase inhibitor was carried out in cell-free metabolic liquid by adding hydrochloric acid until pH 3.0 was achieved. Then, two volumes of ethyl acetate were added and maintained under orbital agitation at 180 rpm for 2 h at 28 °C. Subsequently, the samples were centrifuged at 5000 rpm for 30 min to separate the organic phase, which was evaporated and resuspended in acetonitrile (1:2) v/v []. Then, the sample was resuspended with 1% trifluoroacetic acid at a ratio of 1:1 and left to stand for 10 min for lactonization of any eventual hydroxylic acid form of statin. The crude extract containing simvastatin was purified on aluminum chromatofolios using silica as the stationary phase by the thin-layer chromatography (TLC) method. To perform this, the extracted simvastatin solution from the metabolic liquid was applied to the silica chromatofolios. The plates were eluted in hexane/ethyl acetate (1:2) with 2% acetic acid. The presence of simvastatin was observed under UV light according to the methodology described by [], with modifications.
2.11. Characterization of HMG-CoA Reductase Inhibitor
2.11.1. Identification of HMG-CoA Reductase Inhibitors by Thin-Layer Chromatography (TLC)
The identification of HMG-CoA reductase inhibitors in the extracts of A. terreus UCP 1276 and A. flavus UCP 0316 strains was conducted using TLC employing silica gel chromatographic plates with a chloroform and methanol solvent system (90:10 v/v) as the mobile phase. Five milligrams of simvastatin were dissolved in ethyl acetate (100 mL) for use as a standard. Ten microliters of the standard along with 10 µL of each extract were applied to the chromatographic plate. After drying, it was revealed under UV light, following the physical method described by [,]. The retention factor (Rf) of the extract was determined based on the distance traveled by the simvastatin sample, according to Equation (2)
where
Rf = dc/ds
dc = distance traveled by the sample component;
ds = total distance between the marked lines.
2.11.2. Identification and Quantification of HMG-CoA Reductase Inhibitor by UV–Visible Spectrophotometry
The quantification of the HMG-CoA reductase inhibitor was performed on the sample resuspended in 1% trifluoroacetic acid after the addition of acetonitrile in a 1:10 ratio. Simvastatin was detected at a low scanning rate in the wavelength range of λ 200 nm to 400 nm using a UV–visible spectrophotometer [,] and quantified by the equation of the line obtained through Scheme 1.
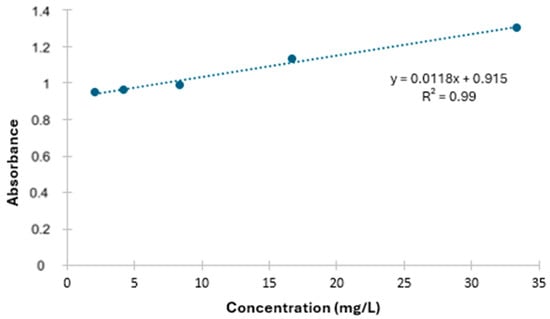
Scheme 1.
Graph of the relationship between absorbance and concentration of HMG-CoA reductase inhibitors.
To verify if acetonitrile influenced the results, a scan of acetonitrile alone was performed in the same wavelength range (λ 200 nm to 240 nm) seeking a reading at the wavelength of 237 nm, which corresponds to simvastatin.
2.11.3. Identification by Fourier-Transform Infrared Spectroscopy (FT-IR)
To identify the HMG-CoA reductase inhibitor produced, the analysis of the main functional groups was carried out by Fourier-transform infrared spectroscopy (FTIR) using an ATR accessory. Spectra were recorded in the wavelength range λ 4000 to 400 cm−1, using solutions of simvastatin (standard) compared to the solution containing the sample produced by A. terreus UCP 1276 and A. flavus UCP 0316. The scanned infrared spectra were also compared with the spectrum of the official reference standard provided by the pharmacopoeia.
2.12. Antimicrobial Assay
For the study of antimicrobial activity, a agar diffusion method was employed. Clinical bacteria Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Salmonella sp., and Staphylococcus aureus, and yeast strains Candida albicans, C. tropicalis, and C. guillermondii, were used as test organisms. Thus, 100 μL of standardized bacterial and yeast suspensions at a concentration of 1.5 × 108 CFU/mL (MacFarland scale 0.5) were spread on the surface of Muller Hinton Agar (bacteria) and Sabouraud Agar (yeasts) media using the spread-plate technique with swabs. The volume of the analyzed extract was 100 μL, ethyl acetate was used as a negative control, and the well diameter was 10 mm. All plates were incubated at 37 °C for 24 h. Antibacterial activity was expressed by the diameter of the zones of inhibition (mm) of bacterial growth. Tests were performed in triplicate.
3. Results
3.1. Growth Profile of A. terreus UCP 1276 and A. flavus UCP 0316 Strains in Modified Medium III with Soluble Starch
Figure 1A shows biomass production (g/L) over time by strains A. terreus UCP 1276 and A. flavus UCP 0316 for 21 days in Modified Medium III. As can be seen, A. flavus UCP 0316 produced biomass quickly in the first days, reaching a peak of approximately 1.0 g/L around the 9th day, followed by a slight decrease and stabilization until the end of the period (21 days). This behavior indicates rapid initial growth, followed by a stationary phase and a slight decrease, possibly due to nutrient depletion or accumulation of inhibitory metabolic products. In comparison, biomass production by A. terreus UCP 1276 was significantly lower, reaching a maximum of approximately 0.1 g/L over the 21 days. This slow growth may be indicative of a lower growth rate or efficiency in the use of soluble starch as a carbon source by this strain.
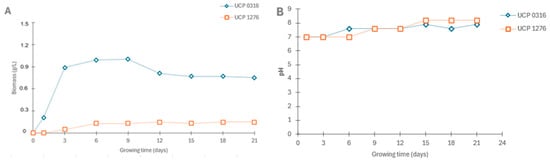
Figure 1.
Profiles of fungal growth (A) and pH (B) by A. terreus UCP 1276 and A. flavus UCP 0316 in Modified Medium III containing soluble starch.
On the other hand, Figure 1B shows the variation in pH for both strains during the 21 days of cultivation. For A. flavus UCP 0316, the initial pH was around 7.0, gradually increasing to approximately 8.0 over the 21 days of growth. This fact suggests a slight alkalinization of the medium, possibly due to the production of basic metabolites or the consumption of acids from the culture medium. For A. terreus UCP 1276, the initial pH was also around 7.0, showing a similar increase to approximately 8.0 during the same period. The pH variation for this strain was quite stable over time, similar to that observed for UCP 0316.
3.2. Production of HMG-CoA Reductase Inhibitors
Table 3 presents the yields of HMG-CoA reductase inhibitors (mg/g) produced by the strains A. terreus UCP 1276 and A. flavus UCP 0316 in different production media.

Table 3.
Strain selection for production of HMG-CoA reductase inhibitor.
In the synthetic control medium, A. terreus UCP 1276 produced 0.24 mg/g of inhibitors, while A. flavus UCP 0316 produced 0.20 mg/g of inhibitors. This medium presented a similar yield for A. terreus UCP 1276 compared to A. flavus UCP 0316. However, it is worth noting that 0.24 mg/g is higher than 0.20 mg/g.
In Modified Medium I, both strains produced 0.10 mg/g of inhibitors. This medium did not show a significant difference in yield between the strains, indicating a low and uniform production of inhibitors. However, in Modified Medium II, A. terreus UCP 1276 produced 0.20 mg/g of inhibitors, while A. flavus UCP 0316 produced 0.18 mg/g of inhibitors. Both strains showed similar results in this medium. In contrast, in Modified Medium III, A. terreus UCP 1276 produced 0.18 mg/g of inhibitors, while A. flavus UCP 0316 produced 0.24 mg/g of inhibitors. This medium was the most effective for A. flavus UCP 0316, which showed the highest yield among all tested media.
Both strains produced 0.20 mg/g of inhibitors in Modified Medium IV. As with Modified Medium I, there was no significant difference between the strains, but the yield was relatively better than in some other media. In Modified Medium V, A. terreus UCP 1276 produced 0.17 mg/g of inhibitors, while A. flavus UCP 0316 produced 0.20 mg/g of inhibitors.
3.3. Growth Kinetics and Productivity of HMG-CoA Reductase Inhibitors
In the present study, an increase in the biosynthesis of HMG-CoA reductase inhibitors (0.24 mg/g) was observed with the availability of the substrate, i.e., in Modified Medium III consisting of SS (4.5%) as the sole carbon source and salt base by the strain A. flavus UCP 0316.
From this result, it was possible to identify that by substituting the synthetic carbon source (glucose) with the alternative carbon source (SS), the ability of A. flavus UCP 0316 in the production of HMG-CoA reductase inhibitors was increased compared to the synthetic medium (2.0 mg/g).
The kinetic parameters of A. flavus UCP 0316 cultivated in SS are shown in Table 4. The final biomass was 2.43 g/L. The yield coefficients of HMG-CoA reductase inhibitors for biomass and for the substrate were found to be 0.0150 and 0.040 g−1, respectively. According to Mielcarek; Prasanna Latha and Bizukojc [,,] found lovastatin yield coefficients of 0.0065 and 0.0050 g−1 per A. terreus using lactose (10 g) and glycerol (5 g) as substrates.

Table 4.
Kinetic parameters of HMG-CoA reductase inhibitor production by A. flavus UCP 0316 in soluble starch.
In the present study, the biomass yield coefficient for the substrate starch and the maximum concentration of HMG-CoA reductase inhibitors were 0.273 g−1. According to Jaivel and Marimuthu [], glucose was used as the sole carbon source to evaluate the capacity of 10 fungal strains for lovastatin production in submerged fermentation, and A. terreus JPM3 was identified as the best lovastatin producer with a yield of 138.4 mg/L.
3.4. Production of HMG-CoA Reductase Inhibitors by A. flavus UCP 0316 Using Alternative Substrates
The production of HMG-CoA reductase inhibitors by A. flavus UCP 0316 depends, among other factors, on substrates used as carbon and nitrogen sources in the culture medium. According to the results presented in Table 5, the production of HMG-CoA reductase inhibitors was evidenced under condition 2, with a yield of 0.24 mg/g in a medium composed of SS (4.5%), and under condition 7, with a yield of 0.20 mg/g in a medium composed of SS (2.0%), CW (2.52%), and CSL (2.52%). The selection of carbon and nitrogen sources for production media is crucial for regulating the yield of HMG-CoA reductase inhibitors.

Table 5.
Yield of HMG-CoA reductase inhibitors production obtained by A. flavus UCP 0316 using 23 full-factorial design ().
3.5. Influence of Concentrations of Alternative Substrates Used in the Production of HMG-CoA Reductase Inhibitors by A. flavus UCP 0316
Figure 2 shows the Pareto chart of the standardized effects of SS), CW, and CSL, as well as their interactions, on the production of HMG-CoA reductase inhibitors by A. flavus s UCP 0316.
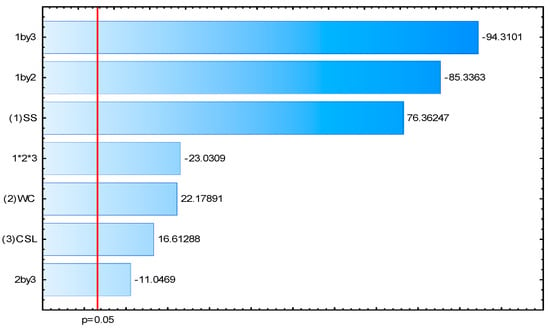
Figure 2.
Pareto chart of standardized effects of soluble starch (1), cassava wastewater (2), and corn steep liquor (3), and their interactions on the production of HMG-CoA reductase inhibitors by A. flavus UCP 0316. The point at which the effect estimates were statistically significant (p = 0.05) is indicated by the red line.
SS demonstrated a significant positive effect on the production of HMG-CoA reductase inhibitors, indicating it to be a promising carbon source. Interactions between the different substrates (especially between SS and other substrates) showed significant negative effects, suggesting that the combination of these substrates may not be beneficial for production. CW and CSL, although showing positive effects, were not significant.
Thus, SS stood out as the most effective substrate, with a significant positive effect on the production of HMG-CoA reductase inhibitors by A. flavus UCP 0316. The interactions between the different substrates had negative effects, suggesting that the use of soluble starch as the only carbon source may be the best strategy to optimize the production of these inhibitors.
3.6. Detection of HMG-CoA Reductase Inhibitors Using Thin-Layer Chromatography (TLC)
To characterize the biomolecule produced by the strains A. terreus UCP 1276 and A. flavus UCP 0316, TLC was employed, thus identifying that both strains could produce HMG-CoA reductase, as shown in Table 6.

Table 6.
Comparison of thin-layer chromatography (TLC) data against A. terreus UCP 1276 and A. flavus UCP 0316 with the standard drug for biomolecule identification in Modified Medium III (soluble starch).
3.7. Identification and Quantification of the Production of a Biomolecule Similar to Simvastatin by UV–Visible Absorption Spectrum
The commercial simvastatin extracted from tablets (standard) was analyzed by the UV–visible method and exhibited an absorption peak at 237 nm. Based on this result, a scan was performed on the synthetic medium and Modified Medium III, which was selected for its higher yield in HMG-CoA reductase inhibitor production (both in synthetic and modified media I, II, III, IV, and V), and compared to the spectra of commercial simvastatin. According to the results obtained, in all tested media, the absorption peak was at 237 nm, indicating the presence of a molecule with a structure similar to simvastatin produced by A. flavus UCP 0316.
The quantification of the HMG-CoA reductase inhibitor similar to simvastatin was performed using the linear equation: y = 0.0118x + 0.915 obtained from the standard curve, and the yield demonstrated as shown in Table 7.

Table 7.
Yield of simvastatin in the synthetic medium and in Modified Medium III (soluble starch).
3.8. Bioassay of the Biomolecule with a Structure Similar to Simvastatin Using C. albicans UCP0993
In this study, the bioassay with commercial simvastatin (standard) resulted in the formation of a small-diameter inhibition zone (halo) in the growth of C. albicans (Figure 3A). According to the results obtained, after cultivation of A. flavus, in the synthetic medium (Figure 2B) and in modified media I (Figure 3C), II (Figure 3D), III (Figure 3E), IV (Figure 3F), and V (Figure 3G), halo formation occurred with larger diameters compared to commercial simvastatin.
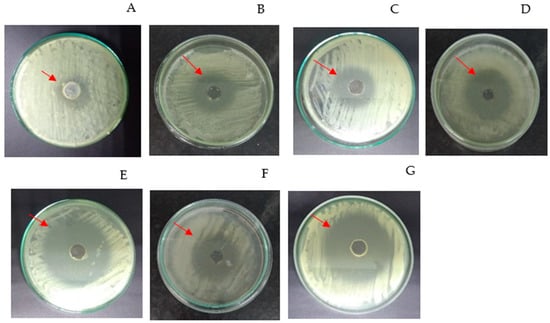
Figure 3.
Bioassay test: (A) Commercial simvastatin (standard); (B) synthetic medium (glucose + glutamate); (C) Modified Medium I (cassava wastewater only), (D) Modified Medium II (cassava wastewater + soluble starch), (E) Modified Medium III (soluble starch), (F) Modified Medium IV (cassava wastewater + corn steep liquor), (G) Modified Medium V (soluble starch + corn steep liquor). The red arrow indicates the inhibition halo.
Modified Medium III (4.5% SS + saline base) and Modified Medium V (4.5% SS and 1.26% CSL) were the media that maximally induced the production of the biomolecule with a structure similar to simvastatin by A. flavus.
The data presented in Table 8 indicate that Modified Medium III was the most effective in inducing the production of crude statins, with a concentration of 256.41 µg/mL and an inhibition zone diameter of 50 mm against C. albicans. Modified Medium V also demonstrated high efficiency, with a concentration of 243.60 µg/mL and an inhibition zone diameter of 47.5 mm. Comparatively, the synthetic control medium presented a statin concentration of 205.13 µg/mL with an inhibition zone diameter of 40 mm.

Table 8.
Concentration of crude statins produced from different media.
The results indicate significant variation in statin production depending on the medium used. Modified Medium III, with SS as the main component, and Modified Medium V, which combines SS and CSL, were the most efficient, suggesting that these components are particularly effective in inducing statin production by A. flavus. The other modified media, although less effective, still showed the capacity to produce statins but in lower concentrations.
These findings suggest that optimizing the cultivation medium can lead to more efficient production of biomolecules with structures similar to simvastatin, with potential applications in both the pharmaceutical and biotechnology fields.
3.9. Identification of the Biomolecule with a Structure Similar to Simvastatin by Fourier-Transform Infrared Spectroscopy (FT-IR)
The transmittance peaks shown in the absorption spectrum in the infrared region of the standard simvastatin were compared with the peaks presented in the sample (Modified Medium III (SS)) selected according to the yield of HMG-CoA reductase inhibitors.
The spectra confirming the functional groups of standard simvastatin are presented in Table 9, with the hydroxyl (OH) group at the wavelength of 2925.10 cm−1 corresponding to stretching vibrations, the methyl (CH) group at the wavelength of 1725.72 cm−1 referring to olefinic stretching, the carbonyl (C=O) group at the wavelength of 1052.21 cm−1, and the ether (C-O-C) group at the wavelength of 925.07 cm−1.

Table 9.
Analysis of the infrared spectra of the production of the biomolecule with a structure similar to simvastatin in Modified Medium III (soluble starch).
3.10. Antimicrobial Assay
The antimicrobial activity of fungal extracts from A. terreus UCP 1276 and A. flavus UCP 0316 strains against test bacteria and yeasts is presented in Table 10. Among the tested strains, both showed antimicrobial activity against all tested bacteria and yeasts, with most demonstrating broad-spectrum antibacterial activity by inhibiting both Gram-negative and Gram-positive bacteria.

Table 10.
Evaluation of the antimicrobial potential of A. terreus UCP 1276 and A. flavus UCP 0316 strains against bacteria and yeasts.
4. Discussion
4.1. Growth Profile of A. terreus UCP 1276 and A. flavus UCP 0316 Strains in Modified Medium III with Soluble Starch
The growth profiles of the two fungal strains, A. terreus UCP 1276 and A. flavus UCP 0316, in terms of biomass production and pH variation over a 21-day period, reveal distinct physiological behaviors and metabolic efficiencies. The biomass data indicate a marked difference in the growth patterns and efficiencies of the two strains when cultured in Modified Medium III with SS as the primary carbon source.
For UCP 0316, the rapid increase in biomass during the initial days, peaking at approximately 1.0 g/L around the ninth day, demonstrates a strong initial growth phase. This early phase of exponential growth suggests that UCP 0316 efficiently utilizes the SS available in the medium. The subsequent stabilization and slight decrease in biomass could be attributed to nutrient depletion or the accumulation of metabolic byproducts that inhibit further growth. This pattern is characteristic of many microbial growth curves, where an initial exponential phase is followed by a stationary phase and eventually a decline phase.
In contrast, UCP 1276 exhibited significantly lower biomass production, with a peak of only about 0.1 g/L over the 21-day period. This slow growth rate suggests that UCP 1276 may have a lower affinity for SS or a reduced metabolic efficiency in converting starch to biomass. Alternatively, UCP 1276 could have slower intrinsic growth kinetics or different metabolic pathways that do not favor rapid biomass accumulation under the given conditions.
These differences in biomass production are critical for understanding the potential applications of each strain. UCP 0316, with its rapid growth and higher biomass yield, may be more suitable for industrial processes requiring fast and efficient biomass production. On the other hand, the lower growth rate of UCP 1276 might be advantageous in processes where slower growth is desired to control biomass accumulation and associated metabolic by products.
The pH profiles of both strains showed similar trends, with the initial pH of around 7.0 gradually increasing to approximately 8.0 over the 21-day period. For UCP 0316, the gradual alkalinization of the medium could be indicative of the production of basic metabolites or the consumption of acidic components from the culture medium.
The increase in pH might also be due to the release of ammonia or other basic compounds as a result of metabolic activities. The stability of this pH trend suggests a consistent metabolic pattern that does not lead to significant fluctuations in the medium’s acidity. Similarly, UCP 1276 exhibited a stable increase in pH, indicating comparable metabolic activities concerning acid and base production. The pH stability over time suggests that both strains maintain a balanced production and consumption of acidic and basic compounds, which is beneficial for maintaining an optimal growth environment over extended periods [,].
4.2. Production of HMG-CoA Reductase Inhibitors
The use of glucose, lactose, fructose, and glycerol as carbon sources is considered more advantageous in biotechnological processes due to their high availability and efficiency in promoting microbial growth and metabolite production. These carbon sources are easily metabolizable by a wide variety of microorganisms, resulting in high yields of desired products [,].
In particular, the utilization of sucrose as a carbon source has proven effective in lovastatin production, yielding a maximum of 2.0 g/L, as reported by []. In comparison, the use of glycerol as a carbon source resulted in a lovastatin production of 0.5 g/L. These data highlight the importance of selecting the appropriate carbon source to optimize lovastatin production, demonstrating that, although glycerol is a viable source, sucrose is significantly more efficient for this purpose.
According to Kapoor; Saxena; Chaurasia [], glucose and lactose are more suitable at low concentrations. A higher concentration of glucose facilitates fungal growth but decreases the level of lovastatin due to ethanol synthesis as a byproduct. The authors of [] reported obtaining the highest yields in lovastatin production using lactose as the carbon source.
The composition of the culture medium significantly influences the production of HMG-CoA reductase inhibitors by the strains A. terreus UCP 1276 and A. flavus UCP 0316. Selecting an appropriate culture medium is crucial for optimizing the production of specific metabolites, and the data suggest that A. flavus UCP 0316 responds better to modifications in the medium, particularly in Modified Medium III. These insights are valuable for the development of industrial and biotechnological processes aimed at maximizing the production of HMG-CoA reductase inhibitors for pharmaceutical applications.
4.3. Growth Kinetics and Productivity of HMG-CoA Reductase Inhibitors
The study reveals a comprehensive analysis of the biosynthesis of HMG-CoA reductase inhibitors by the strain A. flavus UCP 0316, highlighting the importance of culture medium composition in metabolite production. Several key points emerge from the analysis of different media used, with particular emphasis on the efficacy of soluble starch as a carbon source in Modified Medium III.
An increase in the production of HMG-CoA reductase inhibitors (0.24 mg/g) was observed when A. flavus UCP 0316 was cultured in Modified Medium III, which utilized SS (4.5%) as the sole carbon source. This suggests that the substitution of the synthetic carbon source (glucose) with soluble starch significantly enhances the production capabilities of this strain. This finding aligns with the general understanding that different carbon sources can greatly influence microbial metabolite production.
The comparison between Modified Medium III and the synthetic medium, which achieved a production of 2.0 mg/g, demonstrates that the use of SS is not only viable but also preferable in terms of efficiency in producing HMG-CoA reductase inhibitors. This underscores the importance of carefully selecting the carbon source to optimize metabolic yields. The substitution of glucose with SS proved beneficial, aligning with the literature suggesting the significant influence of carbon sources on fungal biosynthesis of products.
Kinetic parameters indicate that A. flavus UCP 0316, when cultivated in soluble starch, achieved a final biomass of 2.43 g/L, with yield coefficients for HMG-CoA reductase inhibitors of 0.0150 g/g for biomass and 0.040 g/g for the substrate. These values suggest an efficient conversion of starch into biomass and metabolic products. Comparative studies, such as those by [], showed lower yield coefficients for lovastatin in A. terreus using lactose and glycerol, further highlighting the effectiveness of SS as a substrate.
The biomass yield coefficient for starch was 0.273 g/g, and the final biomass was 2.43 g/L. Comparatively, A. terreus JPM3 using glucose as the sole carbon source achieved a lovastatin yield of 138.4 mg/L, indicating that while glucose supports high lovastatin production, SS offers a competitive and potentially more sustainable alternative for producing HMG-CoA reductase inhibitors by A. flavus UCP 0316.
Thus, it is possible to highlight the critical influence of culture medium composition on the production of HMG-CoA reductase inhibitors by A. flavus UCP 0316. SS proved to be an effective carbon source, significantly increasing both biomass production and inhibitor yield. These findings are essential for optimizing industrial and biotechnological processes aimed at maximizing the production of valuable metabolites. The insights obtained provide a solid foundation for future research and development in microbial biotechnology, emphasizing the need for careful substrate selection to optimize the production of specific products.
4.4. Influence of Concentrations of Alternative Substrates Used in the Production of HMG-CoA Reductase Inhibitors by A. flavus UCP 0316
The selection of carbon and nitrogen sources for the production medium is of paramount importance for regulating statin production. Combinations of these sources have been reported under submerged fermentation (SF) conditions by Lima et al. and Jia; Zhang; Cao [,].
The selection of alternative substrates as raw materials for obtaining high-value-added products aims to ensure the principles of circular economy necessary for the sustainability of any bioprocess []. In this context, the study focused on the evaluation of alternative substrates for the production of HMG-CoA reductase inhibitors by A. flavus UCP 0316.
4.5. Detection of HMG-CoA Reductase Inhibitors Using Thin-Layer Chromatography (TLC)
The Rf value of simvastatin found in the extract of Aspergillus flavus UCP 0316 was equal to the standard simvastatin value, corroborating the studies conducted by Kazmi; Tahir; Mukhtar [] Al-Saman et al. and Balraj et al. [,] detected lovastatin with an Rf of 0.72, a value very similar to that found in the extract of A. terreus UCP 1276 (Rf 0.76), indicating the possibility of the presence of lovastatin.
Identification and Quantification of the Production of the Biomolecule Similar to Simvastatin through UV–Visible Absorption Spectrum
Polonini et al. [] determined simvastatin in UV/vis spectrophotometry at 237 nm through assays validated for linearity, specificity, accuracy, precision, performance, and robustness. According to Gulyamova et al. [], native strains of A. terreus were capable of producing simvastatin (1290 mg/L) as a fermentation product (intracellular). There are no reports in the literature regarding the direct production of simvastatin by A. niger.
4.6. Bioassay of the Biomolecule with a Structure like Simvastatin Using C. albicans UCP0993
Nigam [] assert that the zone of inhibition of Candida albicans growth is proportional to the concentration of statin produced in each sample. Therefore, the zone of inhibition formed by commercial simvastatin and the biomolecule produced in this study by A. flavus can be explained by the intense metabolic activity of the biomolecule with a structure similar to simvastatin, which acts by inhibiting the synthesis of ergosterol in the fungal membrane, thus impeding growth, corroborating the results found by Lima et al. and Javed et al. [,].
Gyetvat et al. [] reported that the produced statin generates a fungistatic effect on C. albicans, negatively altering membrane fluidity as a result of adaptive modifications in fatty acid composition, phospholipids, and accumulation of adaptive intermediates such as ergosterol.
The bioassay screening method applied in this study allowed for an assessment of the production of the biomolecule with a structure like simvastatin in a shorter period compared to other existing methods, in addition to demonstrating effectiveness in screening semisynthetic statins, as this method was previously only used in the identification of natural statins. This screening method was also able to provide an insight into the product concentration, in addition to requiring less labor and lower costs.
The present study differs from those by Dhar [] and Vilches et al. [], where the method was performed with discs, while the use of wells was employed in the present study because it allows for greater sample diffusion, thus obtaining better results.
4.7. Antimicrobial Assay
According to Foleto et al. [], the rapid growth of bacterial resistance to conventional antibiotics, coupled with the scarcity in identifying new antibacterial agents, has generated a global crisis in public health. Drug repurposing emerges as an alternative strategy to enhance the accelerated discovery of new antimicrobial agents.
This study highlights that simvastatin, a substance commonly used in the treatment of hyperlipidemia, exhibits broad-spectrum antibacterial activity against Gram-positive pathogens, including S. aureus, as well as against Gram-negative bacteria, due to permeabilization of the barrier imposed by the outer membrane. The inhibition zones range from 10 to 23 mm in diameter.
It is also noteworthy that A. flavus UCP 0316 exhibited the largest inhibition zone with a diameter of 23 mm against B. subtilis. Graziano et al. [], in preliminary screening, also demonstrated broad-spectrum antibacterial action of endophytic fungi against Gram-positive and Gram-negative bacteria and reported inhibition zones ranging from 0 to 36 mm in diameter.
In the last 20 years, six studies have evaluated the antibacterial activity of simvastatin, with notable findings from Ajith and Daivya [], where the inhibition zone ranged from 10.25 to 13 mm (disk diffusion) against strains of E. coli, B. subtillis, S. aureus, and Salmonella typhimurium. According to Barbarossa et al. [], statins act by promoting apoptosis, disrupting the bacterial membrane, inhibiting protein synthesis, or acting on insoluble components of the extracellular polysaccharide (EPS), in addition to stating that the antibacterial action is not related to the inhibition of HMG-CoA reductase.
5. Conclusions
This study aimed to investigate the feasibility of using alternative substrates to produce HMG-CoA reductase inhibitors by Aspergillus spp. strains. The results demonstrated that soluble starch stood out as a highly effective substrate, producing 0.24 mg/g of statin under optimized conditions. Statistical analysis reinforced the significance of soluble starch in inhibitor production, highlighting its potential as a viable and cost-effective alternative to conventional substrates.
Furthermore, the statin isolated, which has structural similarity to simvastatin, exhibited broad-spectrum antimicrobial activity, including efficacy against Gram-negative bacteria, Gram-positive bacteria, and yeasts, indicating its therapeutic potential against antimicrobial resistance. Therefore, this study not only achieved its goal of exploring alternative substrates for the biosynthesis of HMG-CoA reductase inhibitors but also opened new perspectives for the direct, sustainable, and economical production of pharmaceutical compounds, offering a promising solution for the development of new antimicrobial agents.
Author Contributions
U.M.d.B.L.L.: Collection of the information, planning, and drafting of the manuscript. R.d.S.M. and S.S.S.D.: Data curation and formatting of the manuscript. A.F.d.S. and D.M.-R.: Conceptualization, analysis, review and editing. R.F.d.S.A. and G.M.C.-T.: Analysis, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) (Process No. 88887.703430/2022-00), the National Council for Scientific and Technological Development (CNPq) (GMCT Process Nr.312242/2022-4), and the Foundation for the Support of Science and Technology of Pernambuco (FACEPE).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Catholic University of Pernambuco—UNICAP, the Multi-User Center for Analysis and Characterization in Biomolecules and Material Surface (CEMACBIOS), the Federal Rural University of Pernambuco—UFRPE, and the Northeast Biotechnology Network—RENORBIO for providing a suitable environment for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.M.; Cook, G.A. Chapter 33—Medicinal Chemistry and Pharmacology of Statins. In Cholesterol; Bukiya, A.N., Dopico, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 903–926. ISBN 978-0-323-85857-1. [Google Scholar]
- Sadowska, A.; Osiński, P.; Roztocka, A.; Kaczmarz-Chojnacka, K.; Zapora, E.; Sawicka, D.; Car, H. Statins—From Fungi to Pharmacy. Int. J. Mol. Sci. 2024, 25, 466. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Davi, K.G. Bioactive Metabolites from Fungi with Anti-Inflammatory and Antithrombotic Properties: Current Status and Future Perspectives for Drug Development. In Fungi Bioactive Metabolites: Integration of Pharmaceutical Applications; Deshmukh, S.K., Takahashi, J.A., Saxena, S., Eds.; Springer Nature: Singapore, 2024; pp. 427–494. ISBN 978-981-9956-96-8. [Google Scholar]
- Cebrián, M.; Ibarruri, J. 9-Filamentous Fungi Processing by Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Taherzadeh, M.J., Ferreira, J.A., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 251–292. ISBN 978-0-323-91872-5. [Google Scholar]
- Huang, X.; Men, P.; Tang, S.; Lu, X. Aspergillus terreus as an Industrial Filamentous Fungus for Pharmaceutical Biotechnology. Curr. Opin. Biotechnol. 2021, 69, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Asgher, M.; Hussain, F.; Bhatti, H.N. Optimization of Process Variables for Hyper-Production of Lovastatin from Wild Type Aspergillus terreus and Its Efficacy Studies Optimización de Variables de Proceso Para La Hiperproducción de Lovastatina a Partir de Aspergillus terreus Silvestre y Estudios de Su Eficacia. Rev. Mex. Ing. Química 2020, 19, 929–939. [Google Scholar]
- Mouafi, F.E.; Ibrahim, G.S.; Abo Elsoud, M.M. Optimization of Lovastatin Production from Aspergillus Fumigatus. J. Genet. Eng. Biotechnol. 2016, 14, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Suwannarat, S.; Iewkittayakorn, J.; Sukpondma, Y.; Rukachaisirikul, V.; Phongpaichit, S.; Chotigeat, W. Optimization of the Production of Lovastatin from Aspergillus Sclerotiorum PSU-RSPG178 under Static Liquid Culture Using Response Surface Methodology. JSM 2019, 48, 93–102. [Google Scholar] [CrossRef]
- Tavares, M.T.; Primi, M.C.; Carvalho, C.F.d.; Polli, M.C.; Parise-Filho, R. Entendendo o processo químico de bioativação da sinvastatina por métodos experimentais e computacionais: Uma aula prática. Quím. Nova 2016, 39, 502–506. [Google Scholar] [CrossRef]
- Gupta, K.; Mishra, P.K.; Srivastava, P. Enhanced Continuous Production of Lovastatin Using Pellets and Siran Supported Growth of Aspergillus terreus in an Airlift Reactor. Biotechnol. Bioprocess Eng. 2009, 14, 207–212. [Google Scholar] [CrossRef]
- Dhar, R. Studies on Process Parameters for Production of Lovastatin. AJBPS 2015, 05, 30–35. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kumar, P.M.; Sarnaik, H.M.; Sadhukhan, A.K. A Rapid Technique for Screening of Lovastatin-Producing Strains of Aspergillus terreus by Agar Plug and Neurospora crassa Bioassay. J. Microbiol. Methods 2000, 40, 99–104. [Google Scholar] [CrossRef]
- Vilches Ferrón, M.A.; Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Chisti, Y. Rapid Screening of Aspergillus terreus Mutants for Overproduction of Lovastatin. World J. Microbiol. Biotechnol. 2005, 21, 123–125. [Google Scholar] [CrossRef]
- Lima, W.G.; Alves-Nascimento, L.A.; Andrade, J.T.; Vieira, L.; de Azambuja Ribeiro, R.I.M.; Thomé, R.G.; dos Santos, H.B.; Ferreira, J.M.S.; Soares, A.C. Are the Statins Promising Antifungal Agents against Invasive Candidiasis? Biomed. Pharmacother. 2019, 111, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, J.; Naskrent, M.; Grobelny, P. Photochemical Properties of Simvastatin and Lovastatin Induced by Radiation. J. Therm. Anal. Calorim. 2009, 96, 301–305. [Google Scholar] [CrossRef]
- Prasanna Latha, D.; Hemalatha, K.P.J. Production of Lovastatin by Aspergillus Fischeri NCIM 509 Using Barley Bran, Wheat Husk, Rice Bran and Rice Husk under Solid State Fermentation. Eur. J. Exp. Biol. 2015, 5, 8–17. [Google Scholar]
- Bizukojc, M.; Pecyna, M. Lovastatin and (+)-Geodin Formation by Aspergillus terreus ATCC 20542 in a Batch Culture with the Simultaneous Use of Lactose and Glycerol as Carbon Sources. Eng. Life Sci. 2011, 11, 272–282. [Google Scholar] [CrossRef]
- Jaivel, N.; Marimuthu, P. Optimization of Lovastatin Production in Solid State Fermentation by Aspergillus terreus. Int. J. Eng. Sci. Technol. 2010, 2, 2730–2733. [Google Scholar]
- Swain, R.; Pendela, S.; Panda, S. Formulation and Evaluation of Gastro-Bilayer Floating Tablets of Simvastatin as Immediate Release Layer and Atenolol as Sustained Release Layer. Indian J. Pharm. Sci. 2016, 78, 458–468. [Google Scholar] [CrossRef]
- Huang, X.; Liang, Y.; Yang, Y.; Lu, X. Single-Step Production of the Simvastatin Precursor Monacolin J by Engineering of an Industrial Strain of Aspergillus terreus. Metab. Eng. 2017, 42, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Thangavelu, K.; Uthandi, S. Lovastatin Production by an Oleaginous Fungus, Aspergillus terreus KPR12 Using Sago Processing Wastewater (SWW). Microb. Cell Fact. 2022, 21, 22. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Cao, X. Effects of Carbon Sources on Fungal Morphology and Lovastatin Biosynthesis by Submerged Cultivation of Aspergillus terreus. Asia-Pac. J. Chem. Eng. 2009, 4, 672–677. [Google Scholar] [CrossRef]
- Kapoor, S.; Sood, H.; Saxena, S.; Chaurasia, O.P. Green Synthesis of Silver Nanoparticles Using Rhodiola Imbricata and Withania Somnifera Root Extract and Their Potential Catalytic, Antioxidant, Cytotoxic and Growth-Promoting Activities. Bioprocess. Biosyst. Eng. 2022, 45, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.H.A.; Harith, H.H.; Montoya, A.; Abbas, A. Growth and Lovastatin Production by Aspergillus terreus under Different Carbohyrates as Carbon Sources. Biocatal. Agric. Biotechnol. 2017, 10, 379–385. [Google Scholar] [CrossRef]
- Kamath, P.V.; Dwarakanath, B.S.; Chaudhary, A.; Janakiraman, S. Optimization of Culture Conditions for Maximal Lovastatin Production by Aspergillus terreus (KM017963) under Solid State Fermentation. HAYATI J. Biosci. 2015, 22, 174–180. [Google Scholar] [CrossRef][Green Version]
- Ramadan, A.M.A.A.; Shehata, R.M.; EL-Sheikh, H.H.; Ameen, F.; Stephenson, S.L.; Zidan, S.A.H.; Al-Bedak, O.A.M. Exploitation of Sugarcane Bagasse and Environmentally Sustainable Production, Purification, Characterization, and Application of Lovastatin by Aspergillus terreus AUMC 15760 under Solid-State Conditions. Molecules 2023, 28, 4048. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.Z.; Tahir, S.F.; Mukhtar, H. Isolation and screening of lovastatin producing fungi. Int. J. Biol. Biotechnol. 2022, 19, 183–187. [Google Scholar]
- Al-Saman, M.A.; Helmy, M.A.; Abdella, A.; Wilkins, M.R.; El Khalik Gobba, N.A.; Mahrous, H. Optimization of Lovastatin Production by Aspergillus terreus ATCC 10020 Using Solid-State Fermentation and Its Pharmacological Applications. Biocatal. Agric. Biotechnol. 2021, 31, 101906. [Google Scholar] [CrossRef]
- Balraj, J.; Murugesan, T.; Dhanapal, A.R.; Kalieswaran, V.; Jairaman, K.; Archunan, G.; Jayaraman, A. Bioconversion of Lovastatin to Simvastatin by Streptomyces Carpaticus toward the Inhibition of HMG-CoA Activity. Biotechnol. Appl. Biochem. 2023, 70, 1162–1175. [Google Scholar] [CrossRef] [PubMed]
- Polonini, H.C.; Lima, L.L.; Gonçalves, K.M.; do Carmo, A.M.R.; da Silva, A.D.; Raposo, N.R.B. Photoprotective Activity of Resveratrol Analogues. Bioorg. Med. Chem. 2013, 21, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Gulyamova, T.G.; Ruzieva, D.M.; Nasmetova, S.M.; Sattarova, R.S.; Lobanova, K.V.; Abdulmyanova, L.A.; Rasulova, G.A. Lovastatin Production by Aspergillus terreus in Solid State and Submerged Fermentations. Int. J. Eng. Sci. Technol. 2013, 5, 19–24. [Google Scholar] [CrossRef]
- Nigam, V.K. Screening of Different Fungi for Production of Lovastatin. Asian J. Biomed. Pharm. Sci. 2015, 5, 24. [Google Scholar]
- Javed, S.; Meraj, M.; Mahmood, S.; Hameed, A.; Naz, F.; Hassan, S.; Irfan, R. Biosynthesis of Lovastatin Using Agro-Industrial Wastes as Carrier Substrates. Trop. J. Pharm. Res. 2017, 16, 263–269. [Google Scholar] [CrossRef]
- Gyetvai, Á.; Emri, T.; Takács, K.; Dergez, T.; Fekete, A.; Pesti, M.; Pócsi, I.; Lenkey, B. Lovastatin Possesses a Fungistatic Effect against Candida Albicans, but Does Not Trigger Apoptosis in This Opportunistic Human Pathogen. FEMS Yeast Res. 2006, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Hörner, R. Repositioning of Non-Antibiotic Drugs as an Alternative to Microbial Resistance: A Systematic Review. Int. J. Antimicrob. Agents 2021, 58, 106380. [Google Scholar] [CrossRef] [PubMed]
- Graziano, T.S.; Cuzzullin, M.C.; Franco, G.C.; Schwartz-Filho, H.O.; Andrade, E.D.d.; Groppo, F.C.; Cogo-Müller, K. Statins and Antimicrobial Effects: Simvastatin as a Potential Drug against Staphylococcus Aureus Biofilm. PLoS ONE 2015, 10, e0128098. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Divya, K.R. An in Vitro. Comparative Study on the Antioxidant Activity and Determination of Antibacterial Potential of Atorvastatin and Simvastatin. Pharm. Biol. 2007, 45, 683–687. [Google Scholar] [CrossRef]
- Barbarossa, A.; Rosato, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carrieri, A.; Carocci, A. Non-Antibiotic Drug Repositioning as an Alternative Antimicrobial Approach. Antibiotics 2022, 11, 816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).