Abstract
Composting emerges as an effective strategy to eliminate antibiotics and antibiotic resistance genes (ARGs) in animal manure. In this study, chicken manure with the addition of wheat straw and sawdust was used as composting raw materials, and different concentrations of norfloxacin were added to investigate its effects on physicochemical properties, bacterial community, and ARGs during the composting process. Results show that the presence of norfloxacin has obvious effects on the composting physicochemical properties and germination index (GI). A high concentration of norfloxacin influences the succession direction of the bacterial community and promotes the transfers of gyrA, gyrB, parC, qepA, and qnrB. The composting physicochemical properties alter bacterial communities and further influence the fate of ARGs. These results suggest that meticulous management of antibiotic usage and compost conditions are vital strategies for mitigating the influx of antibiotics and ARGs into the environment, both at the source and on the path.
1. Introduction
The emergence of antibiotic residues in the environment has aroused significant concern from the scientific community and the public owing to the uncontrolled use of antibiotics against bacterial infections and livestock production [1,2]. The various antibiotics have been ubiquitously detected in different environmental media, such as the aquatic environment [3,4] and the soil [5,6], where the abundant groups usually are sulfonamides, tetracyclines, fluoroquinolones, macrolides, β-lactams, and aminoglycosides. Due to their high antibacterial activity against both Gram-negative and Gram-positive bacteria through inhibition of DNA gyrase, fluoroquinolones (FQs), one of the most popular antibiotics, are used to control diseases in humans and animals [7,8]. In China, the total usage of FQs was estimated to be approximately 25,500 tons in 2013, and about 87% accounted for animals, mainly pigs and chickens [9]. When used in veterinary medicine to treat bacterial infections in chickens, most of the FQs are excreted unchanged or partially metabolized, ending up in the manure. Chicken manure is commonly used as a valuable organic fertilizer due to its rich content of essential plant nutrients such as nitrogen, phosphorus, and potassium. However, the use of chicken manure as a fertilizer can inadvertently introduce antibiotics, such as norfloxacin, into the environment if the chickens have been treated with antibiotics. Some studies have predicted that the highest concentrations of FQs in soil and sediment range from μg/kg to a few mg/kg [9,10]. It has been found that the presence of antibiotics can contribute to the selection pressure for antibiotic-resistant bacteria [11,12] and promote the spread of antibiotic resistance genes (ARGs) [13,14], threatening the ecological environment and human health [15,16,17]. Thus, it is necessary to efficiently remove antibiotics from chicken manure.
Aerobic composting is an environment-friendly approach for achieving high-efficiency biodegradation of organic solid wastes while simultaneously reducing the level of persistent organic pollutants like antibiotics [18]. Many studies presented that the reduction in antibiotics was associated with a series of composting parameters, such as temperature, moisture, oxygen content, carbon/nitrogen (C/N), and raw materials [19,20,21]. Later, the accumulating studies focused on the fate of antibiotics and resistance genes under controlled composting conditions. For example, Liao et al. (2019) [22] suggested that using hyperthermophilic composting could efficiently reduce antibiotic residues and associated resistance genes in tylosin fermentation waste. Tong et al. (2023) [23] observed the effect of biochar with different particle sizes on the fate of antibiotics during swine manure composting. Wei et al. (2024) [24] tried to accelerate the elimination of antibiotics by adding exogenously antibiotic-degrading bacteria. However, controlling composting conditions means high investment and advanced technology, which may encounter more challenges in actual production. Thus, some publications transferred more attention to the efficiency assessment of routine composting with the different residue levels of antibiotics [25], contributing to guiding the rational use of antibiotics in the livestock industry. Previous studies have indicated that some types of antibiotics, including chlortetracycline, oxytetracycline, roxithromycin, enrofloxacin, and ciprofloxacin, with increasing concentrations, would affect the duration of the thermophilic phase, organic matter degradation, and bacterial community succession in composting [12,26]. However, no information is given in the literature on norfloxacin, one of the top five antibiotics used in China [9].
According to the above, the objectives of this study were: (1) to investigate the degradation of different concentrations of norfloxacin during chicken manure; (2) to assess the effects of different concentrations of norfloxacin on the physiochemical properties and microbial communities; and (3) to evaluate the correlations among antibiotic resistance genes, norfloxacin, bacterial composition, and physicochemical properties.
2. Materials and Methods
2.1. Composting Raw Materials
Norfloxacin (NOR, 98%), purchased from J&K (Beijing, China), has the chemical formula C16H18FN3O3 and works by inhibiting bacterial DNA gyrase and topoisomerase IV, enzymes essential for the replication, transcription, and repair of bacterial DNA. The fresh chicken manure was collected from a chicken farm belonging to the Liuminying ecological farm located in the Daxing District of Beijing. The initial carbon/nitrogen (C/N) ratio was adjusted to ~25 by adding wheat straw and sawdust (1:4 dry weight) cut into 2 cm long pieces. The compost raw materials were analyzed for moisture content (MC), pH, total organic carbon (TOC), and total nitrogen (TN) (Table 1).

Table 1.
Selected physicochemical properties of compost raw materials.
2.2. Experimental Design
To investigate the variation in norfloxacin with different residue levels during chicken manure, six composting treatments were designed: (1) chicken manure + wheat straw + sawdust (NOR0); (2) chicken manure + wheat straw + sawdust + 0.6 mg kg−1 NOR (NOR0.6); (3) chicken manure + wheat straw + sawdust + 25 mg kg−1 norfloxacin (NOR25); (4) chicken manure + wheat straw + sawdust + 50 mg kg−1 norfloxacin (NOR50); (5) chicken manure + wheat straw + sawdust + 75 mg kg−1 norfloxacin (NOR75); (6) chicken manure + wheat straw + sawdust + 100 mg kg−1 norfloxacin (NOR100). The raw materials, including chicken manure, wheat straw, and sawdust at the ratio of 4:1:4 calculated by dry weight, were thoroughly mixed manually and spiked with norfloxacin at five concentration levels (0.6, 25, 50, 75, and 100 mg kg−1), and the control treatment was prepared without the addition of norfloxacin. According to the composting operation developed by our research group [24], the moisture content was adjusted to ~60%. Each of the composting mixtures received 60 kg and was incubated for 45 days in a foam insulation box. During the incubation period, the compost temperature was monitored daily, and air was supplied using manual pile-turning. The compost pile was turned once every two days when the inner temperature was over 50 °C and twice every week when below 50 °C. Sampling events occurred on days 1, 3, 7, 14, 21, 28, 35, and 45, where approximately 300 g of sample was withdrawn from each composter. All experimental treatments were performed in triplicate using three composters operating simultaneously.
2.3. Physicochemical Parameters Analysis and Germination Index
A water extract of the compost sample (1:5 w/v sample-water ratio) after shaking equilibration for approximately 30 min was used for the determination of pH with a pH meter (OHAUS, Shanghai, China) [27]. The moisture content of the samples was determined by the weight loss of a fresh sample after drying at 105 °C in an oven for 8 h [28]. The TOC and TN content have been studied using the potassium dichromate external heating method and the Kjeldahl method [28]. Seed germination tests with Chinese wheat cultivar Zhongmai 175 were used to evaluate the phytotoxicity of the compost. The germination index (GI) was calculated based on Formula (1). Three replicates were conducted for each sample (including the distilled water control).
2.4. Extraction and Analysis of NOR
The residual norfloxacin in the compost was extracted and analyzed according to the description by Feng et al. (2016) [29]: a 1 g lyophilized sample was successively extracted with 20 mL of EDTA-McIlvaine buffer and 10 mL of a solution with methanol, acetonitrile, and acetone at a volume ratio of 2:2:1. The supernatant was degassed with 10 mL of n-hexane through a 0.45 μm filter and concentrated by a rotary evaporator. Then, the extracts passed through Oasis HLB cartridges preconditioned with 5 mL of methanol and 10 mL of DI water. The norfloxacin-loaded cartridge was then washed with 5 mL of a methanol/water solution (25:75, v/v). The elution solution was washed with 10 mL of methanol/water solution (65:35, v/v) and then evaporated to near dryness at 40 °C. The analyte was redissolved in 1 mL of acetonitrile with ultrapure water (20:80, v/v) for HPLC analysis. The HPLC analysis (2695, Waters, Milford, MA, USA) was performed with a Waters 2998 Photo-Diode Array (PDA) detector. The chromatographic separation was carried out with the use of a Waters Atlantis® T3 column (150 × 4.6 mm, 3 μm) at 40 °C. The flow rate was 1.0 mL min−1, the injection volume was 10 μL, and the detection wavelength was 274 nm. The mobile phases A and B were 0.1% formic acid in water (A) and acetonitrile (B), respectively. The mobile phase gradient elution procedure took 35 min, as follows: 0–20 min, 90–80% A; 20–24 min, 80% A; 24–25 min, 80–40% A; 25–31 min, 40% A; 31–32 min, 40–90% A; 32–35 min, 90% A.
The removal percentage (R) of the calculation was calculated using the following Formula (2):
where C0 was the initial concentration of norfloxacin in the compost and Cn was the concentration of norfloxacin in the compost on day n.
R (%) = (Cn − C0) C0−1 × 100,
2.5. DNA Extraction and High-Throughput Sequencing Analysis
The total DNA from a 0.5 g composting sample was extracted using a soil DNA extraction kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s protocol. The purified DNA was detected for quality using a NanoDrop NC2000 Qubit fluorometer (Thermo Scientific, Waltham, MA, USA). The V3-V4 hypervariable regions of the 16S rRNA gene were amplified using bacterial primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). A total of 50-μL PCR mixtures contained 1 μL of template DNA, 2 μL of each primer (10 μM), 4 μL of dNTP mixture, 5 μL of 10 × buffer, 0.3 μL of DNA polymerase (2.5 U/μL), and 35.7 μL ddH2O. The PCR cycling program was the following: initial denaturation at 95 °C for 5 min, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, extension at 72 °C for 40 s, and then final extension at 72 °C for 10 min [30]. The negative control reactions, containing all components except for the template, were performed. PCR products were determined using 2% agarose gels and quantified with the NanoDrop NC2000 Qubit, and then they were submitted to Beijing Allwegene Company (Beijing, China) for paired-end sequencing on the Illumina platform.
Further sequencing was performed using the software QIIME 2 2019.4 [31]. To clean the reads, the raw paired-end reads were optimized by removing low-quality reads, primers, and ambiguous base calls. Then, operation taxonomy units (OTUs) were clustered at a 0.97 similar level, and a sequence represented for each OTU was classified to a taxonomic identity, indicating community composition in each sample [32]. Shannon and Chao 1 indices were calculated using Mothur to estimate bacterial diversity and richness, respectively [33].
2.6. ARGs Analysis
Seven fluoroquinolone resistance genes (gyrA, gyrB, parC, qepA, qnrA, qnrB, and qnrS) and 16S rRNA were quantified by qPCR. The qPCR reaction mixture (10 μL) comprised 0.5 μL of DNA sample, 0.75 μL of primer F and primer R, 5 μL of Roche FastStart Universal SYBR Green Master (ROX), and 3 μL of ddH2O. The qPCR conditions comprised an initial hold for 10 min at 95 °C, followed by 40 cycles for 30 s at 95 °C, 40 cycles for 30 s at 60 °C, and then 10 min at 72 °C. The qPCR was performed using an ABI VIIA TM 7 Real-Time PCR system (Applied Biosystems, Life Technologies, Foster City, CA, USA). A threshold cycle (Ct) < 31 and an amplifying efficiency range of 80–120% in technical triplicates for each sample were regarded as positive amplifications, and the average Ct values from the technical triplicates were used for further calculations [28,30]. The relative copy number of ARGs was calculated by Formula (3).
where Ct(ARG) was the threshold cycle of ARG; Ct(16S) was the threshold cycle of 16S rDNA.
2.7. Statistical Analysis
The data were processed using Excel 2019 (Microsoft, Redmond, WA, USA). SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) was used to analyze the significant differences and correlations. The figures were presented using Origin 2024 (OriginLab, Hampton, MA, USA) and Canoco version 5.0.
3. Results and Discussion
3.1. Variations in Physicochemical Properties of Composting
Temperature is one of the most important parameters indicating compost maturity and has a direct influence on microbial activity during composting [28]. During composting, the ambient temperature ranged from 3 °C to 12 °C. Due to the quick establishment of microbial activities, the temperature of all treatments sharply rose above 50 °C on day 3 and then entered the thermophilic phase (Figure 1a). The pile temperature of six treatments peaked on days 4, 8, 5, 6, 6, and 4 of composting, respectively, reaching 64.4 °C (NOR0), 59.1 °C (NOR0.6), 63.0 °C (NOR25), 62.2 °C (NOR50), 63.9 °C (NOR75), and 68.5 °C (NOR100). The temperature of all treatments above 50 °C lasted for 14-16 days, which basically destroyed all potential pathogens. At the same time, the duration of high temperatures above 55 °C for each treatment had an obvious difference. Specifically, the pile temperature of the treatments with low concentrations of norfloxacin (NOR0.6 and NOR 25) remained above 55 °C for 7 days as the control treatment (NOR0), and the three treatments with higher concentrations of norfloxacin (NOR50, NOR75, and NOR100) kept above 55 °C for 10, 10, and 9 days, respectively. This can potentially be attributed to the presence of a diverse microbial population in the treatments with the higher concentration of norfloxacin, which swiftly metabolized the readily biodegradable organic compounds, consequently producing a significant amount of heat [34]. Then, the temperature of five treatments except NOR100 started to drop, tending daily to that of the ambiance, while the temperature of NOR100 was gradually close to that of the ambiance at day 35 of composting. This phenomenon indicated that the occurrence of norfloxacin with elevated concentration hastened microbial activities at the thermophilic phase, which is different from previous studies in that the addition of norfloxacin did not influence the temperature profiles of the composting [35]. This disparity may be related to the composition of compost raw materials, which requires further study.
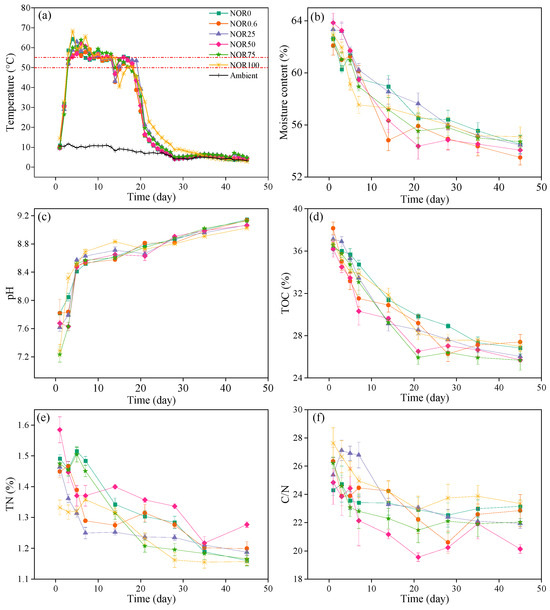
Figure 1.
Dynamic changes in the temperature (a), moisture content (b), pH (c), TOC content (d), TN content (e), and C/N (f) during the composting process. Results are the mean of three replicates; bars indicate the standard error. TOC: total organic carbon; TN: total nitrogen; NOR0: no norfloxacin; NOR0.6: spiked with 0.6 mg kg−1 of norfloxacin; NOR25: spiked with 25 mg kg−1 of norfloxacin; NOR50: spiked with 50 mg kg−1 of norfloxacin; NOR75: spiked with 75 mg kg−1 of norfloxacin; NOR100: spiked with 100 mg kg−1 of norfloxacin, similarly hereinafter.
High temperatures and aeration accelerate the evaporation of moisture. Figure 1b shows the change in moisture content during chicken composting. The moisture content rapidly decreased at the thermophilic phase with continuously dramatic microbial activity and decomposition of organic matter. At day 14 of composting, the depletion rate of moisture content in all treatments ranged from 3.70 to 7.51%. The addition of norfloxacin accelerated moisture evaporation at high temperatures. By the end of the composting, the moisture content had dropped to 54.51%, 53.51%, 54.45%, 54.07%, 54.72%, and 55.11%, respectively. There was no obvious difference in the moisture content among the six treatments, which was consistent with Chen et al. (2020) [35]. The pH of all treatments presented as slightly alkaline, ranging from 7.23 to 7.82 at the initial stage of composting, and kept increasing during the entire process (Figure 1c). The pH increased sharply to above 8.4 in the first 5 days due to the decomposition of organic nitrogen compounds and the production of NH4+-N [18]. The treatments with higher concentrations of norfloxacin possessed a faster ascent pH, which was probably because the presence of norfloxacin promoted the decomposition of organic nitrogen and the formation of NH4+-N to a certain extent [36].
The microbial activity for the decomposition of organic matter requires constant consumption of carbon and nitrogen sources. The TOC and TN contents showed decreasing trends during the whole process of composting (Figure 1d,e), converting into the forms of CO2 and NH3. In this study, the TOC content showed a sharp decrease during the first 21 days and then a slow downward trend after day 21 of composting, which was also observed by the previous studies [24,37]. It was also found that the TOC in three treatments with higher concentrations of NOR (NOR50, NOR75, and NOR100) dropped more markedly than the other three treatments during days 7–21 of composting, which was probably associated with the duration of high temperature (Figure 1a). High temperatures implied strong microbial activity and the rapid decomposition of organic matter. Compared with the initial stage, the TOC content of each treatment decreased by 25.90% (NOR0), 28.20% (NOR0.6), 29.86% (NOR25), 28.94% (NOR50), 29.77% (NOR75), and 26.65% (NOR100) at the end of composting. The change in TN content had a slight difference from the TOC. The TN content of the piles dropped by 22.23% (NOR0), 17.23% (NOR0.6), 18.83% (NOR25), 19.42% (NOR50), 20.95% (NOR75), and 13.16% (NOR100) by the end of composting. The reduction rate of the TN in treatments with norfloxacin was lower than that of the control group, which was probably because the presence of norfloxacin inhibited the volatilization of NH4+-N in the form of NH3 and promoted its conversion to NO3−-N [38]. As shown in Figure 1f, the pile C/N ratio decreased overall during the composting. At the end of composting, the C/N of all treatments was in the suitable range of 20–23 for maturity assessment [19,32], and it decreased by 4.73% (NOR0), 13.25% (NOR0.6), 13.59% (NOR25), 18.91% (NOR50), 16.00% (NOR75), and 15.54% (NOR100). Compared to the control treatment, the norfloxacin-adding treatments showed a significant decline in the C/N since the effective microbial activity required more abundant carbon energy.
3.2. Change in Germination Index during Composting
The GI is a sensitive indicator that can reflect the maturity and phytotoxicity of composting by observing the effects of compost products on seed germination rate [39]. The GI of each treatment showed a trend of unabiding decrease and then continuous increase (Figure 2). The piles of all treatments possessed high biotoxicity, with GIs of 44.70% (NOR0), 44.47% (NOR0.6), 37.97% (NOR25), 43.02% (NOR50), 36.21% (NOR75), and 32.25% (NOR100). During the first three days of composting, the GI showed a more obvious decrease in the treatment containing the higher concentration of norfloxacin. There may be two reasons for this phenomenon. One is that norfloxacin itself aggravates the biological toxicity of the compost, and the other is that norfloxacin mobilizes microbial activity, producing more poisonous substances like NH3, volatile fatty acids (VFAs), and phenols [21]. After the thermophilic stage, the GI of all treatments increased visibly to above 50%. At the end of composting, the GI of each treatment (NOR0, NOR0.6, NOR25, NOR50, NOR75, and NOR100) is 85.27%, 87.87%, 85.49%, 80.38%, 76.47%, and 78.81%, respectively. However, compost products of all treatments satisfied the standard requirement of NY/T 525–2021, which is supposed to have a GI of at least 70%. Just two treatments containing norfloxacin (NOR0.6 and NOR25) had nonsignificantly different GIs from the control, indicating that the low concentration of norfloxacin (≤25 mg kg−1) had no negative influence on the release of phytotoxicity. A similar result was reported by Shi et al. (2016) [40], which also reported that tetracycline at elevated levels of up to 500 mg/kg would hinder the maturity of the feces compost and the release of phytotoxicity.
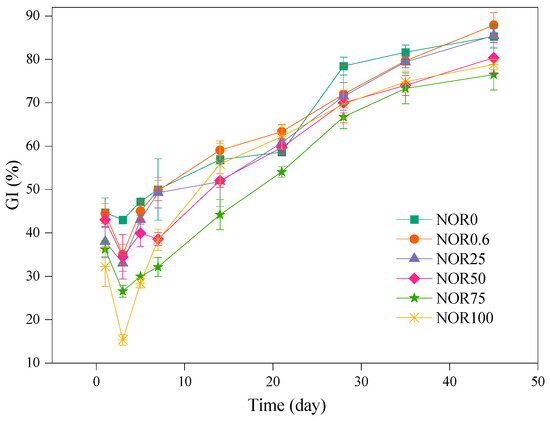
Figure 2.
Dynamic changes in the GI of the compost. Results are the mean of three replicates; bars indicate the standard error.
3.3. Fate of Norfloxacin during Composting
Composting can effectively remove antibiotics such as tetracyclines, sulfonamides, fluoroquinolones, and macrolides from manure [41,42,43]. The variation in norfloxacin in the composting process is illustrated in Figure 3. In consideration of the great contribution of the thermophilic phase to the elimination of antibiotics, we compared the removal efficiency of norfloxacin in different treatments during the first 14 days of composting. Overall, the norfloxacin removal of each treatment increased to a different extent over time. On day 14, the removal rates of norfloxacin in these treatments were 18.01% (NOR0), 28.77% (NOR0.6), 71.84% (NOR25), 70.17% (NOR50), 63.72% (NOR75), and 54.73% (NOR100), respectively. Then, all treatments presented an obvious slowdown in norfloxacin degradation. At the end of composting, norfloxacin in all treatments decreased by 18.01% (NOR0), 28.77% (NOR0.6), 77.86% (NOR25), 80.78% (NOR50), 77.48% (NOR75), and 75.00% (NOR100), respectively. The results showed that norfloxacin in the NOR0 and NOR0.6 treatments was not completely degraded as hypothesized, which probably was because trace norfloxacin would be adsorbed by macromolecular organic matter in the compost [14]. The absorption in compost involves several mechanisms, like sorption, complexation, and co-precipitation, which could negatively influence the biodegradation of antibiotics. Sun et al. (2022) [13] thought the fate of antibiotics was not determined only by high temperature and biodegradation but also by adsorption during the composting process. Meanwhile, the degradation rate of norfloxacin in the other four treatments appeared to have a decrescent tendency with the higher concentrations of norfloxacin. The reason was that the selective pressure of excessive norfloxacin would induce the accelerating spread of resistant bacteria in the compost [44].
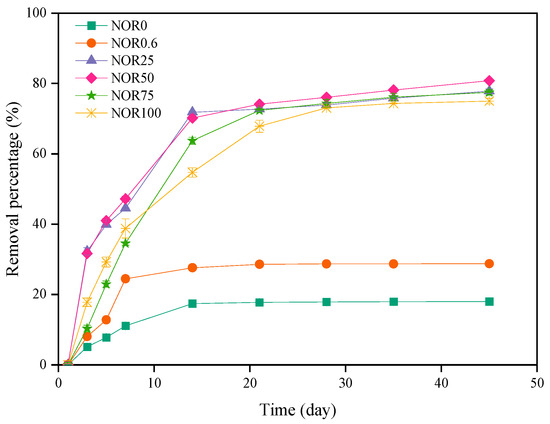
Figure 3.
The fate of NOR throughout the composting process. Results are the mean of three replicates; bars indicate the standard error.
Chen et al. (2021) [42] confirmed that the half-lives of antibiotics had a relationship with different concentrations. Previous studies have also verified that the biodegradation of antibiotics during composting is fitted with a pseudo-first-order kinetic equation [24,45]. Similarly, the degradation of norfloxacin during this composting conformed to the equation as shown in Table 2, with the correlation coefficients (R) between 0.7357 and 0.9396. Among the six treatments, NOR0 and NOR0.6 had lengthy half-lives, reaching 216.56 days and 141.43 days, respectively. A similar phenomenon was also reported by Bourdat-Deschamps et al. (2017) [46], which found that fluoroquinolones usually had the longest half-lives above 200 days compared to other types of common antibiotics. However, the other four treatments (NOR25, NOR50, NOR75, and NOR100) exhibited much shorter half-lives in the range of 20.44–25.96 days, indicating that aerobic composting has a better elimination effect on high concentrations of norfloxacin residues in chicken manure.

Table 2.
Pseudo-first-order kinetic equations for norfloxacin degradation during composting.
3.4. Succession of Bacterial Community during Composting
The composting process, inherently reliant on progressive microbial community dynamics [47], was scrutinized for its response to applied norfloxacin at high concentrations, with a particular focus on shifts within the microbial population structure. Among the five treatments supplemented with norfloxacin, the NOR100 treatment exhibited more pronounced effects on the physicochemical properties of the compost. Consequently, NOR100 treatment was selected for an in-depth analysis to elucidate the differential bacterial community composition compared to the control group.
To evaluate bacterial alpha diversity, the Chao1 and Shannon indexes were utilized to assess bacterial community richness and diversity, respectively [48]. As presented in Figure 4a, the Chao1 and Shannon indexes in two treatments show trends of increasing at first and then slightly decreasing during composting, which is probably related to the availability of nutrients involved in microbial reproduction. In contrast to the control, the NOR100 treatment had lower bacterial richness and diversity at the initial phase of composting but higher richness and diversity than NOR0 at the thermophilic phase. This is because the presence of norfloxacin stimulates the growth and reproduction of thermophilic bacteria to a certain extent. During the whole composting process, there were no statistically significant differences observed in bacterial richness and diversity between the NOR100 and NOR0 treatments, which indicated that norfloxacin had no evident influence on bacterial alpha diversity. A similar result was also observed by Zhao et al. (2022) [49].
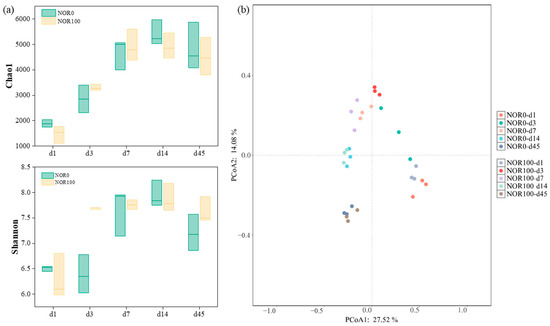
Figure 4.
Alpha diversity, including Chao1 and Shannon indexes (a) and beta diversity (b), of bacterial communities of different treatments.
Principal Coordinate Analysis (PCoA) evaluating beta diversity showed the dynamic succession of bacterial community structure between NOR0 and NOR100 treatments in different composting periods. The PCoA based on Bray–Curtis distance showed that the combined effects of PCO1 and PCO2 explained 41.60% of the total variation in the microbial community species composition. As shown in Figure 4b, distinct temporal successions were observed in bacterial community structures across different composting phases, suggesting that composting significantly altered the composition of the bacterial community in the piles [42]. Comparing NOR0 and NOR100, it was found that the diversity of the bacterial community showed a clear response to high concentrations of norfloxacin on the first week of composting, and there was obvious clustering after 14 days. This result proposes that thermophilic bacteria are critical to the elimination of high concentrations of norfloxacin, and then low concentrations of norfloxacin residue after the thermophilic period have a conservative effect on microbial succession in the piles.
Moreover, the bacterial community structure at the phylum and genus levels during composting is illustrated in Figure 5. The dominant four phyla were Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria (Figure 5a), which accounted for 86.19–99.01% in NOR0 and 92.54–99.42% in NOR100 throughout the composting process. And the top 30 most primary genera in both treatments all belonged to four dominant phyla. These frequently observed phyla are consistently documented as dominant players in the composting process due to their inherent affiliation with the degradation of macromolecular organic matter during composting [13,22,50]. In both treatments, the relative abundance of Firmicutes increased to the peaks of 65% (NOR0) and 81% (NOR100) on day 7 of composting, respectively, and then gradually decreased. The variation for Firmicutes was consistent with the thermophilic period of composting, attributed to their tolerance of high temperatures through spore formation [13,51]. Most of the top 30 genera were members of Firmicutes, in which Exiguobacterium, Tepidimicrobium, Bacillus, and Virgibacillus presented the highest relative abundance on day 7 than on other periods.
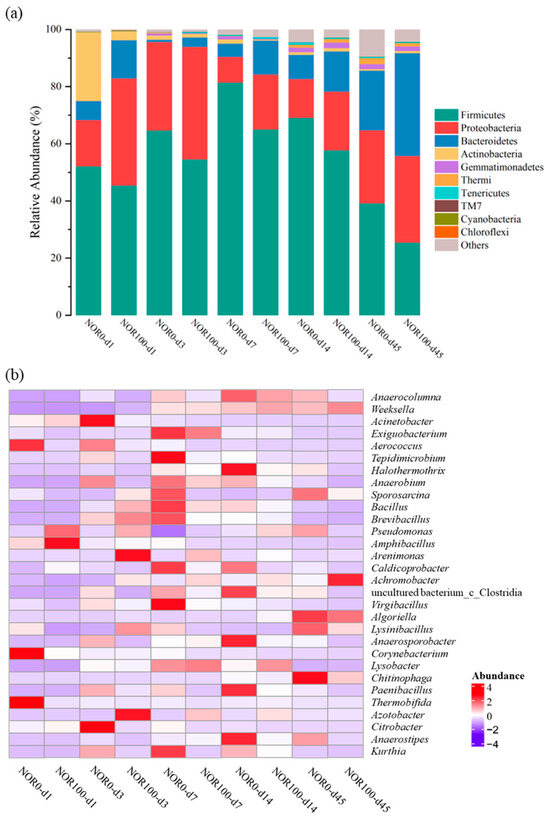
Figure 5.
Bacterial community composition at the phylum (a) and genus (b) level during the composting process. NOR0 and NOR100 represent treatments without norfloxacin and with norfloxacin concentrations of 100 mg kg−1, respectively. And d1, d3, d7, d14, and d45 represent day 1, day 3, day 7, day 14, and day 45 of composting, respectively.
Contrary to the variation in Firmicutes, the relative abundances of Proteobacteria and Bacteroidetes in the two treatments dropped to the minimum on day 7 and then increased. Some evidence suggests that these two phyla play pivotal roles in the degradation of refractory macromolecular substances such as cellulose, which is predominantly active during the later period of composting [42]. As members of these two phyla, the relative abundances of Weeksella, Achromobacter, Algoriella, and Chitinophaga were higher at the end of composting. Significantly, this study displayed that the relative abundance of Actinobacteria gradually decreased in composting processes, different from the previous reports [48,52], in which Actinobacteria was an indicator for composting maturity. This phenomenon was probably explained by the fact that a pH higher than 9.0 inhibited the reproduction of Actinobacteria (Figure S1).
Figure 5 also provided evidence that high concentrations of norfloxacin affected microbial community succession. As shown in Figure 5a, the relative abundance of Proteobacteria and Bacteroidetes in NOR100 treatment was higher than in NOR0 during the whole composting, while Firmicutes showed the opposite trend. Additionally, norfloxacin exerted comparable effects on the top 30 bacterial genera, mirroring the impacts observed at the phylum level to which these genera belong. Through the redundancy analysis (RDA), we found that TN was the most dominant factor affecting the bacterial community among the physicochemical properties, followed by temperature, TOC, moisture content, pH, and GI (Figure S1). The dynamics of physicochemical properties play a pivotal role in the succession of bacterial communities during composting, which might directly or indirectly influence variations in ARGs.
3.5. Behavior of Targeted Fluoroquinolone Resistance Genes and the Relationship with Composting Environmental Factors and Bacterial Communities
The application of antibiotics could lead to the emergence and spread of ARGs. The relative abundances of fluoroquinolone resistance genes (bacterial gyrA, gyrB, parC, qepA, qnrA, qnrB, and qnrS) were determined in the composting process, as illustrated in Table S1. In the initial composting, qepA was observed with the highest relative abundances, followed by gyrB and qnrS. And the other four genes (gyrA, parC, qnrA, and qnrB) had lower relative abundances. In both treatments, these resistance genes varied as composting progressed. Specifically, in the NOR0 treatment, qepA with the highest abundance showed a trend of decreasing first and then increasing, while gyrA showed a reverse trend; the other five genes continuously declined and even were completely removed. The abundance of all targeted ARGs on day 45 was significantly lower than on day 1, which was consistent with previous findings that composting could efficiently eliminate ARGs from animal manure [53,54]. However, in NOR100, most of the ARGs had higher abundance than NOR0 during the same period, apart from qnrA and qnrS, because of horizontal gene transfer and reinforced interconnections among the bacterial community, which collectively facilitated the persistence of ARGs at high antibiotic levels during composting [55].
To further find out the effect of physicochemical properties, norfloxacin, and bacterial community on targeted ARG evolution during composting, a correlation analysis was conducted, as shown in Figure 6. In this study, there were no obvious correlations (p > 0.05) between physicochemical properties (moisture content, pH, TOC, and TN) and most of the targeted ARGs. Only qnrS was positively associated with moisture content (p < 0.05), TOC (p < 0.01), and TN (p < 0.001) and negatively associated with pH (p < 0.01). Meanwhile, qnrS had a significantly positive correlation with Aerococcus (p < 0.001). Meanwhile, the other six ARGs all had significant positive correlations with specific genera. Concretely, gyrA was notably correlated with both Exiguobacterium and Brevibacillus; gyrB, parC, and qepA were associated with Pseudomonas, while qnrA and qnrB were correlated with Arenimonas. It was deduced that Aerococcus, Exiguobacterium, Brevibacillus, Pseudomonas, and Arenimonas were the dominant host bacteria of ARGs. The composting physicochemical properties indirectly influenced the profiles of ARGs by exerting changes on the bacterial community. The ARGs were spread among bacterial communities throughout plasmid-mediated horizontal gene transfer [56,57]. Consequently, as an economically and technically feasible method for resource utilization of animal manure, composting should be ameliorated through meticulous management like the adjustment of moisture content, pH, and C/N, which is vital for ensuring the activities of microorganisms and the efficient elimination of ARGs. Moreover, precautions like the promotion of antibiotic alternatives should be taken to hinder the abuse of antibiotics and prevent antibiotics from entering the environment to reduce the risk of bacteria developing antibiotic resistance.
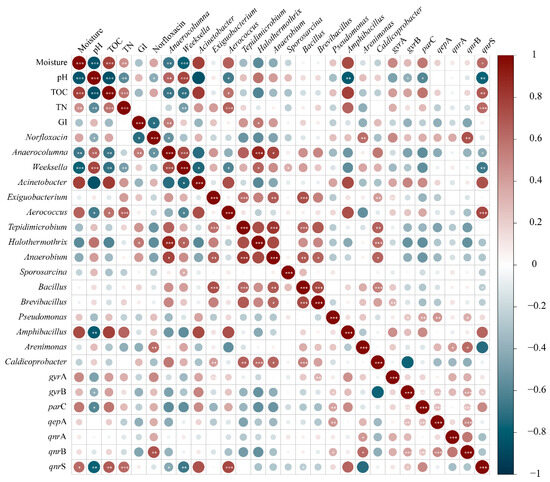
Figure 6.
The correlation heatmap among composting environmental factors, norfloxacin, bacterial genera, and ARGs. “*,**,***” represents that the correlation is significant at the 0.05, 0.01, and 0.001 level, respectively.
4. Conclusions and Future Research Directions
The presence of norfloxacin and affiliated ARGs in animal manure poses a considerable threat to the ecological environment and human health. Composting could eliminate norfloxacin and reduce ARG prevalence. This investigation revealed dynamic variations in norfloxacin levels during composting, with high concentrations being efficiently eliminated and lower concentrations tending towards adsorption by the compost matrix. All the targeted ARGs were reduced after composting. Notably, norfloxacin led to an enrichment of specific ARGs such as gyrA, gyrB, parC, qepA, and qnrB. Firmicutes, Proteobacteria, and Bacteroidetes were the main bacterial phyla in the piles, and norfloxacin significantly affected the succession of the bacterial community. Key bacterial hosts identified for these ARGs included Aerococcus, Exiguobacterium, Brevibacillus, Pseudomonas, and Arenimonas, which were identified as the dominant host bacteria of ARGs. The composting conditions influence the spread of ARGs indirectly by altering bacterial community structure. Therefore, it is important to adjust composting parameters to optimize the efficacy of both antibiotic and ARG removal. In the future, the removal mechanism of ARGs during composting should be further studied.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10070366/s1, Figure S1: Redundancy analysis (RDA) displaying the correlations among composting physicochemical properties, norfloxacin, and bacterial phyla; Table S1: Abundance change in targeted resistance genes in the two treatments during chicken manure composting (n = 3).
Author Contributions
Conceptualization, Z.L. and H.S.; methodology, Y.F. (Yao Feng); software, Y.F. (Yao Feng); validation, Y.F. (Yang Fei); formal analysis, Y.F. (Yao Feng); investigation, Y.F. (Yao Feng); data curation, Y.F. (Yao Feng); writing—original draft preparation, Y.F. (Yao Feng); writing—review and editing, Y.F. (Yang Fei) and Z.L.; visualization, Y.F. (Yao Feng); supervision, H.S. and Z.L.; project administration, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the first/corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Namrata, R.; Sruthi, A.A.; Chandrasekaran, N.; Amitava, M.; Krishnan, K. A comprehensive update on antibiotics as an emerging water pollutant and their removal using nano-structured photocatalysts. J. Environ. Chem. Eng. 2021, 9, 104796. [Google Scholar]
- Shi, J.Y.; Dong, Y.B.; Shi, Y.Y.; Yin, T.T.; He, W.; An, T.Y.; Tang, Y.L.; Hou, X.W.; Chong, S.J.; Chen, D.N.; et al. Groundwater antibiotics and microplastics in a drinking-water source area, northern China: Occurrence, spatial distribution, risk assessment, and correlation. Environ. Res. 2022, 210, 112855. [Google Scholar] [CrossRef] [PubMed]
- Jurado, A.; Margareto, A.; Pujades, E.; Vázquez-Suñé, E.; Diaz-Cruz, M.S. Fate and risk assessment of sulfonamides and metabolites in urban groundwater. Environ. Pollut. 2020, 267, 115480. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, L.; Yen, H.; Zhao, F.K.; Wang, X.M.; Zhou, T.H.; Feng, Q.Y.; Chen, L.D. Occurrence, spatial distribution and ecological risks of antibiotics in soil in urban agglomeration. J. Environ. Sci. 2023, 125, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Nkoh, J.N.; Shang, C.J.; Okeke, E.S.; Ejeromedoghene, O.; Oderinde, O.; Etafo, N.O.; Mgbechidinma, C.L.; Bakare, O.C.; Meugang, E.F. Antibiotics soil-solution chemistry: A review of environmental behavior and uptake and transformation by plants. J. Environ. Manage 2024, 354, 120312. [Google Scholar] [CrossRef] [PubMed]
- Sunidhi, B.; Subhankar, C. Fluoroquinolone antibiotics: Occurrence, mode of action, resistance, environmental detection, and remediation—A comprehensive review. Environ. Pollut. 2022, 315, 120440. [Google Scholar]
- Gbylik-Sikorska, M.; Posyniak, A.; Sniegocki, T.; Zmudzki, J. Liquid chromatography–tandem mass spectrometry multiclass method for the determination of antibiotics residues in water samples from water supply systems in food-producing animal farms. Chemosphere 2015, 119, 8–15. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Wang, R.; Feng, F.; Chai, Y.; Meng, X.S.; Sui, Q.W.; Chen, M.X.; Wei, Y.S.; Qi, K.M. Screening and quantitation of residual antibiotics in two different swine wastewater treatment systems during warm and cold seasons. Sci. Total Environ. 2019, 660, 1542–1554. [Google Scholar] [CrossRef]
- Xiong, W.G.; Sun, Y.X.; Ding, X.Y.; Wang, M.Z.; Zeng, Z.L. Selective pressure of antibiotics on ARGs and bacterial c ommunities in manure-polluted fresh water-sediment microcosms. Front. Microbiol. 2015, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Qian, X.; Gu, J.; Wang, X.J.; Gao, H. Effects of oxytetracycline on the abundance and community structure of nitrogen-fixing bacteria during cattle manure composting. Bioresour. Technol. 2016, 216, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Y.; Liu, B.T.; Imtiaz, A.; Yang, J.; Zhang, B. Composting effect and antibiotic removal under a new temperature control strategy. Waste Manag. 2022, 153, 89–98. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Guo, J.; Sun, H.; Aboagye, D.; Sobhi, M.; Muhmood, A.; Dong, R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021, 333, 125069. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Boonsaner, M.; Hawker, D.W. Transfer of oxytetracycline from swine manure to three different aquatic plants: Implications for human exposure. Chemosphere 2015, 122, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, S.; Chen, Z.; Wen, Q.; Wang, Y. Maturity and security assessment of pilot-scale aerobic co-composting of penicillin fermentation dregs (PFDs) with sewage sludge. Bioresour. Technol. 2016, 204, 185–191. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.X.; Zhang, X.L.; Feng, C.H.; Gao, M.; Shen, Q. Effects of composting process on the dissipation of extractable sulfonamides in swine manure. Bioresour. Technol. 2015, 175, 284–290. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Ok, Y.S.; Park, W.; Lee, D.B.; Kwon, S.I. Decline in extractable antibiotics in manure-based composts during composting. Waste Manage. 2012, 32, 110–116. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, G.Z.; Liu, Y.W.; Cheng, D.M.; Fan, S.H.; Zhao, Q.S.; Xue, J.M.; Zhang, S.Q.; Li, Z.J. The impacts of oxytetracycline on humification during manure composting can be alleviated by initial moisture contents as illustrated by NMR. J Integr. Agr. 2020, 19, 2–13. [Google Scholar] [CrossRef]
- Liao, H.P.; Zhao, Q.; Cui, P.; Chen, Z.; Yu, Z.; Stefan, G.; Ville-Petri, F.; Zhou, S.G. Efficient reduction of antibiotic residues and associated resistance genes in tylosin antibiotic fermentation waste using hyperthermophilic composting. Environ. Int. 2019, 133 Pt B, 105203. [Google Scholar] [CrossRef]
- Tong, Z.Y.; Liu, F.W.; Sun, B.; Tian, Y.; Zhang, J.Z.; Duan, J.Z.; Bi, W.L.; Qin, J.M.; Xu, S.Z. Effect of biochars with different particle sizes on fates of antibiotics and antibiotic resistance genes during composting of swine manure. Bioresour. Technol. 2023, 370, 128542. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.H.; Wang, X.X.; Feng, Y.; Ren, Y.F.; He, J.Y.; Li, Z.J. Dynamics of physicochemical properties, microbial composition, and antibiotic and antibiotic resistance genes during chicken manure composting with strain T4. J. Soils Sediments 2024, 24, 1750–1763. [Google Scholar] [CrossRef]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Felsa, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Ezzariai, A.; Barret, M.; Merlina, G.; Pinelli, E.; Hafidi, M. Evaluation of the antibiotics effects on the physical and chemical parameters during the co-composting of sewage sludge with palm wastes in a bioreactor. Waste Manage. 2017, 68, 388–397. [Google Scholar] [CrossRef]
- Li, C.N.; Li, H.Y.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J.H.; Lan, X.J.; Zhang, Y.C.; Yang, X.M.; et al. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.M.; Feng, Y.; Liu, Y.W.; Xue, J.M.; Li, Z.J. Dynamics of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting. J. Environ. Manage. 2019, 230, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, C.J.; Zhang, W.J.; Liu, Y.W.; Li, Z.J.; Hu, H.Y.; Xue, J.M.; Davis, M. A simple and economic method for simultaneous determination of 11 antibiotics in manure by solid-phase extraction and high-performance liquid chromatography. J. Soils Sediments 2016, 16, 2242–2251. [Google Scholar] [CrossRef]
- Liu, Y.W.; Feng, Y.; Cheng, D.M.; Xue, J.M.; Wakelin, S.A.; Li, Z.J. Dynamics of bacterial composition and the fate of antibiotic resistance genes and mobile genetic elements during the co-composting with gentamicin fermentation residue and lovastatin fermentation residue. Bioresour. Technol. 2018, 261, 249–256. [Google Scholar] [CrossRef]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Fierer, N.; Gonzalez Pena, A.; Goodrich, J.; Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Sun, L.F.; Zhang, Y.F.; Zhang, X.L.; Qiao, J.J. Conversion of spent mushroom substrate to biofertilizer using a stress-tolerant phosphatesolubilizing Pichia farinose FL7. Bioresour. Technol. 2012, 111, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Wu, Y.Q.; Wen, Q.X.; Bao, H.Y.; Fu, Q.Q. Insight into the effects of sulfamethoxazole and norfloxacin on nitrogen transformation functional genes during swine manure composting. Bioresour. Technol. 2020, 297, 122463. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Ying, G.G.; Liu, S.; Zhou, L.J.; Zhao, J.L.; Tao, R.; Peng, P.A. Biological degradation and microbial function effect of norfloxacin in a soil under different conditions. J. Environ. Sci. Health B 2012, 47, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, X.; Cui, P.; Lin, J.; Zeng, J.; Lin, H.; Zhou, S. Nitrification plays a key role in N2O emission in electric-field assisted aerobic composting. Bioresour. Technol. 2020, 297, 122470. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Ma, B.R.; She, Z.L.; Guo, L.; Zhao, Y.G.; Jin, C.J.; Gao, M.C. The presence of norfloxacin inhibited the volatilization of NH4+-N in the form of NH3 and promote its conversion to NO3−-N. Environ. Technol. Inno. 2020, 18, 100726. [Google Scholar] [CrossRef]
- Qu, Y.; Qu, J.; Yan, W.; Yue, T.; Zhang, Q.; Yi, W.; Liu, X.; Sun, Y. Influence of biochar on physico-chemical, microbial community and maturity during biogas residue aerobic composting process. Fermentation 2022, 8, 623. [Google Scholar] [CrossRef]
- Shi, H.L.; Wang, X.C.; Li, Q.; Jiang, S.Q. Effects of elevated tetracycline concentrations on aerobic composting of human feces: Composting behavior and microbial community succession. Indian J. Microbiol. 2018, 58, 423–432. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ma, W.J.; Zhou, K.X.; An, B.Y.; Huo, M.X.; Lin, X.D.; Wang, L.; Wang, H.Y.; Liu, Z.L.; Cheng, G.Y.; et al. Effects of composting on the fate of doxycycline, microbial community, and antibiotic resistance genes in swine manure and broiler manure. Sci. Total Environ. 2022, 832, 155039. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Ye, C.; He, X.; Zhang, S. Fate of antibiotics and antibiotic resistance genes during aerobic co-composting of food waste with sewage sludge. Sci. Total Environ. 2021, 784, 146950. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Liu, W.R.; Zhang, J.N.; Chen, J.; He, L.K.; Zhang, Q.Q.; Ying, G.G. Fate of veterinary antibiotics during animal manure composting. Sci. Total Environ. 2019, 650, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Ezugworie, F.N.; Igbokwe, V.C.; Onwosi, C.O. Proliferation of antibiotic-resistant microorganisms and associated genes during composting: An overview of the potential impacts on public health, management and future. Sci. Total Environ. 2021, 784, 147191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Feng, Y.; Cheng, D.M.; Xue, J.M.; Wakelin, S.A.; Hu, H.Y.; Li, Z.J. Gentamicin degradation and changes in fungal diversity and physicochemical properties during composting of gentamicin production residue. Bioresour. Technol. 2017, 244, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Bourdat-Deschamps, M.; Ferhi, S.; Bernet, N.; Feder, F.; Crouzet, O.; Patureau, D.; Montenach, D.; Moussard, G.D.; Mercier, V.; Benoit, P. Fate and impacts of pharmaceuticals and personal care products after repeated applications of organic waste products in long-term field experiments. Sci. Total Environ. 2017, 607, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Peng, Y.; Ye, C.; Zhang, S. Effects of antibiotics on hydrolase activity and structure of microbial community during aerobic co-composting of food waste with sewage sludge. Bioresour. Technol. 2021, 321, 124506. [Google Scholar] [CrossRef]
- Zhao, C.X.; Xin, L.Q.; Xu, X.K.; Qin, Y.; Wu, W.X. Dynamics of antibiotics and antibiotic resistance genes in four types of kitchen waste composting processes. J. Hazard. Mater. 2022, 424 Pt C, 127526. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, Z.; Zhang, Y. Evolution of physicochemical properties and bacterial community in aerobic composting of swine manure based on a patent compost tray. Bioresour. Technol. 2022, 343, 126136. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Chu, S.; Xu, Y.; Su, Y.; Wu, D.; Xie, B. Short-term biodrying achieves compost maturity and significantly reduces antibiotic resistance genes during semi-continuous food waste composting inoculated with mature compost. J. Hazard. Mater. 2022, 427, 127915. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Lin, C.; Hoang, H.G.; Nguyen, M.T.; Kaewlaoyoong, A.; Cheruiyot, N.K.; Bui, X.T.; Vu, C.T. Biodegradation of dioxin-contaminated soil via composting: Identification and phylogenetic relationship of bacterial communities. Environ. Technol. Innovation 2020, 19, 101023. [Google Scholar] [CrossRef]
- Qian, X.; Sun, W.; Gu, J.; Wang, X.; Zhang, Y.; Duan, M.; Li, H.; Zhang, R. Reducing antibiotic resistance genes, integrons, and pathogens in dairy manure by continuous thermophilic composting. Bioresour. Technol. 2016, 220, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.C.; Qin, H.Y.; Zhang, H.; Luo, Y.H.; Ru, Y.N.; Li, J.R.; Kim, W.S.; Wang, L.S.; Yu, X.N.; Guo, W.H. Variations in antibiotic resistance genes and removal mechanisms induced by C/N ratio of substrate during composting. Sci. Total Environ. 2021, 798, 149288. [Google Scholar] [CrossRef]
- Wen, X.; Chen, M.J.; Ma, B.H.; Xu, J.J.; Zhu, T.; Zou, Y.D.; Liao, X.D.; Wang, Y.; Anja, W.; Wu, Y.B. Removal of antibiotic resistance genes during swine manure composting is strongly impaired by high levels of doxycycline residues. Waste Manag. 2024, 177, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Murase, T.; Ozaki, H. Research Note: Longitudinal monitoring of chicken houses in a commercial layer farm for antimicrobial resistance in Escherichia coli with special reference to plasmid-mediated quinolone resistance. Poultry Sci. 2020, 99, 1150–1155. [Google Scholar] [CrossRef]
- Cui, M.Q.; Zhang, P.; Li, J.Y.; Sun, C.T.; Song, L.; Zhang, C.P.; Zhao, Q.; Wu, C.M. Prevalence and characterization of fluoroquinolone resistant Salmonella isolated from an integrated broiler chicken supply chain. Front. Microbiol. 2019, 10, 1865. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).