Revealing the 2300-Year-Old Fermented Beverage in a Bronze Bottle from Shaanxi, China
Abstract
1. Introduction
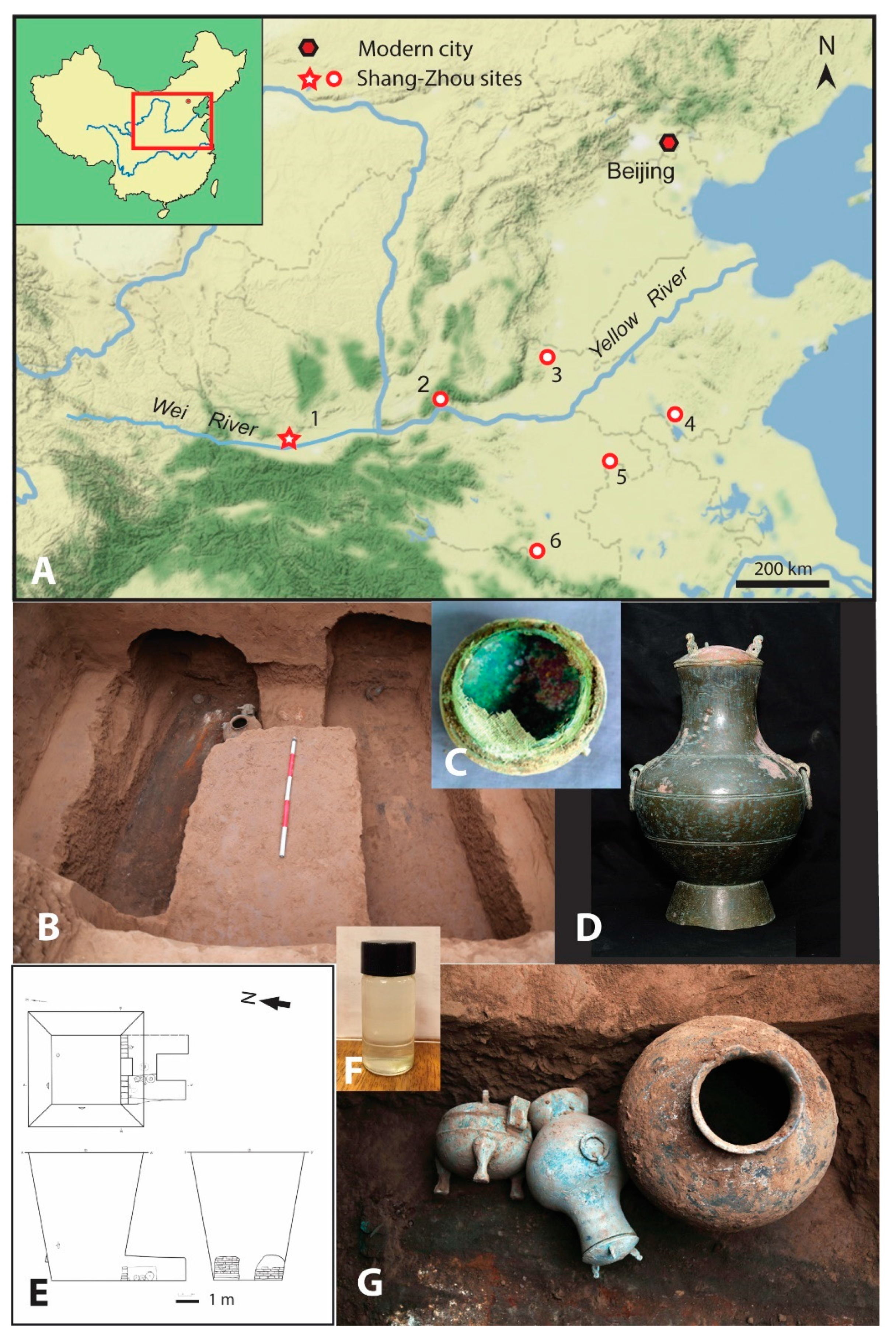
2. Archaeological Materials
3. Research Methods
3.1. Fatty Acid and Amino Acid Tests
3.2. Analyses of the Microfossil Remains
3.3. Identification of Microfossils Based on DNA-Sequenced Modern Samples
3.4. The MS Analyses
3.5. Microfossil Analysis of Control Samples
3.6. Testing pH Values
4. Results
4.1. Fatty Acid and Amino Acid Testing
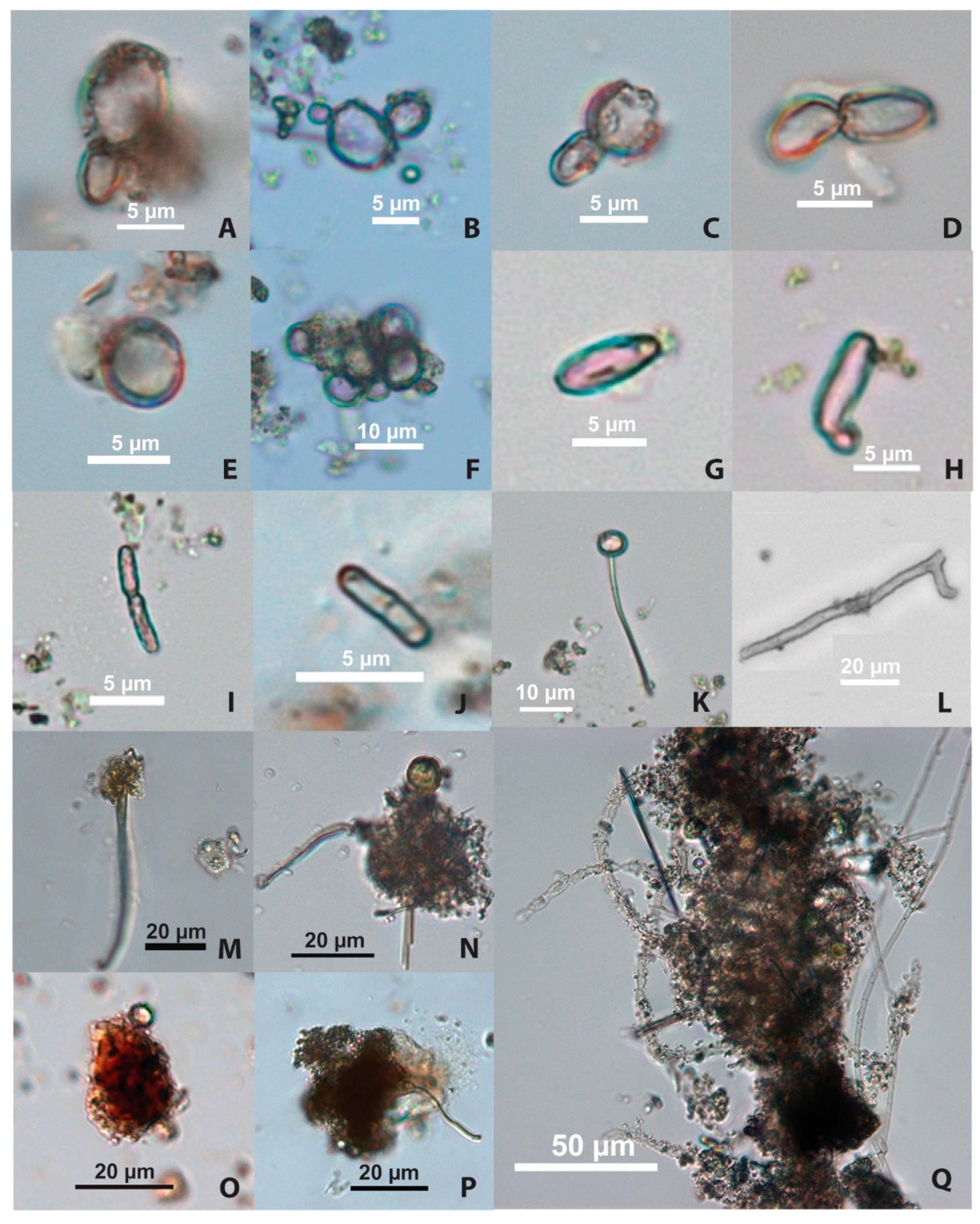
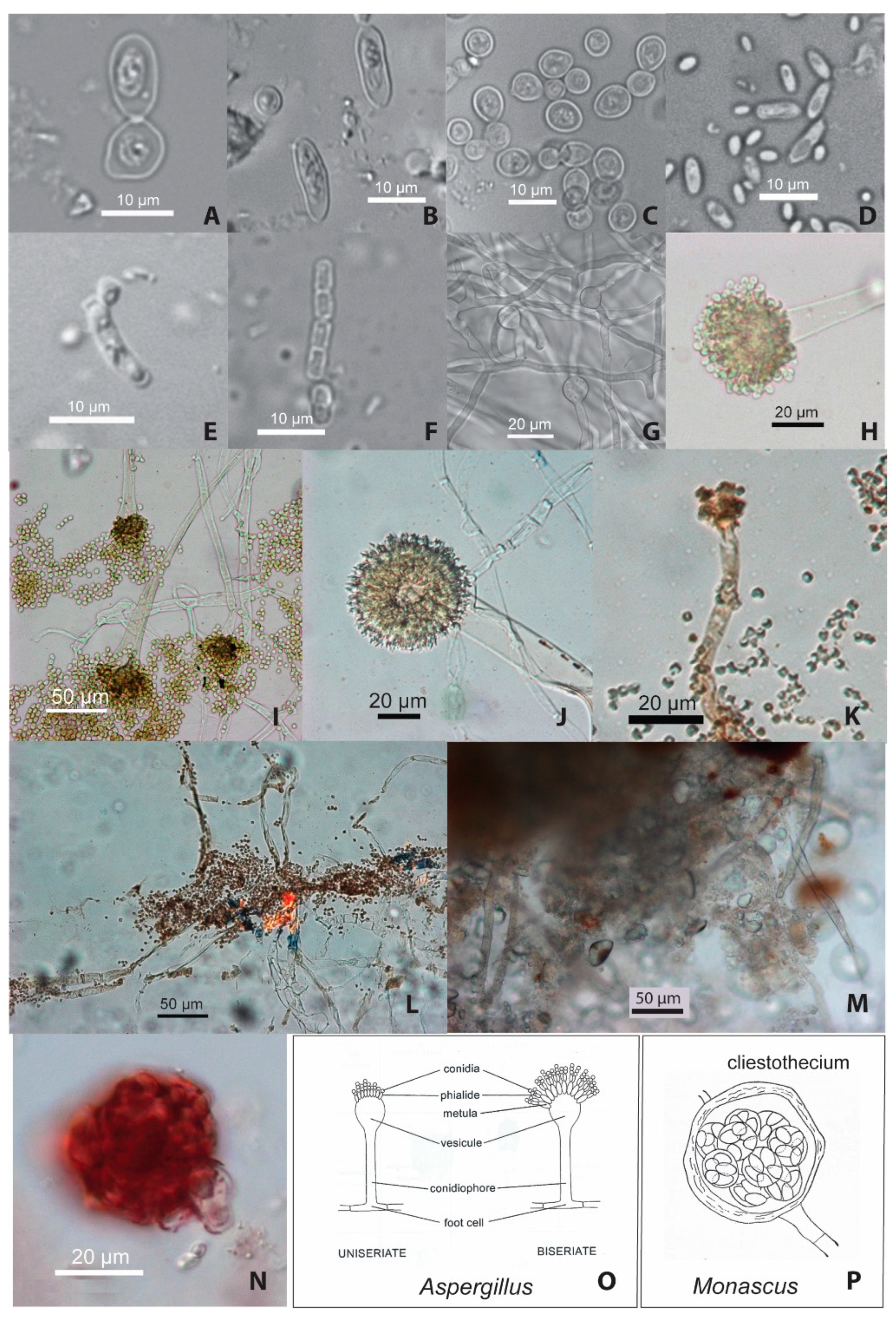
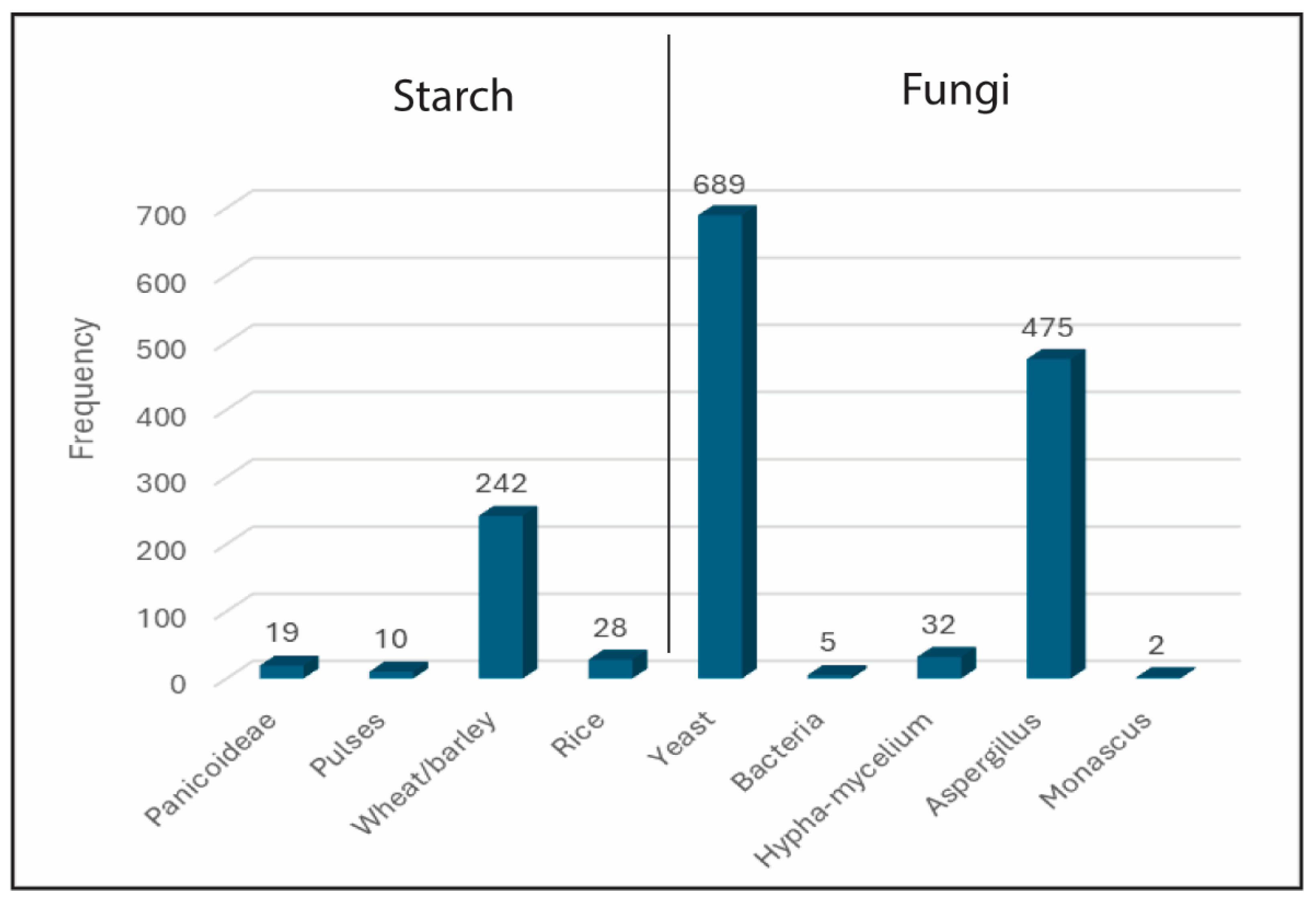
4.2. Microfossil Analyses
4.2.1. Starch Granules
4.2.2. Yeast Cells
4.2.3. Bacteria
4.2.4. Filamentous Fungi (Molds)
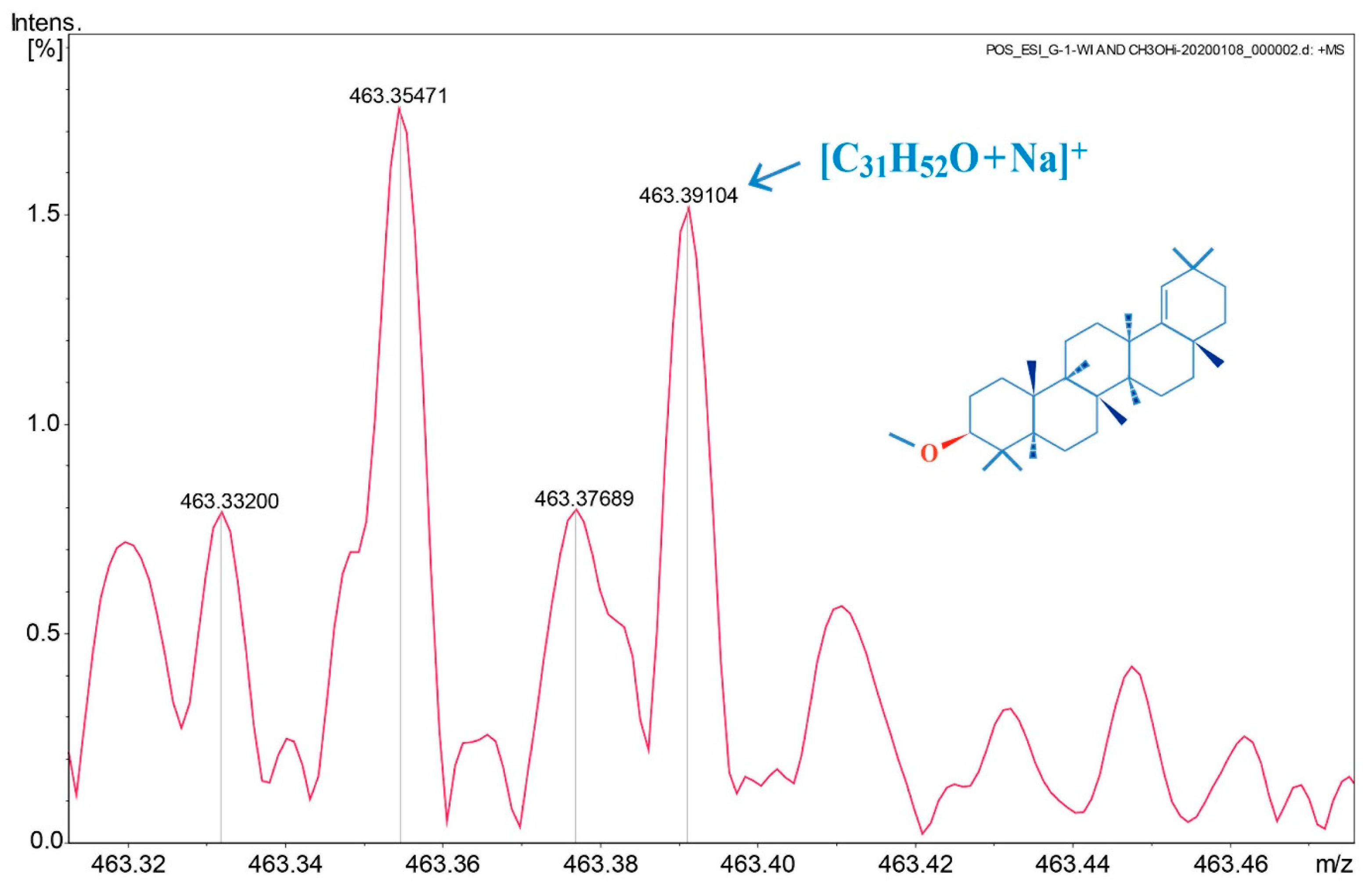
4.3. MS Analyses
4.4. Control Samples
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L. Archaeological evidence for fermented alcoholic beverages in ritual feasts. In Microbial Fermentations in Nature and As Designed Processes; Hurst, C.J., Ed.; Wiley: Hoboken, NJ, USA, 2023; pp. 209–224. [Google Scholar]
- Liu, L.; Wang, J.; Levin, M.J.; Sinnott-Armstrong, N.; Zhao, H.; Zhao, Y.; Shao, J.; Di, N.; Zhang, T. The origins of specialized pottery and diverse alcohol fermentation techniques in Early Neolithic China. Proc. Natl. Acad. Sci. USA 2019, 116, 12767–12774. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5,000-y-old beer recipe in China. Proc. Natl. Acad. Sci. USA 2016, 113, 6444–6448. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Chen, X.; Liang, Z. The quest for red rice beer: Transregional interactions and development of competitive feasting in Neolithic China. Archaeol. Anthropol. Sci. 2022, 14, 78. [Google Scholar] [CrossRef]
- Liu, L. Communal drinking rituals and social formations in the Yellow River valley of Neolithic China. J. Anthropol. Archaeol. 2021, 63, 101310. [Google Scholar] [CrossRef]
- McGovern, P.E.; Underhill, A.; Fang, H.; Luan, F.; Hall, G.; Yu, H.; Wang, C.; Cai, F.; Zhao, Z.; Feinman, G. Chemical identification and cultural implications of a mixed fermented beverage from late prehistoric China. Asian Perspect. 2005, 44, 249–275. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.R.; Wang, C.-S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Zhao, Y.; Chen, X.; Gu, W. Beyond subsistence: Evidence for red rice beer in 8000-year old Neolithic burials, north China. J. Archaeol. Sci. Rep. 2023, 51, 104168. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Bao, Q.; Zhou, J. Niangzao (Fermentation); Daxiang Press: Zhengzhou, China, 2007. [Google Scholar]
- Yu, W. Niangzao Jiangnan mijiu de caoqu (The herb-starter for making rice wine in southern China). Dongfang Meishi Xueshuban 2003, 4, 75–80. [Google Scholar]
- Ling, C. Zhongguo jiu zhi qiyuan (The origins of alcohol in China). Bull. Inst. Hist. Philol. 1958, 29, 883–901. [Google Scholar]
- Wen, S.; Yuan, T. Yinxu Buci Yanjiu: Kexue Jishu Pian (A Study of Oracle Bones from Yinxu: Science and Technolgy); Sichuansheng Shehui Kexueyuan Chubanshe: Chengdu, China, 1983. [Google Scholar]
- Huang, H.T. Science and Civilisation in China: Vol 6, Biology and Biological Technology, Part V: Fermentations and Food Science; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Bao, Q. Zhoudai ji Chunqiu Zhanguo shidai de niangjiu jishu (Alcohol production techniques during the Zhou, Spring-Autumn, and Warring States period). Zhongguo Niangzao 1991, 3, 38–44. [Google Scholar]
- Hong, G. Zhongguo Niangjiu Keji Fazhan Shi (History of Science and Technology of Alcoholic Production in China); Chinese Light Industry Press: Beijing, China, 2001. [Google Scholar]
- Evershed, R.P. Organic residue analysis in archaeology: The archaeological biomarker revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- McGovern, P.E. Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2009. [Google Scholar]
- Gong, Y.; Kong, Y.; Li, C.; Wang, D. Liquid residue analysis of Chinese bronze vessel of the Han Dynasty. J. Archaeol. Sci. Rep. 2024, 55, 104526. [Google Scholar] [CrossRef]
- Jiao, N.; Ma, Y. Xi Han diling xuanzhi yanjiu (On the location choose of West Han imperial mausoleums). Kaogu 2011, 11, 76–82. [Google Scholar]
- Liu, Q. Dixia Chang’an—Gudai Chang’an de Ziran Dili Huanjing (Underground Chang’an—Geographical Envirnment of Ancient Chang’an); Zhonghua Shuju: Beijing, China, 2016. [Google Scholar]
- Zhangyang, L.; Xu, W. Shaanxi Xiangyang Yancun mudi M41 fajue jianbao (Brief report of excavation of tomb M41 at Yancun burial site in Xianyang, Shaanxi). Zhongyuan Wenwu 2020, 1, 31–38. [Google Scholar]
- Chai, Y.; Liu, Z.; Zhao, Z.; Zhang, G.; Chu, Z.; Huang, J. Shaanxi Xixian xinqu konggang xincheng Yancun mudi fajue jianbao (Brief report of excavation at the Yancun cemetery in Konggang Xincheng, Xixian xinqu, Shaanxi). Wenbo 2022, 2, 12–34, 47. [Google Scholar]
- Institute of Archaeology, CASS. Zhongguo Kaoguxue: Liang Zhou Juan (Chinese Archaeology: The Western Zhou and Eastern Zhou Volume); Zhongguo Shehui Kexue Press: Beijing, China, 2004. [Google Scholar]
- Piperno, D.R. Paleoethnobotany in the Neotropics from Microfossils: New Insights into Ancient Plant Use and Agricultural Origins in the Tropical Forest. J. World Prehistory 1998, 12, 393–449. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Georgescu, A.; Le, V.V.; Ota, M.H.; Tang, S.; Vanderbilt, M. Identifying ancient beer brewing through starch analysis: A methodology. J. Archaeol. Sci. Rep. 2017, 15, 150–160. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z. Millet Beer Brewing in North China: Exploring Traditional Methods and their Significance in Archaeological Research. Ethnoarchaeology 2023, 15, 138–152. [Google Scholar] [CrossRef]
- Sinnott-Armstrong, N. DNA Sequencing of Cultured Millet Beer. NIH Sequence Read Archive. 2019. Available online: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA535381 (accessed on 15 May 2019).
- Henry, A.G.; Hudson, H.F.; Piperno, D.R. Changes in starch grain morphologies from cooking. J. Archaeol. Sci. 2009, 36, 915–922. [Google Scholar] [CrossRef]
- St-Germain, G.; Summerbell, R. Identifying Fungi: A Clinical Laboratory Handbook; Star Publishing Company: Belmont, CA, USA, 2011. [Google Scholar]
- Smith, C.K.; McGrath, D.A. The alteration of soil chemistry through shell deposition on a Georgia (USA) barrier island. J. Coast. Res. 2011, 27, 103–109. [Google Scholar]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China. Vol. 22 (Poaceae); Science Press and Missouri Botanical Garden Press: Beijing, China; St. Louis, CA, USA, 2006. [Google Scholar]
- Liu, L.; Ma, S.; Cui, J. Identification of starch granules using a two-step identification method. J. Archaeol. Sci. 2014, 52, 421–427. [Google Scholar] [CrossRef]
- Northwest Institute of Botany CAS. Qinling Zhiwuzhi (Flora of Qinling Mountains) Vol. 1.3; Kexue Press: Beijing, China, 1981. [Google Scholar]
- Yuan, M. Shijing Yizhu (Translation of Classic of Poetry); Qingdao Chubanshe: Qingdao, China, 1999. [Google Scholar]
- Zhao, Z. Weihe pingyuan gudai nongye de fazhan yu bianhua (Development and change of ancient agriculture in the Wei River plain). Huaxia Kaogu 2019, 5, 70–84. [Google Scholar]
- Zhao, Z. New archaeobotanic data for the study of the origins of agriculture in China. Curr. Anthropol. 2011, 52, S295–S306. [Google Scholar] [CrossRef]
- Liu, X. Xian Qin liang Han nongzuowu fenbu zuhe de kaoguxue yanjiu (Distribution and assemblage of crops during the pre-Qin and Han dynasties). Kaogu Xuebao 2016, 4, 465–494. [Google Scholar]
- Douglass, A.P.; Offei, B.; Braun-Galleani, S.; Coughlan, A.Y.; Martos, A.A.R.; Ortiz-Merino, R.l.A.; Byrne, K.P.; Wolfe, K.H. Population genomics shows no distinction between pathogenic Candida krusei and environmental Pichia kudriavzevii: One species, four names. PLoS Pathog. 2018, 14, e1007138. [Google Scholar] [CrossRef] [PubMed]
- Essayag, S.M.; Baily, G.G.; Denning, D.W.; Burnie, J.P. Karyotyping of Fluconazole-Resistant Yeasts with Phenotype Reported as Candida krusei or Candida inconspicua. Int. J. Syst. Bacteriol. 1996, 46, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; Blackwell Science Ltd.: Oxford, UK, 2001. [Google Scholar]
- Zheng, X.-W.; Tabrizi, M.R.; Nout, M.J.R.; Han, B.-Z. Daqu—A Traditional Chinese Liquor Fermentation Starter. J. Inst. Brew. 2011, 117, 82–90. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, Q.; Zhang, Y.; Xua, Y. In Situ Analysis of Metabolic Characteristics Reveals the Key Yeast in the Spontaneous and Solid-State Fermentation Process of Chinese Light-Style Liquor. Appl. Environ. Microbiol. 2014, 80, 3667–3676. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Babbar, N.; Sandhu, S.K.; Dhaliwal, S.S.; Kaur, U.; Chadha, B.S.; Bhargav, V.K. Ethanol production from alkali-treated rice straw via simultaneous saccharification and fermentation using newly isolated thermotolerant Pichia kudriavzevii HOP-1. J. Ind. Microbiol. Biotechnol. 2012, 39, 557–566. [Google Scholar] [CrossRef]
- Moon, S.H.; Chang, M.; Kim, H.Y.; Chang, H.C. Pichia kudriavzevii is the Major Yeast Involved in Film-formation, Off-odor Production, and Texture-softening in Over-ripened Kimchi. Food Sci. Biotechnol. 2014, 23, 489–497. [Google Scholar] [CrossRef]
- He, G.; Jia, Y.; Ding, L. Shipin Weishengwuxue (Food Microbiology); China Agricultural University Press: Beijing, China, 2016. [Google Scholar]
- Wang, H.-Y.; Gao, Y.-B.; Fan, Q.-W.; Xu, Y. Characterization and comparison of microbial community of different typical Chinese liquor Daqus by PCR–DGGE. Lett. Appl. Microbiol. 2011, 53, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-l.; Shi, D.-j.; Gong, G.-l. Microorganisms in Daqu: A starter culture of Chinese Maotai-flavor liquor. World J. Microbiol. Biotechnol 2008, 24, 2183–2190. [Google Scholar] [CrossRef]
- Bao, Q. Hongqu de yuanyuan jiqi peiyang jishu de fazhan (The origins and development of techniques of red qu starter). Zhongguo Niangzao 2001, 1, 5–10. [Google Scholar]
- Liu, L.; Wang, J.; Liu, H. The brewing function of the first amphorae in the Neolithic Yangshao culture, North China. J. Anthropol. Archaeol. Sci. 2020, 12, 118. [Google Scholar] [CrossRef]
- Bossard, N.; Jacob, J.; Le Milbeau, C.; Sauze, J.; Terwilliger, V.; Poissonnier, B.; Vergès, E. Distribution of miliacin (olean-18-en-3β-ol methyl ether) and related compounds in broomcorn millet (Panicum miliaceum) and other reputed sources: Implications for the use of sedimentary miliacin as a tracer of millet. Org. Geochem. 2013, 63, 48–55. [Google Scholar] [CrossRef]
- Jacob, J.; Disnar, J.R.; Bardoux, G. Carbon isotope evidence for sedimentary miliacin as a tracer of Panicum miliaceum (broomcorn millet) in the sediments of Lake le Bourget (French Alps). Org. Geochem. 2008, 39, 1077–1080. [Google Scholar] [CrossRef][Green Version]
- Jacob, J.; Disnar, J.R.; Arnaud, F.; Chapron, E.; Debret, M.; Lallier-Vergès, E.; Desmet, M.; Revel-Rolland, M. Millet cultivation history in the French Alps as evidenced by a sedimentary molecule. J. Archaeol. Sci. 2008, 35, 814–820. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Liu, K.-b.; Wu, N.; Li, Y.; Zhou, K.; Ye, M.; Zhang, T.; Zhang, H.; Yang, X.; et al. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 6425–6426. [Google Scholar] [CrossRef]
- Blom, K.F. Utility of peak shape analyses in determining unresolved interferences in exact mass measurements at low resolution. J. Am. Soc. Mass Spectrom. 1998, 9, 789–798. [Google Scholar] [CrossRef][Green Version]
- Ganorkar, M.C.; Rao, V.P.; Gayathri, P.; Rao, T.A.S. A novel method for conservation of copper-based artifacts. Stud. Conserv. 1988, 33, 97–101. [Google Scholar] [CrossRef]
- MacLeod, I.D. Conservation of corroded copper alloys: A comparison of new and traditional methods for removing chloride ions. Stud. Conserv. 1987, 32, 25–40. [Google Scholar] [CrossRef]
- Bååth, E. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Duan, S.-F.; Han, P.-J.; Wang, Q.-M.; Liu, W.-Q.; Shi, J.-Y.; Li, K.; Zhang, X.-L.; Bai, F.-Y. The origin and adaptive evolution of domesticated populations of yeast from Far East Asia. Nature 2018, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, L.; Zhang, Y.-G.; Tu, T.-Y.; Wang, S.-T.; Shen, C.-H.; Yuan, H.-W.; Zhong, X.-Z. A comparison of microbial communities and volatile compounds in wheat Qu from different geographic locations. Lwt 2021, 148, 111752. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Fan, W.L.; Mu, X.Q.; Chen, J. Traditional Chinese biotechnology. In Biotechnology in China II; Tsao, G.T., Ouyang, P., Chen, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 189–233. [Google Scholar]
- Meng, X. 1983-1986 nian Anyang Liujiazhuang Yindai muzang fajue baogao (Excavations of Yin tombs at Liujiazhuang, Anyang, in 1983-1986). Huaxia Kaogu 1997, 2, 8–27. [Google Scholar]
- Anyang Working Team, I.o.A.C. Anyang Yinxu Liujiazhuang bei 1046 hao mu (Tomb 1046 at Liujiazhuang north, Yinxu, in Anyang). Kaoguxue Jikan 2004, 15, 359–390. [Google Scholar]
- Yue, H.; Yue, Z.; He, Y. Yinxu Dasikong M303 fajue baogao (Excavation report of Tomb 303 at Dasikong in Yinxu). Kaogu Xuebao 2008, 3, 353–394. [Google Scholar]
- Cotsen Institute of Archaeology Press Monographs. Tengzhou Qianzhangda Mudi (Qianzhangda Burial Site in Tengzhou); Wenwu Press: Beijing, China.
- Henan Provincial Institute of Archaeology; Zhoukou City Cultural Bureau. Luyi Taiqinggong Changzikou Mu (The Changzikou Tomb at Taiqinggong in Luyi); Zhongzhou Guji Press: Zhengzhou, China, 2000. [Google Scholar]
- Ou, T. Luoshanxian Mangzhang Louli Shang Zhou mudi dierci fajue jianbao (Second excavation report of the Shang and Zhou burial site at Houli in Mangzhang, Luoshan county). Zhongyuan Wenwu 1981, 4, 4–13. [Google Scholar]
- Wen, R.; Li, J. Kaogu yicun zhong jiulei danliuwu de yanjiu jinzhan (Research progree of wine residues from archaeological remains). Xibei Daxue Xuebao 2017, 47, 160–166. [Google Scholar]
- Li, J.; Yang, J.; Cao, J.; Nan, P.; Gao, J.; Shi, D.; Han, B.; Yang, Y. Characterization of liquor remains in Beibaie site, central China during the 8th century BCE. Microchemical Journal 2022, 177. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Nan, P.; Yang, J.; Cao, J.; Ma, Z.; Ge, W.; Wen, R. Wine or Beer? A reinvestigation of residues from bronze vessels from the Beibai’e cemetery, Shanxi China. Heritage Science 2023, 11. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, Y.; Ge, W.; Lin, Z.; Sinnott-Armstrong, N.; Yang, L. Revealing the 2300-Year-Old Fermented Beverage in a Bronze Bottle from Shaanxi, China. Fermentation 2024, 10, 365. https://doi.org/10.3390/fermentation10070365
Liu L, Zhang Y, Ge W, Lin Z, Sinnott-Armstrong N, Yang L. Revealing the 2300-Year-Old Fermented Beverage in a Bronze Bottle from Shaanxi, China. Fermentation. 2024; 10(7):365. https://doi.org/10.3390/fermentation10070365
Chicago/Turabian StyleLiu, Li, Yanglizheng Zhang, Wei Ge, Zhiwei Lin, Nasa Sinnott-Armstrong, and Lu Yang. 2024. "Revealing the 2300-Year-Old Fermented Beverage in a Bronze Bottle from Shaanxi, China" Fermentation 10, no. 7: 365. https://doi.org/10.3390/fermentation10070365
APA StyleLiu, L., Zhang, Y., Ge, W., Lin, Z., Sinnott-Armstrong, N., & Yang, L. (2024). Revealing the 2300-Year-Old Fermented Beverage in a Bronze Bottle from Shaanxi, China. Fermentation, 10(7), 365. https://doi.org/10.3390/fermentation10070365





