The Antilisterial Effect of Latilactobacillus sakei CTC494 in Relation to Dry Fermented Sausage Ingredients and Temperature in Meat Simulation Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Bacterial Strains and Inoculum Preparation
2.3. Experiments with L. sakei Strains and L. monocytogens in Mono and Coculture in Meat Simulation Media
2.4. Microbiological Analyses
2.5. Physicochemical and Metabolite Determinations
2.6. Assessment of Growth and Inactivation Parameters
2.7. Response Surface Methodology and Data Analysis
3. Results and Discussion
3.1. Assessment of the Effect of NaCl, Mn, Glucose, Temperature and Coculture in the Acidification and Bacteriocin Production in Meat Simulation Media
3.1.1. pH Decrease and Lactic Acid Formation
3.1.2. Sakacin K Activity
3.2. Impact of NaCl, Mn, Glucose, Temperature and Coculture in the Behaviour of L. sakei and L. monocytogenes
3.2.1. Bacterial Growth under DFS Fermentation Conditions in MSM
3.2.2. L. monocytogenes Inactivation by the Sakacin K Producer L. sakei CTC494
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warriner, K.; Namvar, A. What Is the Hysteria with Listeria? Trends Food Sci. Technol. 2009, 20, 245–254. [Google Scholar] [CrossRef]
- Gutiérrez-Chocoza, M.A.; López-Romero, J.C.; García-Galaz, A.; González-Ríos, H.; Peña-Ramos, A.; Juneja, V.K.; Pérez-Báez, A.J.; Valenzuela-Melendres, M. Modeling the Effects of Temperature and PH on Listeria monocytogenes Growth in Mexican-Style Pork Chorizo. Appl. Food Res. 2023, 3, 100336. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, PMC9745727. [Google Scholar] [CrossRef]
- Mataragas, M.; Rovetto, F.; Bellio, A.; Alessandria, V.; Rantsiou, K.; Decastelli, L.; Cocolin, L. Differential Gene Expression Profiling of Listeria monocytogenes in Cacciatore and Felino Salami to Reveal Potential Stress Resistance Biomarkers. Food Microbiol. 2015, 46, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Possas, A.; Valdramidis, V.; García-Gimeno, R.M.; Pérez-Rodríguez, F. High Hydrostatic Pressure Processing of Sliced Fermented Sausages: A Quantitative Exposure Assessment for Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2019, 52, 406–419. [Google Scholar] [CrossRef]
- European Commission RASFF—Food and Feed Safety Alerts. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 5 March 2024).
- Martín, B.; Perich, A.; Gómez, D.; Yangüela, J.; Rodríguez, A.; Garriga, M.; Aymerich, T. Diversity and Distribution of Listeria monocytogenes in Meat Processing Plants. Food Microbiol. 2014, 44, 119–127. [Google Scholar] [CrossRef]
- Lagarde, J.; Feurer, C.; Denis, M.; Douarre, P.E.; Piveteau, P.; Roussel, S. Listeria monocytogenes Prevalence and Genomic Diversity along the Pig and Pork Production Chain. Food Microbiol. 2024, 119, 104430. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Meloni, D. Presence of Listeria monocytogenes in Mediterranean-Style Dry Fermented Sausages. Foods 2015, 4, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- ICMSF. Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Blackie Academic & Professional: London, UK, 1996; ISBN 041247350X. [Google Scholar]
- European Commission. Commission regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Comm. 2005, I, 1–26. [Google Scholar]
- European Food Safety Authority Dashboard on Listeria monocytogenes. Available online: https://www.efsa.europa.eu/en/microstrategy/listeria-dashboard (accessed on 24 April 2024).
- Martin, B.; Garriga, M.; Aymerich, T. Prevalence of Salmonella spp. and Listeria monocytogenes at Small-Scale Spanish Factories Producing Traditional Fermented Sausages. J. Food Prot. 2011, 74, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Lücke, F.-K. Fermented Sausages. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Blackie Academic and Professional: London, UK, 1998; pp. 441–483. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-To-Eat Meat-and Dairy-Ripened Products. Foods 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Serra-Castelló, C.; Costa, J.C.C.P.; Jofré, A.; Bolívar, A.; Pérez-Rodríguez, F.; Bover-Cid, S. A Mathematical Model to Predict the Antilisteria Bioprotective Effect of Latilactobacillus sakei CTC494 in Vacuum Packaged Cooked Ham. Int. J. Food Microbiol. 2022, 363, 109491. [Google Scholar] [CrossRef]
- Austrich-Comas, A.; Serra-Castelló, C.; Jofré, A.; Gou, P.; Bover-Cid, S. Control of Listeria monocytogenes in Chicken Dry-Fermented Sausages with Bioprotective Starter Culture and High-Pressure Processing. Front. Microbiol. 2022, 13, 983265. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Garriga, M.; Aymerich, M.T.; Monfort, J.M. Inhibition of Listeria in Dry Fermented Sausages by the Bacteriocinogenic Lactobacillus sake CTC494. J. Appl. Bacteriol. 1995, 79, 322–330. [Google Scholar] [CrossRef]
- Leroy, F.; Lievens, K.; De Vuyst, L. Interactions of Meat-Associated Bacteriocin-Producing Lactobacilli with Listeria innocua under Stringent Sausage Fermentation Conditions. J. Food Prot. 2005, 68, 2078–2084. [Google Scholar] [CrossRef]
- Aymerich, M.T.; Garriga, M.; Monfort, J.M.; Nes, I.; Hugas, M. Bacteriocin-Producing Lactobacilli in Spanish-Style Fermented Sausages: Characterization of Bacteriocins. Food Microbiol. 2000, 17, 33–45. [Google Scholar] [CrossRef]
- Urso, R.; Rantsiou, K.; Cantoni, C.; Comi, G.; Cocolin, L. Technological Characterization of a Bacteriocin-Producing Lactobacillus sakei and Its Use in Fermented Sausages Production. Int. J. Food Microbiol. 2006, 110, 232–239. [Google Scholar] [CrossRef]
- Verluyten, J.; Messens, W.; De Vuyst, L. Sodium Chloride Reduces Production of Curvacin A, a Bacteriocin Produced by Lactobacillus curvatus Strain LTH 1174, Originating from Fermented Sausage. Appl. Environ. Microbiol. 2004, 70, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Jurkiewicz, C.; Landgraf, M.; Todorov, S.D.; Franco, B.D.G.M. Effect of Proteins, Glucose and NaCl on Growth, Biosynthesis and Functionality of Bacteriocins of Lactobacillus sakei Subsp. Sakei 2a in Foods during Storage at 4 °C: Tests in Food Models. LWT 2018, 95, 167–171. [Google Scholar] [CrossRef]
- Zaika, L.L.; Kissinger, J.C. Fermentation Enhancement by Spices: Identification of Active Component. J. Food Sci. 1984, 49, 5–9. [Google Scholar] [CrossRef]
- Hagen, B.F.; Naes, H.; Holck, A.L. Meat Starters Have Individual Requirements for Mn2+. Meat Sci. 2000, 55, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. The Presence of Salt and a Curing Agent Reduces Bacteriocin Production by Lactobacillus sakei CTC 494, a Potential Starter Culture for Sausage Fermentation. Appl. Environ. Microbiol. 1999, 65, 5350–5356. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Simulation of the Effect of Sausage Ingredients and Technology on the Functionality of the Bacteriocin-Producing Lactobacillus sakei CTC 494 Strain. Int. J. Food Microbiol. 2005, 100, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Bustins, N.; Martín, B.; Llauger, M.; Bou, R.; Bover-Cid, S.; Jofré, A. Dynamics of Microbial Communities in Nitrite-Free and Nutritionally Improved Dry Fermented Sausages. Fermentation 2023, 9, 403. [Google Scholar] [CrossRef]
- Giovanni, M. Response Surface Methodology and Product Optimization. Food Technol. 1983, 37, 41–45. [Google Scholar]
- Dussault, D.; Vu, K.D.; Lacroix, M. Development of a Model Describing the Inhibitory Effect of Selected Preservatives on the Growth of Listeria monocytogenes in a Meat Model System. Food Microbiol. 2016, 53, 115–121. [Google Scholar] [CrossRef]
- Seman, D.L.; Borger, A.C.; Meyer, J.D.; Hall, P.A.; Milkowski, A.L. Modeling the Growth of Listeria monocytogenes in Cured Ready-to-Eat Processed Meat Products by Manipulation of Sodium Chloride, Sodium Diacetate, Potassium Lactate, and Product Moisture Content. J. Food Prot. 2002, 65, 651–658. [Google Scholar] [CrossRef]
- NIST NIST/SEMATECH e-Handbook of Statistical Methods. Available online: http://www.itl.nist.gov/div898/handbook/ (accessed on 8 April 2024).
- FDA. Bad Bug Book: Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd ed.; Lampel, K.A., Al-Khaldi, S., Cahill, S.M., Eds.; Center for Food Safety and Applied Nutrition (CFSAN), U.S. Department of Health and Human Services: College Park, MD, USA, 2012. [Google Scholar]
- Chaillou, S.; Champomier-Vergès, M.-C.; Cornet, M.; Crutz-Le Coq, A.-M.; Dudez, A.-M.; Martin, V.; Beaufils, S.; Darbon-Rongère, E.; Bossy, R.; Loux, V.; et al. The Complete Genome Sequence of the Meat-Borne Lactic Acid Bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 2005, 23, 1527–1533. [Google Scholar] [CrossRef]
- Sánchez Mainar, M.; Matheuse, F.; De Vuyst, L.; Leroy, F. Effects of Glucose and Oxygen on Arginine Metabolism by Coagulase-Negative Staphylococci. Food Microbiol. 2017, 65, 170–178. [Google Scholar] [CrossRef]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric Determination of Lactic Acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Groff, M.C.; Scaglia, G.; Ortiz, O.A.; Noriega, S.E. Modification of the Luedeking and Piret Model with a Delay Time Parameter for Biotechnological Lactic Acid Production. Biotechnol. Lett. 2022, 44, 415–427. [Google Scholar] [CrossRef]
- Rosso, L.; Bajard, S.; Flandrois, J.P.; Lahellec, C.; Fournaud, J.; Veit, P. Differential Growth of Listeria monocytogenes at 4 and 8 °C: Consequences for the Shelf Life of Chilled Products. J. Food Prot. 1996, 59, 944–949. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Temperature and PH Conditions That Prevail during Fermentation of Sausages Are Optimal for Production of the Antilisterial Bacteriocin Sakacin K. Appl. Environ. Microbiol. 1999, 65, 974–981. [Google Scholar] [CrossRef]
- Barbieri, F.; Laghi, L.; Montanari, C.; Lan, Q.; Levante, A.; Gardini, F.; Tabanelli, G. Insights into the Metabolomic Diversity of Latilactobacillus sakei. Foods 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic Acid Bacteria as Starter Cultures: An Update in Their Metabolism and Genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.-D.; Herskovits, A.A.; O’Riordan, M.X.D. Metabolism of the Gram-Positive Bacterial Pathogen Listeria monocytogenes. Microbiol. Spectr. 2019, 7, 864–872. [Google Scholar] [CrossRef]
- Maldonado-Barragán, A.; Caballero-Guerrero, B.; Lucena-Padrós, H.; Ruiz-Barba, J.L. Induction of Bacteriocin Production by Coculture Is Widespread among Plantaricin-Producing Lactobacillus plantarum Strains with Different Regulatory Operons. Food Microbiol. 2013, 33, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Aasen, I.M.; Møretrø, T.; Katla, T.; Axelsson, L.; Storrø, I. Influence of Complex Nutrients, Temperature and PH on Bacteriocin Production by Lactobacillus sakei CCUG 42687. Appl. Microbiol. Biotechnol. 2000, 53, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Sabharwal, V.; Pragya, K.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- Pleasants, A.B.; Soboleva, T.K.; Dykes, G.A.; Jones, R.J.; Filippov, A.E. Modelling of the Growth of Populations of Listeria monocytogenes and a Bacteriocin-Producing Strain of Lactobacillus in Pure and Mixed Cultures. Food Microbiol. 2001, 18, 605–615. [Google Scholar] [CrossRef][Green Version]
- Giello, M.; La Storia, A.; De Filippis, F.; Ercolini, D.; Villani, F. Impact of Lactobacillus Curvatus 54M16 on Microbiota Composition and Growth of Listeria monocytogenes in Fermented Sausages. Food Microbiol. 2018, 72, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.E. A Discussion of the Dynamics of Salmonella Enrichment. J. Hyg. 1962, 60, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Lizarazo, C.M.; Sotelo-Díaz, I.; Llorente-Bousquets, A. In Vitro Modelling of Simultaneous Interactions of Listeria monocytogenes, Lactobacillus sakei, and Staphylococcus carnosus. Food Sci. Biotechnol. 2016, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Pragalaki, T.; Bloukas, J.G.; Kotzekidou, P. Inhibition of Listeria monocytogenes and Escherichia coli O157: H7 in Liquid Broth Medium and during Processing of Fermented Sausage Using Autochthonous Starter Cultures. Meat Sci. 2013, 95, 458–464. [Google Scholar] [CrossRef]
- Siddi, G.; Piras, F.; Spanu, V.; Meloni, M.P.; Sanna, R.; Carta, N.; Errico, M.; Cuccu, M.; De Santis, E.P.L.; Scarano, C. Selection of Commercial Protective Cultures to Be Added in Sardinian Fermented Sausage to Control Listeria monocytogenes. Ital. J. Food Saf. 2022, 11. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]



| Levels a | CCD1 Factors | CCD2 Factors | ||
|---|---|---|---|---|
| x1 b | x2 c | x3 d | x2 c | |
| −1.6818 | 0.00 | 0.08 | 0.00 | 3.01 |
| −1.0000 | 8.18 | 0.13 | 8.18 | 9.90 |
| 0.0000 | 20.18 | 0.20 | 20.18 | 20.00 |
| +1.0000 | 32.18 | 0.27 | 32.18 | 30.11 |
| +1.6818 | 40.36 | 0.32 | 40.36 | 37.00 |
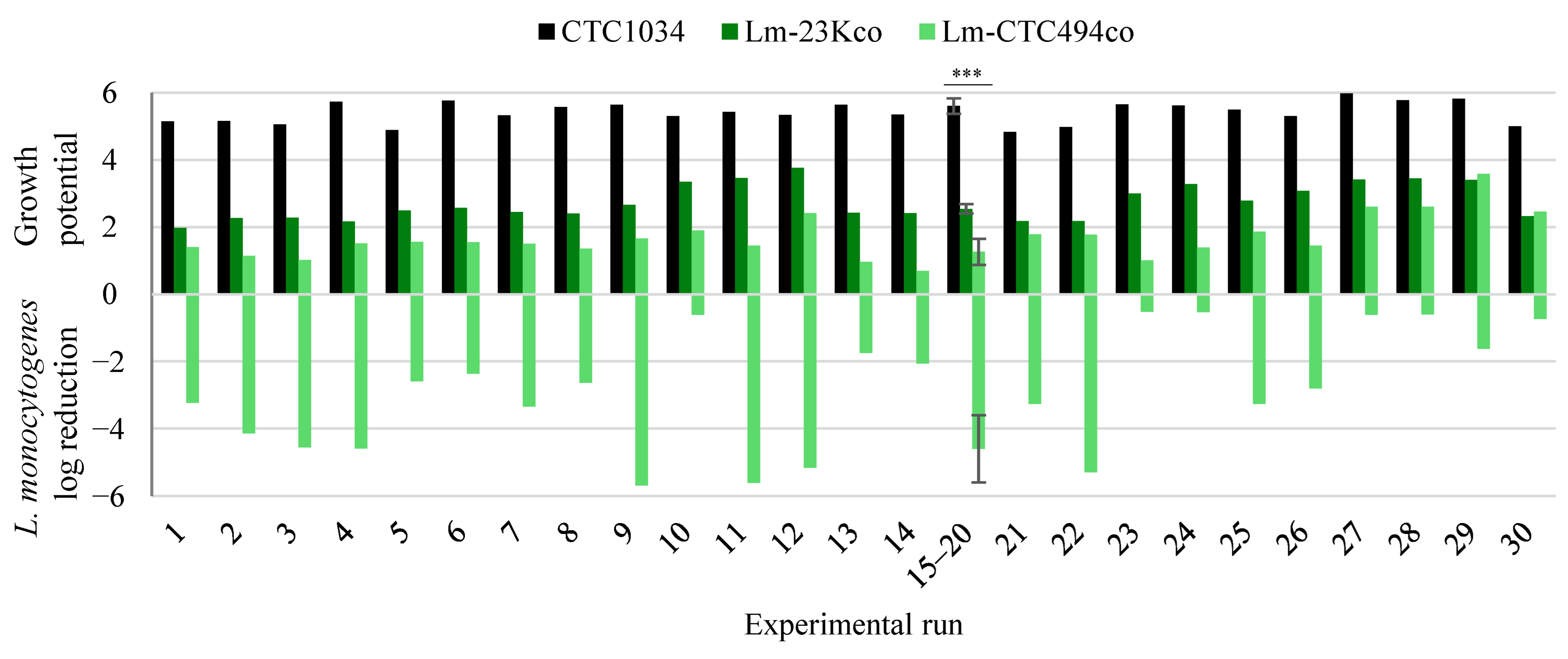
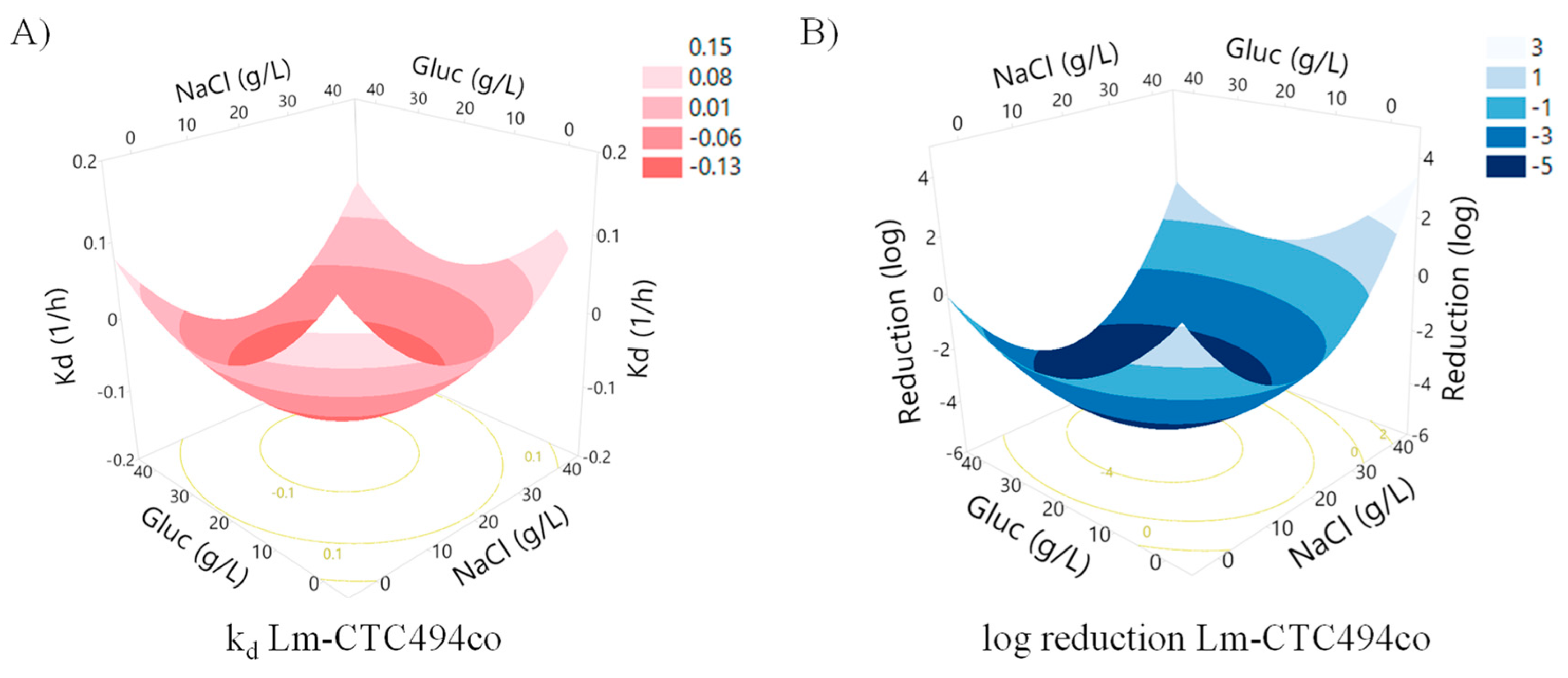
| Experiment | x1 | x2 | x3 | NaCl (g/L) | Mn (g/L) | T (°C) | Gluc (g/L) | Observed Reduction (log) 1 | Predicted Reduction (log) 1 | Observed kd (h−1) 1 | Predicted kd (h−1) 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1.00 | −1.00 | −1.00 | 8.18 | 0.13 | NE 2 | 8.18 | −3.24 | −2.81 | −0.12 | −0.06 |
| 2 | −1.00 | −1.00 | 1.00 | 8.18 | 0.13 | NE | 32.18 | −4.14 | −4.29 | −0.17 | −0.09 |
| 3 | −1.00 | 1.00 | −1.00 | 8.18 | 0.27 | NE | 8.18 | −4.56 | −2.81 | −0.20 | −0.06 |
| 4 | −1.00 | 1.00 | 1.00 | 8.18 | 0.27 | NE | 32.18 | −4.59 | −4.29 | −0.15 | −0.09 |
| 5 | 1.00 | −1.00 | −1.00 | 32.18 | 0.13 | NE | 8.18 | −2.59 | −2.39 | −0.07 | −0.06 |
| 6 | 1.00 | −1.00 | 1.00 | 32.18 | 0.13 | NE | 32.18 | −2.37 | −3.87 | −0.06 | −0.09 |
| 7 | 1.00 | 1.00 | −1.00 | 32.18 | 0.27 | NE | 8.18 | −3.34 | −2.39 | −0.08 | −0.06 |
| 8 | 1.00 | 1.00 | 1.00 | 32.18 | 0.27 | NE | 32.18 | −2.64 | −3.87 | −0.07 | −0.09 |
| 9 | 0.00 | 0.00 | 1.68 | 20.18 | 0.20 | NE | 40.36 | −5.59 | −5.46 | −0.13 | −0.09 |
| 10 | 0.00 | 0.00 | −1.68 | 20.18 | 0.20 | NE | 0.00 | 0.35 | −2.96 | −0.01 | −0.03 |
| 11 | 0.00 | 1.68 | 0.00 | 20.18 | 0.32 | NE | 20.18 | −5.62 | −4.21 | −0.12 | −0.13 |
| 12 | 0.00 | −1.68 | 0.00 | 20.18 | 0.08 | NE | 20.18 | −5.16 | −4.21 | −0.19 | −0.13 |
| 13 | 1.68 | 0.00 | 0.00 | 40.36 | 0.20 | NE | 20.18 | −1.75 | −1.40 | −0.06 | −0.04 |
| 14 | −1.68 | 0.00 | 0.00 | 0.00 | 0.20 | NE | 20.18 | −2.07 | −2.11 | −0.04 | −0.04 |
| 15 | 0.00 | 0.00 | 0.00 | 20.18 | 0.20 | NE | 20.18 | −5.69 | −4.21 | −0.14 | −0.13 |
| 16 | 0.00 | 0.00 | 0.00 | 20.18 | 0.20 | NE | 20.18 | −5.56 | −4.21 | −0.10 | −0.13 |
| 17 | 0.00 | 0.00 | 0.00 | 20.18 | 0.20 | NE | 20.18 | −5.61 | −4.21 | −0.13 | −0.13 |
| 18 | 0.00 | 0.00 | 0.00 | 20.18 | 0.20 | NE | 20.18 | −3.72 | −4.21 | −0.16 | −0.13 |
| 19 | 0.00 | 0.00 | 0.00 | 20.18 | 0.20 | NE | 20.18 | −3.10 | −4.21 | −0.14 | −0.13 |
| 20 | 0.00 | 0.00 | 0.00 | 20.18 | 0.20 | NE | 20.18 | −4.15 | −4.21 | −0.10 | −0.13 |
| 21 | 0.00 | −1.00 | 0.00 | 20.18 | NE | 9.90 | 20.18 | −3.26 | −2.48 | −0.02 | −0.01 |
| 22 | 0.00 | −1.00 | 0.00 | 20.18 | NE | 9.90 | 20.18 | −5.29 | −3.97 | −0.04 | −0.04 |
| 23 | 0.00 | 1.00 | 0.00 | 20.18 | NE | 30.11 | 20.18 | −0.52 | −0.89 | −0.03 | −0.02 |
| 24 | 0.00 | 1.00 | 0.00 | 20.18 | NE | 30.11 | 20.18 | −0.53 | −2.37 | −0.03 | −0.05 |
| 25 | 0.00 | −1.00 | 0.00 | 20.18 | NE | 9.90 | 20.18 | −3.26 | −2.06 | −0.02 | −0.01 |
| 26 | 0.00 | −1.00 | 0.00 | 20.18 | NE | 9.90 | 20.18 | −2.81 | −3.55 | −0.02 | −0.04 |
| 27 | 0.00 | 1.00 | 0.00 | 20.18 | NE | 30.11 | 20.18 | −0.60 | −0.47 | −0.02 | −0.02 |
| 28 | 0.00 | 1.00 | 0.00 | 20.18 | NE | 30.11 | 20.18 | −0.60 | −1.95 | −0.02 | −0.05 |
| 29 | 0.00 | 1.68 | 0.00 | 20.18 | NE | 37.00 | 20.18 | −1.62 | 0.31 | −0.05 | −0.02 |
| 30 | 0.00 | −1.68 | 0.00 | 20.18 | NE | 3.01 | 20.18 | −0.02 | −2.37 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Bustins, N.; Costa, J.C.C.P.; Pérez-Rodríguez, F.; Martín, B.; Bover-Cid, S.; Jofré, A. The Antilisterial Effect of Latilactobacillus sakei CTC494 in Relation to Dry Fermented Sausage Ingredients and Temperature in Meat Simulation Media. Fermentation 2024, 10, 326. https://doi.org/10.3390/fermentation10060326
Ferrer-Bustins N, Costa JCCP, Pérez-Rodríguez F, Martín B, Bover-Cid S, Jofré A. The Antilisterial Effect of Latilactobacillus sakei CTC494 in Relation to Dry Fermented Sausage Ingredients and Temperature in Meat Simulation Media. Fermentation. 2024; 10(6):326. https://doi.org/10.3390/fermentation10060326
Chicago/Turabian StyleFerrer-Bustins, Núria, Jean Carlos Correia Peres Costa, Fernando Pérez-Rodríguez, Belén Martín, Sara Bover-Cid, and Anna Jofré. 2024. "The Antilisterial Effect of Latilactobacillus sakei CTC494 in Relation to Dry Fermented Sausage Ingredients and Temperature in Meat Simulation Media" Fermentation 10, no. 6: 326. https://doi.org/10.3390/fermentation10060326
APA StyleFerrer-Bustins, N., Costa, J. C. C. P., Pérez-Rodríguez, F., Martín, B., Bover-Cid, S., & Jofré, A. (2024). The Antilisterial Effect of Latilactobacillus sakei CTC494 in Relation to Dry Fermented Sausage Ingredients and Temperature in Meat Simulation Media. Fermentation, 10(6), 326. https://doi.org/10.3390/fermentation10060326









