A Mixture of Prebiotics, Essential Oil Blends, and Onion Peel Did Not Affect Greenhouse Gas Emissions or Nutrient Degradability, but Altered Volatile Fatty Acids Production in Dairy Cows Using Rumen Simulation Technique (RUSITEC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Substrate Preparation
2.3. Determination of Nutrient Composition
2.4. Ingredients and Experimental Design
- (1)
- Control [TMR only],
- (2)
- GEO [TMR + GOS + EOB + OPE],
- (3)
- MEO [TMR + MOS + EOB + OPE],
- (4)
- OLEO [TMR + OLG +EOB + OPE].
2.5. In Vitro RUSITEC Fermentation
2.6. In Vitro Fermentation
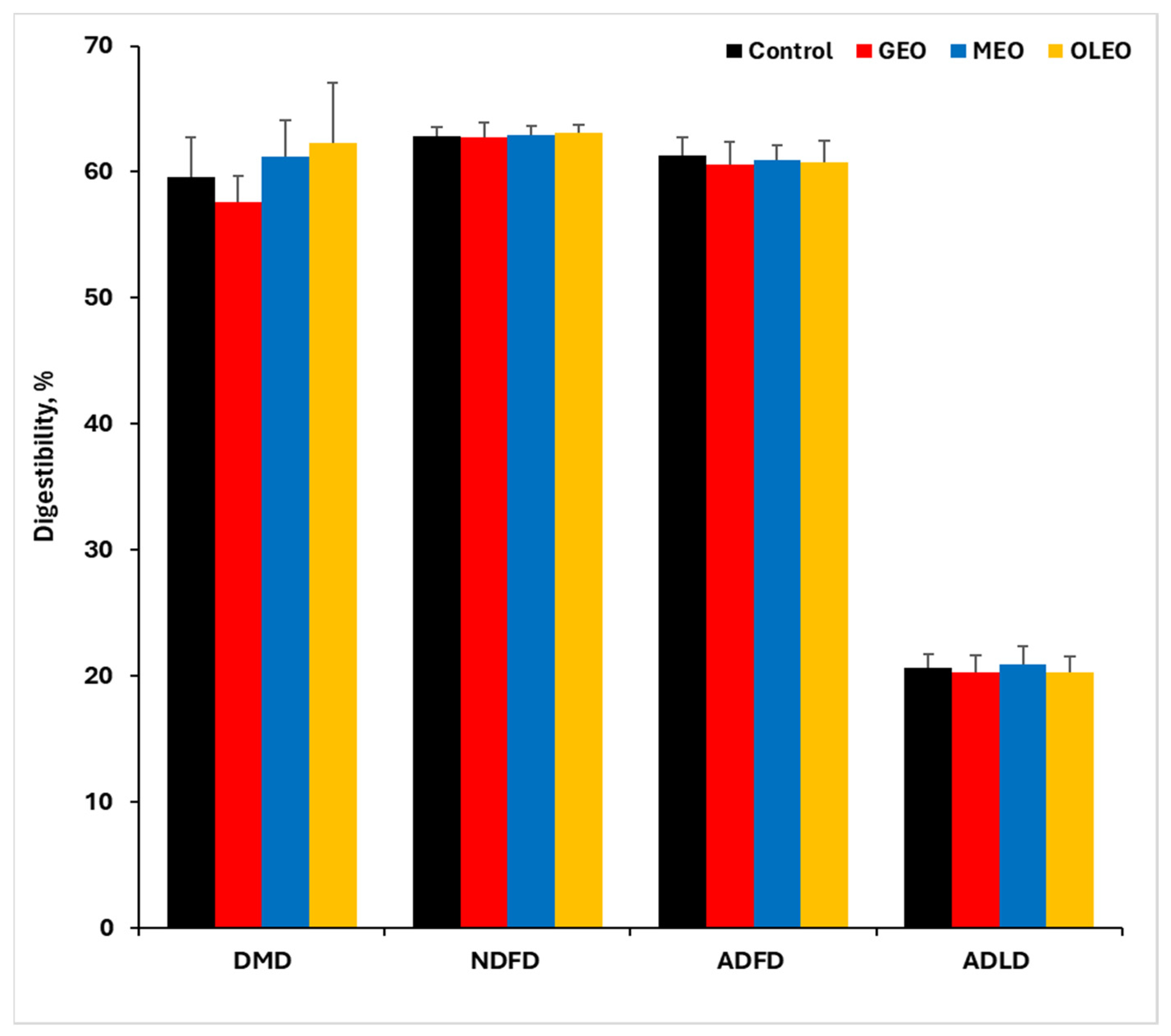
2.7. Dry Matter and Fiber Fractions Digestibility
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, B.R.; Lee, S.; Jung, H.; Miller, D.N.; Chen, R. Enteric Methane Emissions and Animal Performance in Dairy and Beef Cattle Production: Strategies, Opportunities, and Impact of Reducing Emissions. Animals 2022, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- US EPA Understanding Global Warming Potentials; United States Environmental Protection Agency: Washington, DC, USA, 2024. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 1 April 2024).
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological Insights into Anaerobic Digestion for Biogas, Hydrogen or Volatile Fatty Acids (VFAs): A Review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Panitsidis, I.; Barbe, F.; Chevaux, E.; Giannenas, I.; Demey, V. Probiotics, Prebiotics, Paraprobiotics, Postbiotics. In Sustainable Use of Feed Additives in Livestock; Springer International Publishing: Cham, Switzerland, 2023; pp. 173–227. [Google Scholar]
- Zheng, C.; Zhou, J.; Zeng, Y.; Liu, T. Effects of Mannan Oligosaccharides on Growth Performance, Nutrient Digestibility, Ruminal Fermentation and Hematological Parameters in Sheep. PeerJ 2021, 9, e11631. [Google Scholar] [CrossRef] [PubMed]
- Dagnaw Fenta, M.; Gebremariam, A.A.; Mebratu, A.S. Effectiveness of Probiotic and Combinations of Probiotic with Prebiotics and Probiotic with Rumenotorics in Experimentally Induced Ruminal Acidosis Sheep. Vet. Med. Res. Rep. 2023, 14, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Mwenya, B.; Santoso, B.; Sar, C.; Gamo, Y.; Kobayashi, T.; Arai, I.; Takahashi, J. Effects of Including Β1-4 Galacto-Oligosaccharides, Lactic Acid Bacteria or Yeast Culture on Methanogenesis as Well as Energy and Nitrogen Metabolism in Sheep. Anim. Feed. Sci. Technol. 2004, 115, 313–326. [Google Scholar] [CrossRef]
- Garcia Diaz, T.; Ferriani Branco, A.; Jacovaci, F.A.; Cabreira Jobim, C.; Bolson, D.C.; Pratti Daniel, J.L. Inclusion of Live Yeast and Mannan-Oligosaccharides in High Grain-Based Diets for Sheep: Ruminal Parameters, Inflammatory Response and Rumen Morphology. PLoS ONE 2018, 13, e0193313. [Google Scholar] [CrossRef]
- Franklin, S.T.; Newman, M.C.; Newman, K.E.; Meek, K.I. Immune Parameters of Dry Cows Fed Mannan Oligosaccharide and Subsequent Transfer of Immunity to Calves. J. Dairy Sci. 2005, 88, 766–775. [Google Scholar] [CrossRef]
- Linneen, S.K.; Mourer, G.L.; Sparks, J.D.; Jennings, J.S.; Goad, C.L.; Lalman, D.L. Effects of Mannan Oligosaccharide on Beef-Cow Performance and Passive Immunity Transfer to Calves. Prof. Anim. Sci. 2014, 30, 311–317. [Google Scholar] [CrossRef]
- Brice, R.M.; Dele, P.A.; Ike, K.A.; Shaw, Y.A.; Olagunju, L.K.; Orimaye, O.E.; Subedi, K.; Anele, U.Y. Effects of Essential Oil Blends on In Vitro Apparent and Truly Degradable Dry Matter, Efficiency of Microbial Production, Total Short-Chain Fatty Acids and Greenhouse Gas Emissions of Two Dairy Cow Diets. Animals 2022, 12, 2185. [Google Scholar] [CrossRef]
- Khattab, I.M.; Elgandy, M.F. Essential Oils in Animal Diets to Improve the Fatty Acids Composition of Meat and Milk Quality in Ruminant. In Essential Oils—Recent Advances, New Perspectives and Applications; Viskelis, J., Surguchov, A., Eds.; IntechOpen: Rijeka, Croatia, 2024; ISBN 978-0-85014-205-1. [Google Scholar]
- Kholif, A.E.; Olafadehan, O.A. Essential Oils and Phytogenic Feed Additives in Ruminant Diet: Chemistry, Ruminal Microbiota and Fermentation, Feed Utilization and Productive Performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Metwally, A. Effects of a Specific Blend of Essential Oil on Rumen Degradability, Total Tract Digestibility and Fermentation Characteristics in Rumen Fistulated Cows. J. Dairy Vet. Anim. Res. 2016, 3, 51–60. [Google Scholar] [CrossRef]
- Benetel, G.; Silva, T.D.S.; Fagundes, G.M.; Welter, K.C.; Melo, F.A.; Lobo, A.A.G.; Muir, J.P.; Bueno, I.C.S. Essential Oils as In Vitro Ruminal Fermentation Manipulators to Mitigate Methane Emission by Beef Cattle Grazing Tropical Grasses. Molecules 2022, 27, 2227. [Google Scholar] [CrossRef] [PubMed]
- Blanch, M.; Carro, M.D.; Ranilla, M.J.; Viso, A.; Vázquez-Añón, M.; Bach, A. Influence of a Mixture of Cinnamaldehyde and Garlic Oil on Rumen Fermentation, Feeding Behavior and Performance of Lactating Dairy Cows. Anim. Feed. Sci. Technol. 2016, 219, 313–323. [Google Scholar] [CrossRef]
- Alabi, J.O.; Okedoyin, D.O.; Anotaenwere, C.C.; Wuaku, M.; Gray, D.; Adelusi, O.O.; Ike, K.A.; Olagunju, L.K.; Dele, P.A.; Anele, U.Y. Essential Oil Blends with or without Fumaric Acid Influenced In Vitro Rumen Fermentation, Greenhouse Gas Emission, and Volatile Fatty Acids Production of a Total Mixed Ration. Ruminants 2023, 3, 373–384. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha, S.; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) Peels: A Review on Bioactive Compounds and Biomedical Activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.L.; Abd-Elhak, N.A.; Abd El-Rahman, H.S.M. The Utilization of Yellow and Red Onion Peels and Their Extracts as Antioxidant and Antimicrobial in Preservation of Beef Burger during Storage. Am. J. Food Sci. Technol. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Eom, J.S.; Lee, S.J.; Lee, Y.; Kim, H.S.; Choi, Y.Y.; Kim, H.S.; Kim, D.H.; Lee, S.S. Effects of Supplementation Levels of Allium fistulosum L. Extract on in vitro Ruminal Fermentation Characteristics and Methane Emission. PeerJ 2020, 8, e9651. [Google Scholar] [CrossRef] [PubMed]
- Ike, K.A.; Adelusi, O.O.; Alabi, J.O.; Olagunju, L.K.; Wuaku, M.; Anotaenwere, C.C.; Okedoyin, D.O.; Gray, D.; Dele, P.A.; Subedi, K.; et al. Effects of Different Essential Oil Blends and Fumaric Acid on in Vitro Fermentation, Greenhouse Gases, Nutrient Degradability, Total and Molar Proportion of Volatile Fatty Acids Production of a Total Mixed Ration in Dairy Cattle. Agriculture 2024, 14, 876. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of Essential Oils on Methane Production and Fermentation by, and Abundance and Diversity of, Rumen Microbial Populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef]
- Lee, S.J.; Shin, N.H.; Ok, J.U.; Jung, H.S.; Chu, G.M.; Kim, J.D.; Kim, I.H.; Lee, S.S. Effects of Dietary Synbiotics from Anaerobic Microflora on Growth Performance, Noxious Gas Emission and Fecal Pathogenic Bacteria Population in Weaning Pigs. Asian-Australas. J. Anim. Sci. 2009, 22, 1202–1208. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis of AOAC International, 21st ed.; Oxford University Press: Washington, DC, USA, 2019; ISBN 9780197610138.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Alabi, J.O.; Dele, P.A.; Okedoyin, D.O.; Wuaku, M.; Anotaenwere, C.C.; Adelusi, O.O.; Gray, D.; Ike, K.A.; Oderinwale, O.A.; Subedi, K.; et al. Synergistic Effects of Essential Oil Blends and Fumaric Acid on Ruminal Fermentation, Volatile Fatty Acid Production and Greenhouse Gas Emissions Using the Rumen Simulation Technique (RUSITEC). Fermentation 2024, 10, 114. [Google Scholar] [CrossRef]
- Duarte, A.C.; Holman, D.B.; Alexander, T.W.; Durmic, Z.; Vercoe, P.E.; Chaves, A.V. The Type of Forage Substrate Preparation Included as Substrate in a RUSITEC System Affects the Ruminal Microbiota and Fermentation Characteristics. Front. Microbiol. 2017, 8, 704. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. Comparison of Fermentation of Diets of Variable Composition and Microbial Populations in the Rumen of Sheep and Rusitec Fermenters. I. Digestibility, Fermentation Parameters, and Microbial Growth. J. Dairy Sci. 2010, 93, 3684–3698. [Google Scholar] [CrossRef]
- Orzuna-orzuna, J.F.; Dorantes-iturbide, G.; Lara-bueno, A.; Miranda-romero, L.A.; Mendoza-martínez, G.D.; Santiago-figueroa, I. A Meta—Analysis of Essential Oils Use for Beef Cattle Feed: Rumen Fermentation, Blood Metabolites, Meat Quality, Performance and, Environmental and Economic Impact. Fermentation 2022, 8, 254. [Google Scholar] [CrossRef]
- Mei, Z.; Yuan, J.; Li, D. Biological Activity of Galacto-Oligosaccharides: A Review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.A.S.; Grossi, S.; Dell’anno, M.; Compiani, R.; Rossi, L. Effect of a Blend of Essential Oils, Bioflavonoids and Tannins on In Vitro Methane Production and In Vivo Production Efficiency in Dairy Cows. Animals 2022, 12, 728. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Wanapat, M.; Shah, A.M.; Luo, X.; Peng, Q.; Kang, K.; Hu, R.; Guan, J.; Wang, Z. Ruminal PH Pattern, Fermentation Characteristics and Related Bacteria in Response to Dietary Live Yeast (Saccharomyces Cerevisiae) Supplementation in Beef Cattle. Anim. Biosci. 2022, 35, 184–195. [Google Scholar] [CrossRef]
- Khorrami, B.; Vakili, A.R.; Mesgaran, M.D.; Klevenhusen, F. Thyme and Cinnamon Essential Oils: Potential Alternatives for Monensin as a Rumen Modifier in Beef Production Systems. Anim. Feed. Sci. Technol. 2015, 200, 8–16. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Zebeli, Q. Meta-Analysis of the Effects of Essential Oils and Their Bioactive Compounds on Rumen Fermentation Characteristics and Feed Efficiency in Ruminants. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zhao, H.; Tu, Y.; Liu, M.; Jiang, L.; Zhao, Y. Characterization of the Dynamic Changes of Ruminal Microbiota Colonizing Citrus Pomace Waste during Rumen Incubation for Volatile Fatty Acid Production. Microbiol. Spectr. 2023, 11, e03517-22. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Cao, Z.; Wang, Y.; Li, S.; Wang, W. In Vitro Fermentation and Degradation Characteristics of Rosemary Extract in Total Mixed Ration of Lactating Dairy Cows. Fermentation 2022, 8, 461. [Google Scholar] [CrossRef]
- Bateki, C.A.; Wassie, S.E.; Wilkes, A. The Contribution of Livestock to Climate Change Mitigation: A Perspective from a Low-Income Country. Carbon. Manag. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Coşkuntuna, L.; Lackner, M.; Erten, K.; Gül, S.; Palangi, V.; Koç, F.; Esen, S. Greenhouse Gas Emission Reduction Potential of Lavender Meal and Essential Oil for Dairy Cows. Fermentation 2023, 9, 253. [Google Scholar] [CrossRef]
- Baraz, H.; Jahani-Azizabadi, H.; Azizi, O. Simultaneous Use of Thyme Essential Oil and Disodium Fumarate Can Improve in Vitro Ruminal Microbial Fermentation Characteristics. Vet. Res. Forum 2018, 9, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Jemaa, M.B.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.A.; Chaudhary, L.C.; Agarwal, N.; Chaturvedi, V.B.; Kamra, D.N. Effect of Feeding of Blend of Essential Oils on Methane Production, Growth, and Nutrient Utilization in Growing Buffaloes. Asian-Australas. J. Anim. Sci. 2018, 31, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Kamra, D.N.; Bhar, R.; Kumar, R.; Agarwal, N. Effect of Terminalia Chebula and Allium Sativum on in Vivo Methane Emission by Sheep. J. Anim. Physiol. Anim. Nutr. 2011, 95, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, H.; Bai, Y.; Wu, J.; Cheng, S.; He, B.; Casper, D.P. Calf Starter Containing a Blend of Essential Oils and Prebiotics Affects the Growth Performance of Holstein Calves. J. Dairy Sci. 2020, 103, 2315–2323. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy Contributions of Volatile Fatty Acids from the Gastrointestinal Tract in Various Species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Khateri, N.; Azizi, O.; Jahani-Azizabadi, H. Effects of a Specific Blend of Essential Oils on Apparent Nutrient Digestion, Rumen Fermentation and Rumen Microbial Populations in Sheep Fed a 50:50 Alfalfa Hay:Concentrate Diet. Asian-Australas. J. Anim. Sci. 2017, 30, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, N.L.; Harvatine, K.J. Acetate Dose-Dependently Stimulates Milk Fat Synthesis in Lactating Dairy Cows. J. Nutr. 2017, 147, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020, 11, 528227. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; de Lurdes Nunes Enes Dapkevicius, M.; Borba, A.E.S. Alternative Pathways for Hydrogen Sink Originated from the Ruminal Fermentation of Carbohydrates: Which Microorganisms Are Involved in Lowering Methane Emission? Anim. Microbiome 2022, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; López-Ferreras, L.; Andrés, S.; Mateos, I.; Horst, E.H.; López, S. Differential Diet and PH Effects on Ruminal Microbiota, Fermentation Pattern and Fatty Acid Hydrogenation in RUSITEC Continuous Cultures. Fermentation 2023, 9, 320. [Google Scholar] [CrossRef]
- Yi, X.; Wu, B.; Ma, J.; Cui, X.; Deng, Z.; Hu, S.; Li, W.; Runa, A.; Li, X.; Meng, Q.; et al. Effects of Dietary Capsaicin and Yucca Schidigera Extracts as Feed Additives on Rumen Fermentation and Microflora of Beef Cattle Fed with a Moderate-Energy Diet. Fermentation 2022, 9, 30. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K.; Raatikainen, K.; Holder, V.; Moran, C.A. Conversion of Branched-Chain Amino Acids to Corresponding Isoacids—An in Vitro Tool for Estimating Ruminal Protein Degradability. Front. Vet. Sci. 2019, 6, 311. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.P.; Ushida, K. The Role of Protozoa in Feed Digestion. Asian-Australas. J. Anim. Sci. 1999, 12, 113–128. [Google Scholar] [CrossRef]
- Susanto, I.; Rahmadani, M.; Wiryawan, K.G.; Laconi, E.B.; Jayanegara, A. Evaluation of Essential Oils as Additives during Fermentation of Feed Products: A Meta-Analysis. Fermentation 2023, 9, 583. [Google Scholar] [CrossRef]
- Hassan, F.U.; Arshad, M.A.; Ebeid, H.M.; Saif-Ur Rehman, M.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic Additives Can Modulate Rumen Microbiome to Mediate Fermentation Kinetics and Methanogenesis Through Exploiting Diet–Microbe Interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar] [CrossRef] [PubMed]
| Variables | Corn Silage | Alfalfa Hay | Concentrate | TMR |
|---|---|---|---|---|
| Dry matter, % | 38.13 | 83.88 | 90.76 | 68.12 |
| Organic matter, % | 96.49 | 90.95 | 83.28 | 93.08 |
| Crude protein, % | 6.31 | 16.00 | 20.25 | 10.03 |
| Crude fat, % | 4.67 | 3.15 | 8.49 | 4.73 |
| Total ash, % | 3.51 | 9.05 | 16.72 | 6.92 |
| Neutral detergent fiber, % | 59.40 | 49.47 | 74.05 | 61.86 |
| Acid detergent fiber, % | 14.10 | 9.56 | 15.38 | 12.04 |
| Acid detergent lignin, % | 14.51 | 18.24 | 10.45 | 13.70 |
| Dry matter intake | 2.02 | 2.43 | 1.62 | 1.94 |
| Dry matter digestibility | 77.92 | 81.45 | 76.92 | 79.52 |
| Relative forage value | 122.01 | 153.14 | 96.61 | 119.57 |
| Relative forage quality | 142.28 | 182.47 | 111.92 | 140.85 |
| Total digestible nutrients | 86.61 | 92.52 | 84.95 | 89.29 |
| Digestible crude protein | 2.69 | 11.57 | 15.46 | 6.10 |
| Gross energy, MJ/kg | 4.38 | 4.21 | 4.22 | 4.30 |
| Digestible energy, MJ/kg | 3.82 | 4.08 | 3.75 | 3.94 |
| Metabolizable energy, MJ/kg | 3.14 | 3.35 | 3.08 | 3.23 |
| Net energy of maintenance, MJ/kg | 2.38 | 2.62 | 2.35 | 2.49 |
| Net energy of gain, MJ/kg | 1.77 | 2.10 | 1.77 | 1.92 |
| Net energy of lactation, MJ/kg | 1.99 | 2.19 | 1.96 | 2.08 |
| Treatments 1 | pH | Gas Volume (mL) | Volume of Effluent (mL) | Ammonia Nitrogen |
|---|---|---|---|---|
| Control | 7.25 | 1648 | 438 | 433 |
| GEO | 7.30 | 1418 | 476 | 472 |
| MEO | 7.26 | 1487 | 441 | 503 |
| OLEO | 7.22 | 1537 | 479 | 445 |
| SEM | 0.047 | 128.6 | 28.6 | 77.3 |
| p-value | 0.634 | 0.640 | 0.620 | 0.923 |
| Treatments 1 | Methane (mg/g DM) | Carbon Dioxide (mg/g DM) | Ammonia (mmol/g DM) | Hydrogen Sulfide (mg/g DM) |
|---|---|---|---|---|
| Control | 64.1 | 297 | 433 | 3574 |
| GEO | 51.3 | 234 | 472 | 5852 |
| MEO | 61.3 | 289 | 503 | 6658 |
| OLEO | 63.6 | 300 | 445 | 4397 |
| SEM | 10.06 | 39.8 | 77.3 | 1103.1 |
| p-value | 0.786 | 0.617 | 0.923 | 0.198 |
| Treatments 1 | VFA | C2 | C3 | C4 | Iso-C4 | C5 | Iso-C5 | BCVFA | C2:C3 |
|---|---|---|---|---|---|---|---|---|---|
| Control | 62.1 a | 53.7 b | 27.3 | 14.5 | 0.482 ab | 3.85 | 0.181 | 0.6615 ab | 1.99 b |
| GEO | 52.9 b | 55.5 ab | 26.4 | 14.1 | 0.427 c | 3.43 | 0.170 | 0.5975 b | 2.11 ab |
| MEO | 55.4 ab | 54.3 ab | 26.5 | 14.5 | 0.496 a | 4.07 | 0.186 | 0.6805 a | 2.06 ab |
| OLEO | 56.8 ab | 55.7 a | 25.7 | 14.1 | 0.449 bc | 3.84 | 0.173 | 0.6215 ab | 2.20 a |
| SEM | 2.36 | 0.48 | 0.52 | 0.32 | 0.0122 | 0.253 | 0.0078 | 0.01925 | 0.056 |
| p-value | 0.045 | 0.011 | 0.215 | 0.676 | 0.001 | 0.339 | 0.435 | 0.013 | 0.050 |
| Variables | pH | DMD | NDFD | ADFD | ADLD | CH4 | CO2 | NH3 | H2S |
|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | −0.16 | 0.05 | 0.06 | 0.03 | −0.71 *** | −0.72 *** | −0.61 *** | −0.52 *** |
| Gas volume | −0.58 *** | 0.02 | 0.17 | 0.15 | 0.00 | 0.91 *** | 0.93 *** | 0.79 *** | 0.61 *** |
| Effluent volume | −0.56 | 0.01 | −0.10 | 0.01 | −0.13 | 0.42 *** | 0.37 ** | 0.41 *** | 0.31 ** |
| NH3-N | 0.01 | 0.07 | −0.21 | 0.00 | 0.18 | 0.09 | 0.07 | 0.13 | 0.11 |
| TVFA | −0.57 *** | 0.09 | −0.11 | −0.23 | 0.22 | 0.45 *** | 0.47 *** | 0.34 ** | 0.23 * |
| Acetate | −0.33 ** | 0.00 | 0.02 | −0.03 | −0.18 | 0.27 * | 0.28 * | 0.21 | 0.17 |
| Propionate | −0.21 | 0.28 * | −0.11 | −0.03 | 0.13 | 0.26 * | 0.21 | 0.29 * | 0.19 |
| Butyrate | 0.42 *** | −0.34 ** | 0.09 | −0.04 | 0.07 | −0.40 *** | −0.40 *** | −0.32 ** | −0.19 |
| Iso-butyrate | 0.44 *** | 0.05 | 0.08 | −0.07 | 0.38 ** | −0.10 | −0.14 | 0.02 | −0.06 |
| Valerate | 0.52 *** | −0.15 | 0.08 | 0.15 | −0.15 | −0.52 *** | −0.47 *** | −0.54 *** | −0.36 ** |
| Isovalerate | 0.59 *** | −0.12 | −0.03 | −0.18 | 0.28 | −0.30 ** | −0.33 ** | −0.17 | −0.17 |
| BCVFA | 0.52 *** | −0.02 | 0.05 | −0.11 | 0.36 * | −0.18 | −0.22 | −0.05 | −0.10 |
| APR | 0.03 | −0.25 * | 0.08 | 0.00 | −0.14 | −0.06 | −0.02 | −0.11 | −0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabi, J.O.; Wuaku, M.; Anotaenwere, C.C.; Okedoyin, D.O.; Adelusi, O.O.; Ike, K.A.; Gray, D.; Kholif, A.E.; Subedi, K.; Anele, U.Y. A Mixture of Prebiotics, Essential Oil Blends, and Onion Peel Did Not Affect Greenhouse Gas Emissions or Nutrient Degradability, but Altered Volatile Fatty Acids Production in Dairy Cows Using Rumen Simulation Technique (RUSITEC). Fermentation 2024, 10, 324. https://doi.org/10.3390/fermentation10060324
Alabi JO, Wuaku M, Anotaenwere CC, Okedoyin DO, Adelusi OO, Ike KA, Gray D, Kholif AE, Subedi K, Anele UY. A Mixture of Prebiotics, Essential Oil Blends, and Onion Peel Did Not Affect Greenhouse Gas Emissions or Nutrient Degradability, but Altered Volatile Fatty Acids Production in Dairy Cows Using Rumen Simulation Technique (RUSITEC). Fermentation. 2024; 10(6):324. https://doi.org/10.3390/fermentation10060324
Chicago/Turabian StyleAlabi, Joel O., Michael Wuaku, Chika C. Anotaenwere, Deborah O. Okedoyin, Oludotun O. Adelusi, Kelechi A. Ike, DeAndrea Gray, Ahmed E. Kholif, Kiran Subedi, and Uchenna Y. Anele. 2024. "A Mixture of Prebiotics, Essential Oil Blends, and Onion Peel Did Not Affect Greenhouse Gas Emissions or Nutrient Degradability, but Altered Volatile Fatty Acids Production in Dairy Cows Using Rumen Simulation Technique (RUSITEC)" Fermentation 10, no. 6: 324. https://doi.org/10.3390/fermentation10060324
APA StyleAlabi, J. O., Wuaku, M., Anotaenwere, C. C., Okedoyin, D. O., Adelusi, O. O., Ike, K. A., Gray, D., Kholif, A. E., Subedi, K., & Anele, U. Y. (2024). A Mixture of Prebiotics, Essential Oil Blends, and Onion Peel Did Not Affect Greenhouse Gas Emissions or Nutrient Degradability, but Altered Volatile Fatty Acids Production in Dairy Cows Using Rumen Simulation Technique (RUSITEC). Fermentation, 10(6), 324. https://doi.org/10.3390/fermentation10060324









