Biohydrogen Production under Aerial Conditions by a Nitrogen-Fixing Bacterium Isolated from a Steel Signboard
Abstract
1. Introduction
2. Materials and Methods
2.1. Subsection
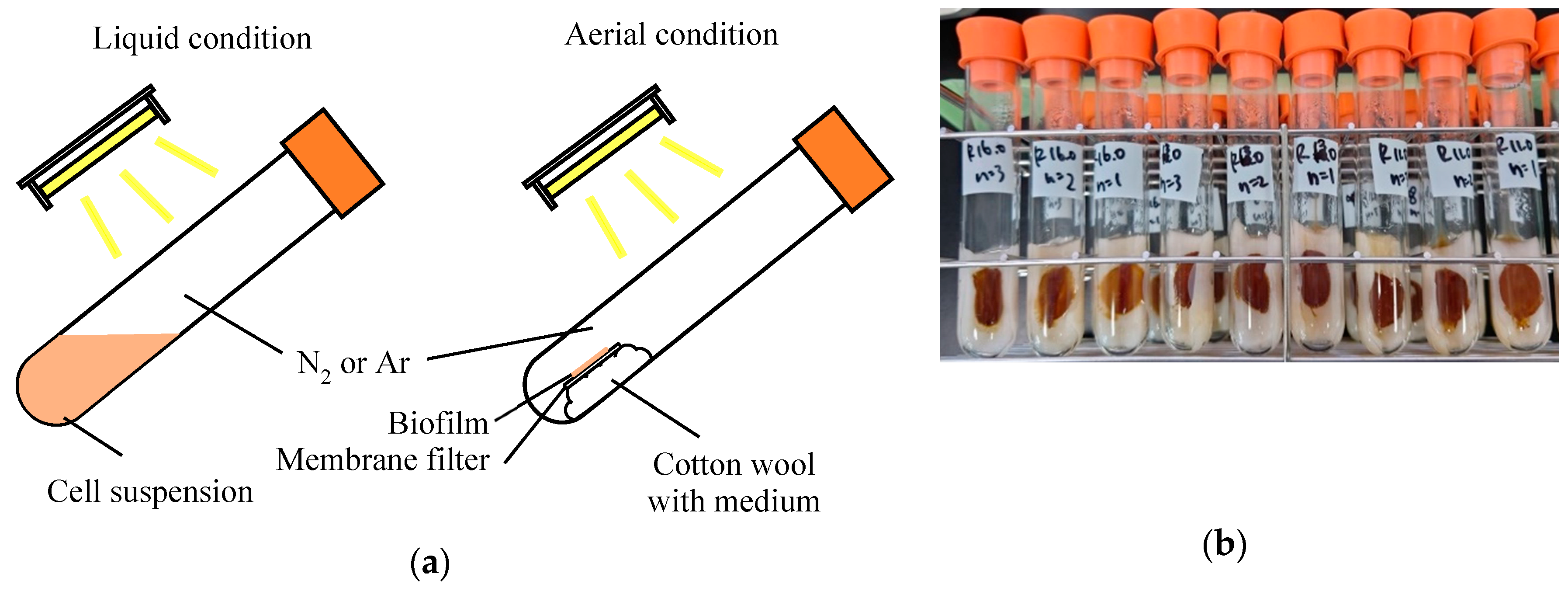
2.2. Liquid and Aerial Culture Conditions
2.3. Hydrogen Analysis
2.4. 16S rRNA Analysis and Phylogenetic Study
2.5. Statistical Analysis
3. Results and Discussion
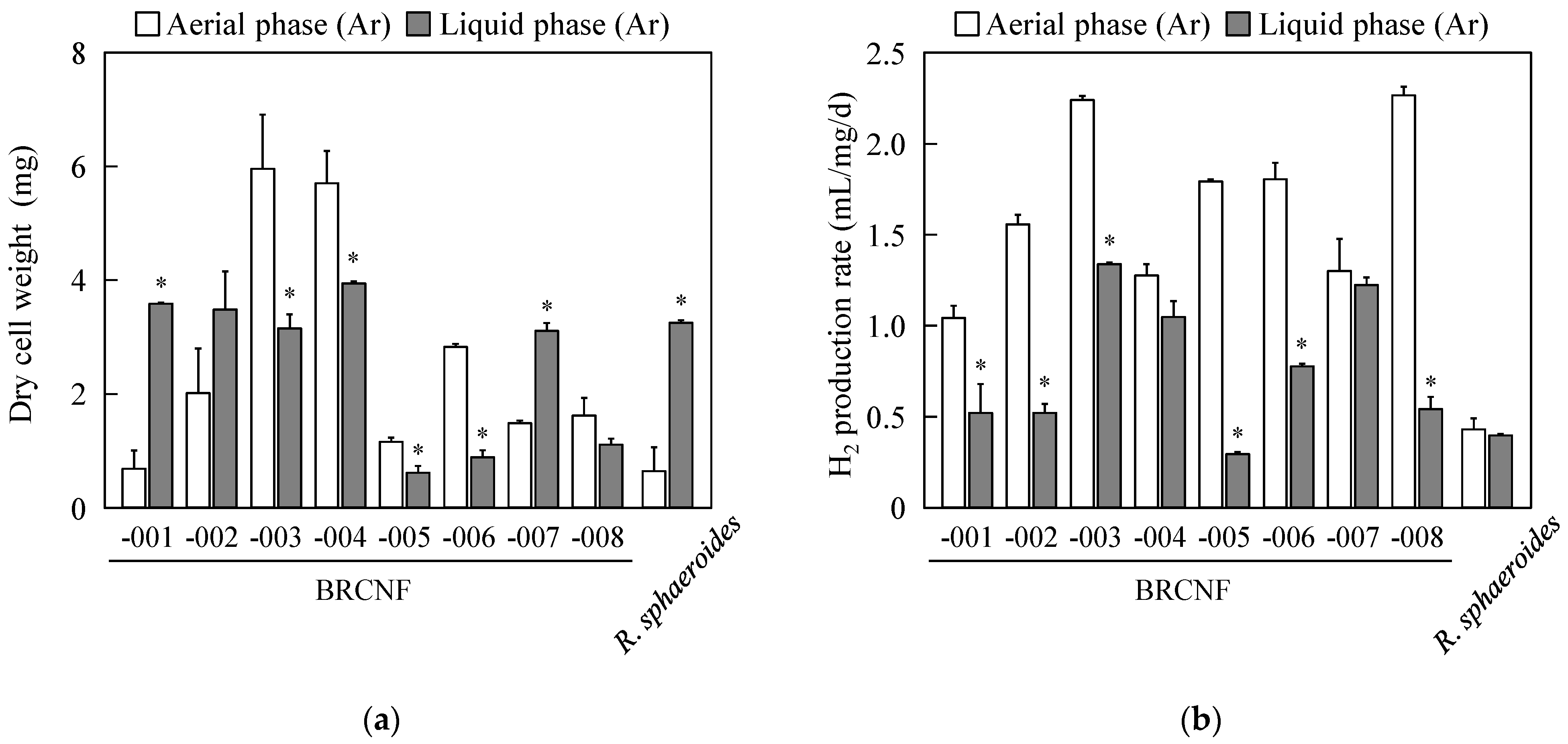
3.1. Biohydrogen Production under Aerial- and Liquid-Phase Conditions
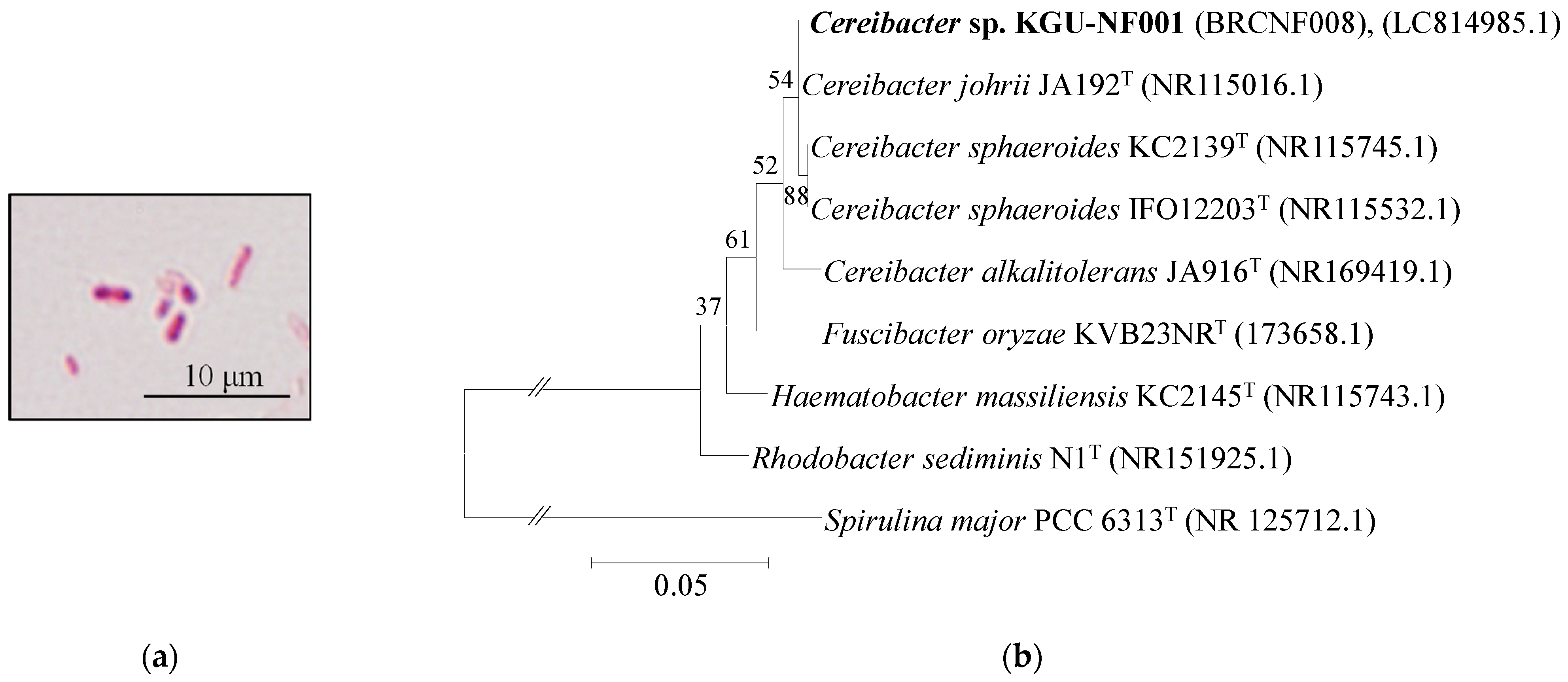
3.2. Phylogenetic Analysis of the Bacterial Strain of BRCNF008
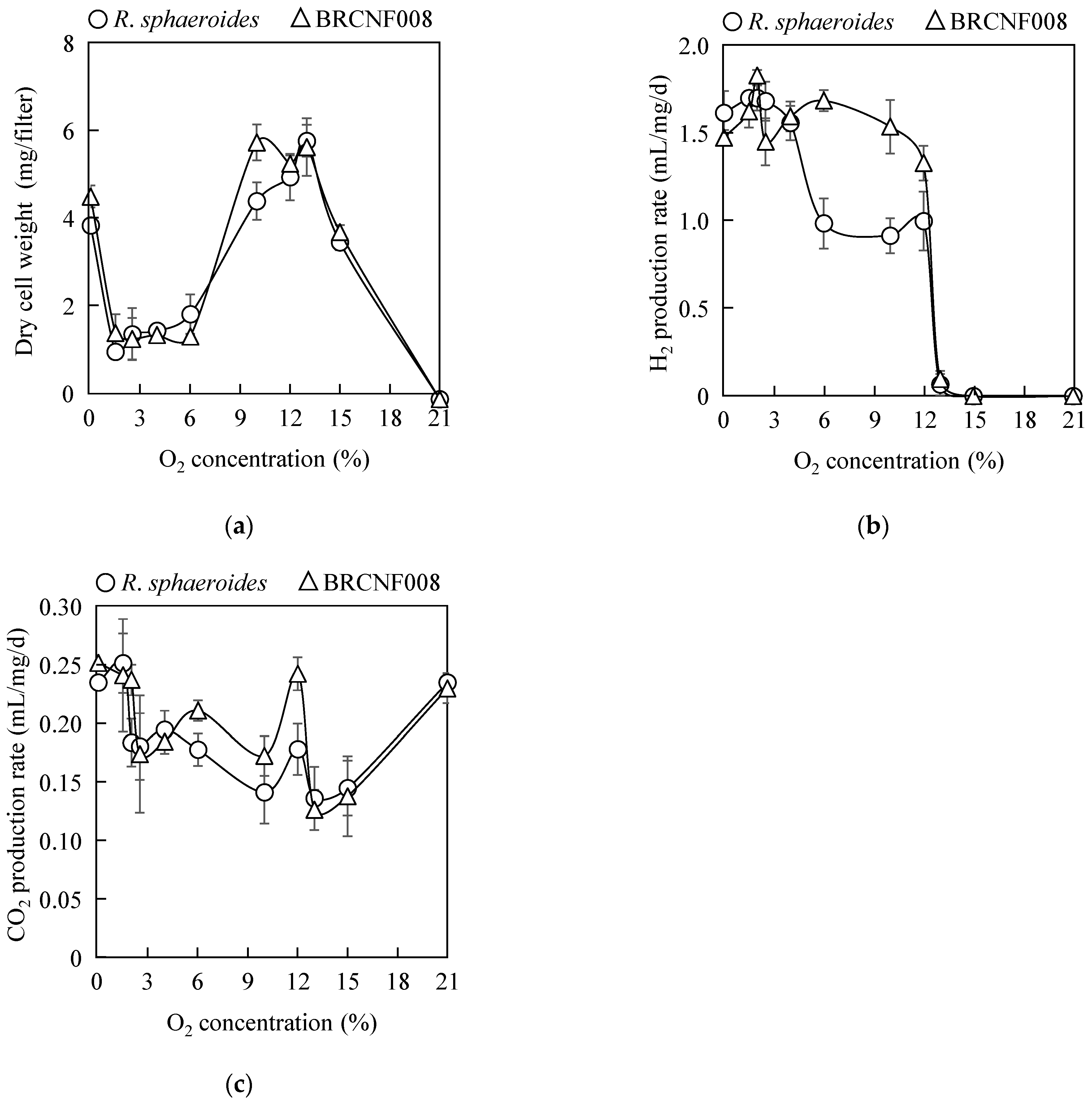
3.3. Influence of O2 on H2 Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Dowell, N.M.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kotay, S.M.; Das, D. Biohydrogen as a renewable energy resource—Prospects and potentials. Int. J. Hydrogen Energy 2008, 33, 258–263. [Google Scholar] [CrossRef]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.; Ma, X.Y.; Hoang, B.N. Biohydrogen production, storage, and delivery: A comprehensive overview of current strategies and limitations. Chem. Eng. J. 2023, 471, 144669. [Google Scholar] [CrossRef]
- Mokheimer, E.M.A.; Shakeel, M.R.; Harale, A.; Paglieri, S.; Mansour, R.B. Fuel reforming processes for hydrogen production. Fuel 2024, 359, 130427. [Google Scholar] [CrossRef]
- Yadav, D.; Lu, X.; Ma, B.-C.; Jing, D. Advancements in microreactor technology for hydrogen production via steam reforming: A comprehensive review of numerical studies. J. Power Sources 2024, 596, 234090. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Kumar, P.S.; Vinayagam, R.; Selvaraj, R.; Rangasamy, G. Recent advances in fermentative biohydrogen production. Int. J. Hydrogen Energy 2024, 54, 200–217. [Google Scholar] [CrossRef]
- Woon, J.M.; Khoo, K.S.; AL-Zahrani, A.A.; Alanazi, M.M.; Lim, J.W.; Cheng, C.K.; Sahrin, N.T.; Ardo, F.M.; Yi-Ming, S.; Lin, K.-S.; et al. Epitomizing biohydrogen production from microbes: Critical challenges vs opportunities. Environ. Res. 2023, 227, 115780. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Dutta, K.; Khoo, K.S.; Soto-Moscoso, M. An overview on light assisted techniques for waste-derived hydrogen fuel towards aviation industry. Fuel 2023, 334, 126645. [Google Scholar] [CrossRef]
- Iragavarapu, G.P.; Imam, S.S.; Sarkar, O.; Mohan, S.V.; Chang, Y.-C.; Reddy, M.V.; Kim, S.-H.; Amradi, N.K. Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy. Energies 2023, 16, 3873. [Google Scholar] [CrossRef]
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogen Energy 2023, 48, 21088–21109. [Google Scholar] [CrossRef]
- Jiao, H.; Tsigkou, K.; Elsamahy, T.; Pispas, K.; Sun, J.; Manthos, G.; Schagerl, M.; Sventzouri, E.; Al-Tohamy, R.; Kornaros, M.; et al. Recent advances in sustainable hydrogen production from microalgae: Mechanisms, challenges, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 270, 115908. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Nizami, M.; Qyyum, M.A.; Iqbal, N.; Al-Muhtaseb, A.H.; Hasan, M. Sustainable hydrogen production via microalgae: Technological advancements, economic indicators, environmental aspects, challenges, and policy implications. Environ. Res. 2024, 244, 117815. [Google Scholar] [CrossRef]
- Suresh, G.; Kumari, P.; Mohan, S.V. Light-dependent biohydrogen production: Progress and perspectives. Bioresour. Technol. 2023, 380, 129007. [Google Scholar] [CrossRef] [PubMed]
- Davila-Vazquez, G.; Arriaga, S.; Alatriste-Mondragón, F.; de León-Rodríguez, A.; Rosales-Colunga, L.; Razo-Flores, E. Fermentative biohydrogen production: Trends and perspectives. Rev. Environ. Sci. Biotechnol. 2008, 7, 27–45. [Google Scholar] [CrossRef]
- Xuan, J.; He, L.; Wen, W.; Feng, Y. Hydrogenase and Nitrogenase: Key Catalysts in Biohydrogen Production. Molecules 2023, 28, 1392. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.; Ribbe, M. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Sgrignani, J.; Franco, D.; Magistrato, A. Theoretical Studies of Homogeneous Catalysts Mimicking Nitrogenase. Molecules 2011, 16, 442–465. [Google Scholar] [CrossRef]
- Rutledge, H.L.; Cook, B.D.; Nguyen, H.P.M.; Herzik, M.A., Jr.; Tezcan, F.A. Structures of the nitrogenase complex prepared under catalytic turnover conditions. Science 2022, 377, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Ruzicka, J.; Kallas, H.; Chica, B.; Mulder, D.W.; Peters, J.W.; Seefeldt, L.C.; Dukovic, G.; King, P.W. Excitation-Rate Determines Product Stoichiometry in Photochemical Ammonia Production by CdS Quantum Dot-Nitrogenase MoFe Protein Complexes. ACS Catal. 2020, 10, 11147–11152. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Ohkubo, K.; Aburai, N.; Miyauchi, H.; Tsuzuki, M.; Abe, K. CO2 fixation and lipid accumulation in biofilms of the aerial microalga Coccomyxa sp. KGU-D001 (Trebouxiophyceae). J. Appl. Phycol. 2017, 29, 1745–1753. [Google Scholar] [CrossRef]
- Aburai, N.; Onda, T.; Fujii, K. Carotenogenesis and carotenoid esterification in biofilms of the microalga Coelastrella rubescens KGU-Y002 in the aerial phase. Algal Res. 2022, 67, 102847. [Google Scholar] [CrossRef]
- Aburai, N.; Morita, R.; Miyauchi, H.; Okada, K.; Sato, N.; Fujiwara, S.; Fujii, K. Acclimation of the Aerial Microalga Coccomyxa subellipsoidea KGU-D001 to Water Stress in the Aerial Phase. Bioenerg. Res. 2023, 17, 622–633. [Google Scholar] [CrossRef]
- Aburai, N.; Tsukagoshi, T.; Sekiguchi, S.; Arakawa, H.; Imamura, Y.; Abe, K. Mutual supply of carbon and nitrogen sources in the co-culture of aerial microalgae and nitrogen-fixing bacteria. Algal Res. 2023, 70, 103001. [Google Scholar] [CrossRef]
- Zhong, N.; Liao, Q.; Zhu, X.; Chen, R. A Fiber-Optic Sensor for Accurately Monitoring Biofilm Growth in a Hydrogen Production Photobioreactor. Anal. Chem. 2014, 86, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Zhong, N.; Zhu, X.; Huang, Y.; Chen, R. Enhancement of hydrogen production by optimization of biofilm growth in a photobioreactor. Int. J. Hydrogen Energy 2015, 40, 4741–4751. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Aburai, N.; Fujii, K. Development of Digested Sludge-Assimilating and Biohydrogen-Yielding Microflorae. Fermentation 2023, 9, 175. [Google Scholar] [CrossRef]
- Sato, S.; Ichiyanagi, N.; Sugiyama, K.; Aburai, N.; Fujii, K. Production of polyglutamic acid-like mucilage protein by Peribacillus simplex strain 8h. Folia Microbiol. 2023, 68, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, L.; Wei, W.; Zhou, Z. Function of glucose catabolic pathways in hydrogen production from glucose in Rhodobacter sphaeroides 6016. Int. J. Hydrogen Energy 2014, 39, 4215–4221. [Google Scholar] [CrossRef]
- Hucker, G.J. A New Modification and Application of the Gram Stain. J. Bacteriol. 1921, 6, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Koku, H.; Eroğlu, İ.; Gündüz, U.; Yücel, M.; Türker, L. Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2002, 27, 1315–1329. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Wahid, Z.A. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strat. Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Parakh, S.K.; Tian, Z.; Wong, J.Z.E.; Tong, Y.W. From Microalgae to Bioenergy: Recent Advances in Biochemical Conversion Processes. Fermentation 2023, 9, 529. [Google Scholar] [CrossRef]
- Akköse, S.; Gündüz, U.; Yücel, M.; Eroglu, I. Effects of ammonium ion, acetate and aerobic conditions on hydrogen production and expression levels of nitrogenase genes in Rhodobacter sphaeroides O.U.001. Int. J. Hydrogen Energy 2009, 34, 8818–8827. [Google Scholar] [CrossRef]
- Girija, K.R.; Sasikala, C.; Ramana, C.V.; Spröer, C.; Takaichi, S.; Thiel, V.; Imhoff, J.F. Rhodobacter johrii sp. nov., an endospore-producing cryptic species isolated from semi-arid tropical soils. Int. J. Syst. Evol. Microbiol. 2010, 60, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.-M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1,000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hashesh, M.; Hallenbeck, P.C. Microaerobic dark fermentative hydrogen production by the photosynthetic bacterium, Rhodobacter capsulatus JP91. Int. J. Low Carbon Technol. 2012, 7, 97–103. [Google Scholar] [CrossRef]
- Goldberg, I.; Nadler, V.; Hochman, A. Mechanism of nitrogenase switch-off by oxygen. J. Bacteriol. 1987, 169, 874–879. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Sargsyan, H.; Trchounian, A. Novel properties of photofermentative biohydrogen production by purple bacteria Rhodobacter sphaeroides: Effects of protonophores and inhibitors of responsible enzymes. Microb. Cell Fact. 2015, 14, 131. [Google Scholar] [CrossRef]
- Yakunin, A.; Hallenbeck, P. Regulation of nitrogenase activity in Rhodobacter capsulatus under dark microoxic conditions. Arch. Microbiol. 2000, 173, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, L.; Sargsyan, H.; Hakobyan, L.; Trchounian, A. Regulation of hydrogen photoproduction in Rhodobacter sphaeroides batch culture by external oxidizers and reducers. Appl. Energy 2014, 131, 20–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aburai, N.; Tanaka, H.; Kohira, H.; Sekine, T. Biohydrogen Production under Aerial Conditions by a Nitrogen-Fixing Bacterium Isolated from a Steel Signboard. Fermentation 2024, 10, 248. https://doi.org/10.3390/fermentation10050248
Aburai N, Tanaka H, Kohira H, Sekine T. Biohydrogen Production under Aerial Conditions by a Nitrogen-Fixing Bacterium Isolated from a Steel Signboard. Fermentation. 2024; 10(5):248. https://doi.org/10.3390/fermentation10050248
Chicago/Turabian StyleAburai, Nobuhiro, Honami Tanaka, Hana Kohira, and Tinami Sekine. 2024. "Biohydrogen Production under Aerial Conditions by a Nitrogen-Fixing Bacterium Isolated from a Steel Signboard" Fermentation 10, no. 5: 248. https://doi.org/10.3390/fermentation10050248
APA StyleAburai, N., Tanaka, H., Kohira, H., & Sekine, T. (2024). Biohydrogen Production under Aerial Conditions by a Nitrogen-Fixing Bacterium Isolated from a Steel Signboard. Fermentation, 10(5), 248. https://doi.org/10.3390/fermentation10050248





