Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enrichment of Alkaliphilic Microalgae
2.3. Isolation of Algal Strains
2.4. Growth Test for Microalgal Isolates at Various pH
2.5. Growth Test for Microalgae under Various CO2 Concentrations
2.6. Phylogenetic Study of the Isolates
2.7. Biogas Upgrading Assay
2.8. Carotenoids Analysis
2.9. Protein and Amino Acids Analysis
2.10. Fatty Acid Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Enrichment of High-Level CO2-Tolerant Alkaliphilic Microalgae
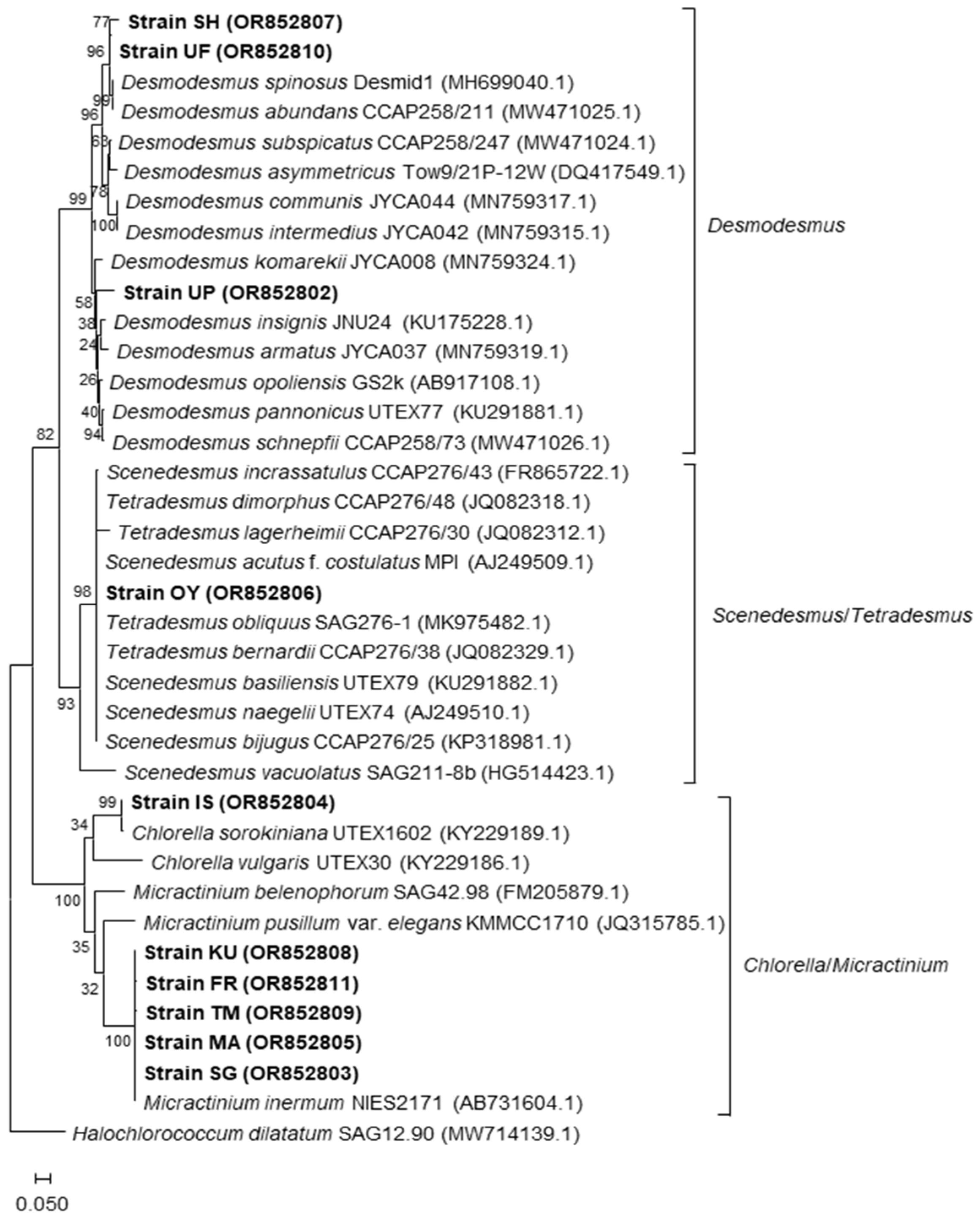
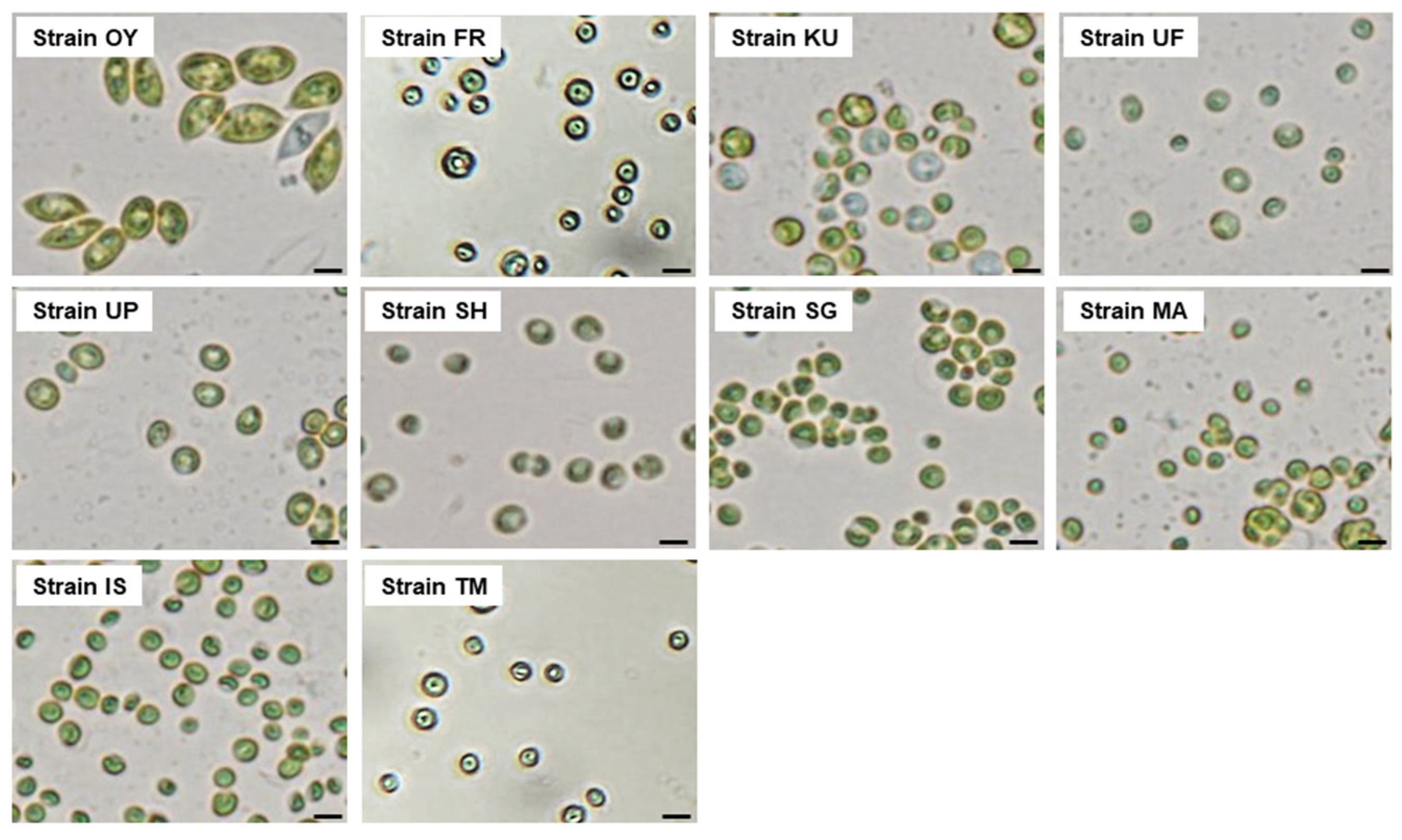
3.2. Phylogenetic Identification of the Microalgal Strains
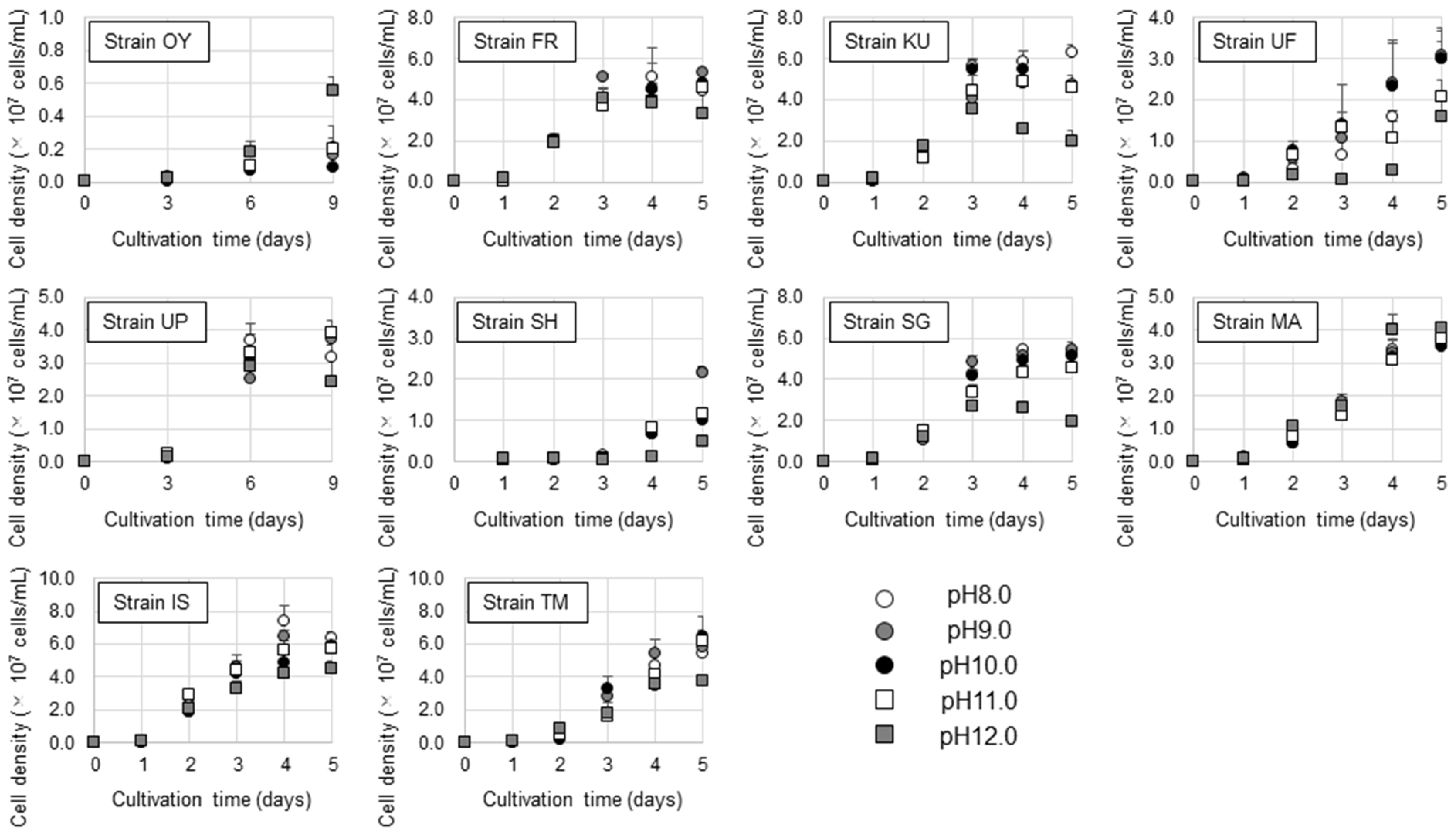
3.3. Growth Characteristics under Various pH and CO2 Levels
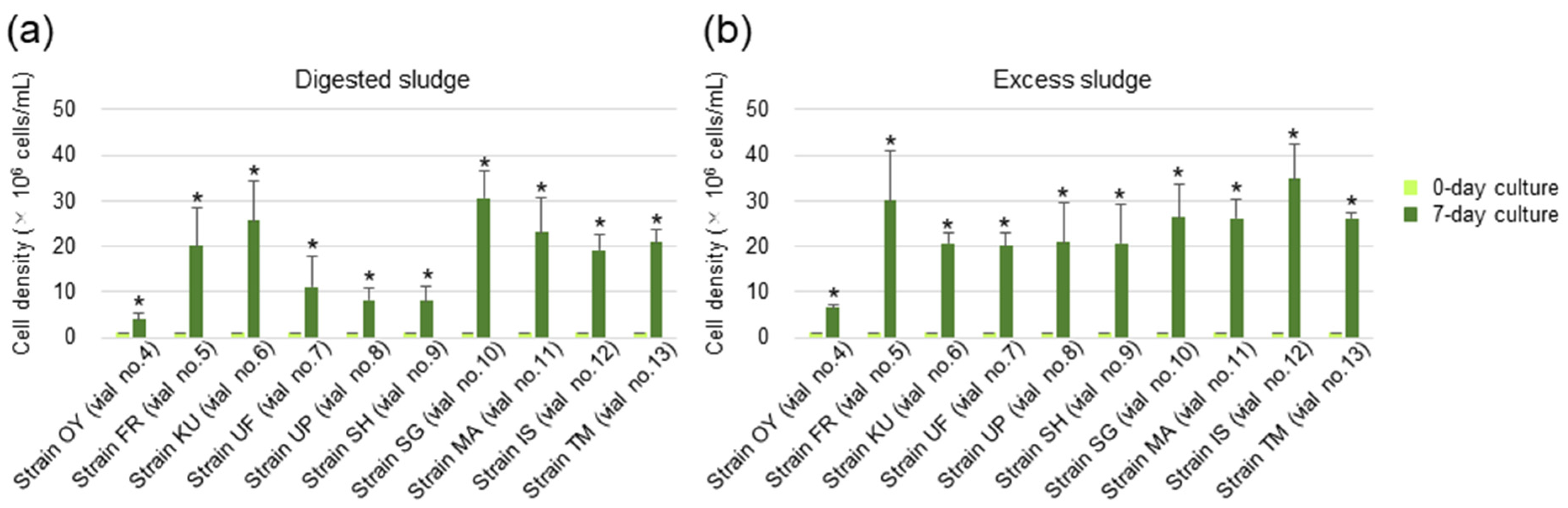
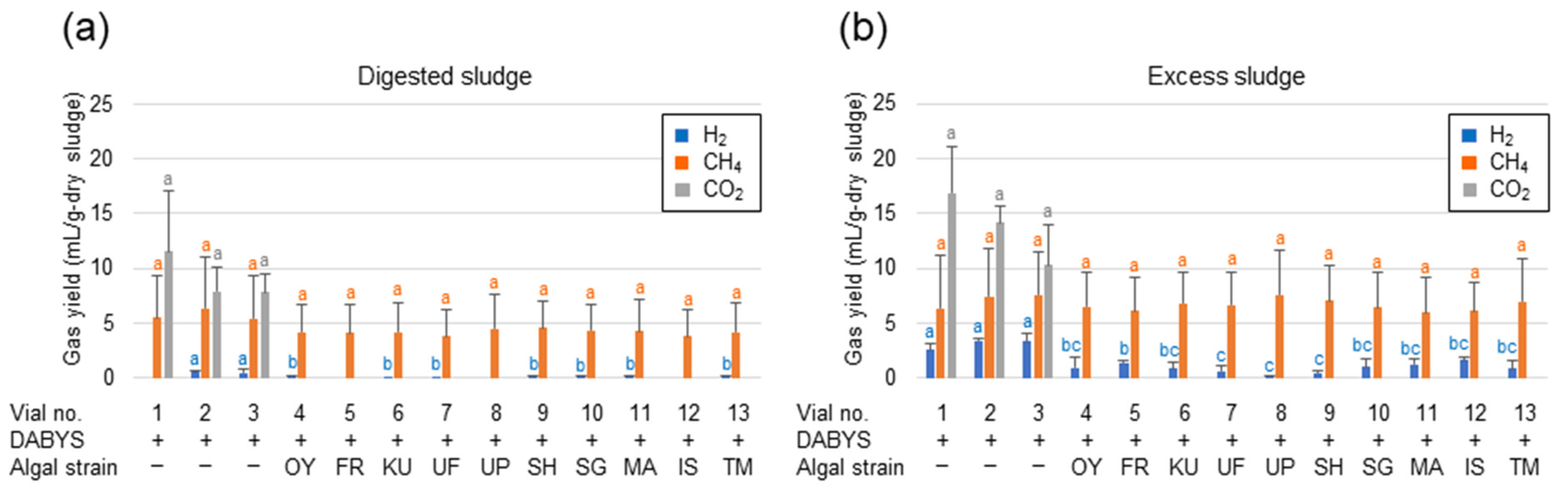
3.4. Upgrading of Biogas by the Algal Strains
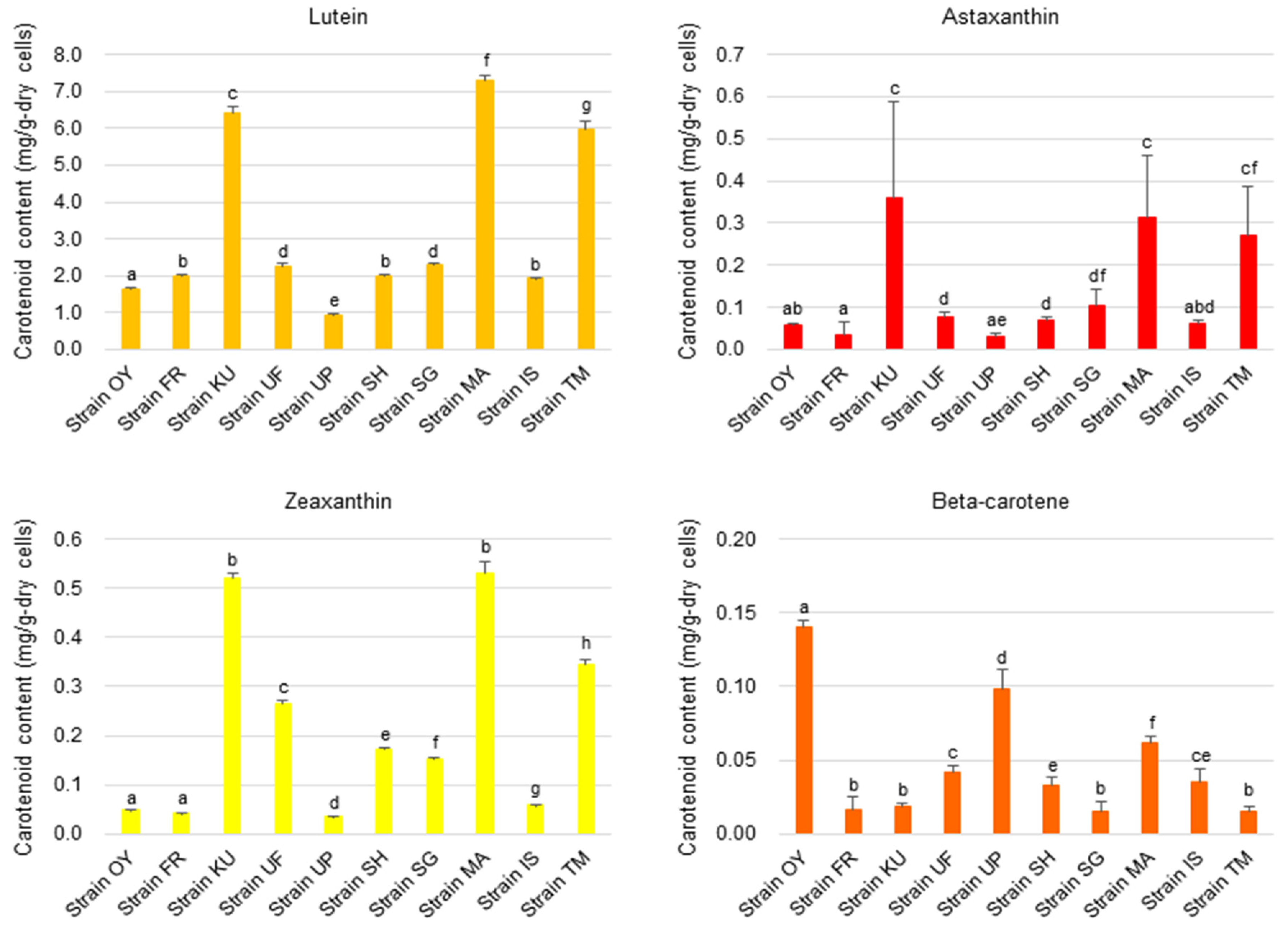
3.5. Chemical Composition of the Algal Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. CO2 Emissions in 2022. 2023. Available online: https://iea.blob.core.windows.net/assets/3c8fa115-35c4-4474-b237-1b00424c8844/CO2Emissionsin2022.pdf (accessed on 30 December 2023).
- Manish Pillai, I.R.; Banerjee, R. Sustainability analysis of renewables for climate change mitigation. Energy Sustain. Develop. 2006, 10, 25–36. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Kemper, J. Biomass and carbon dioxide capture and storage: A review. Int. J. Greenh. Gas Cont. 2015, 40, 401–430. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative techno-economic analysis of carbon capture processes: Pre-combustion, post-combustion, and oxy-fuel combustion operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Yasemi, S.; Khalili, Y.; Sanati, A.; Bagheri, M. Carbon capture and storage: Application in the oil and gas industry. Sustainability 2023, 15, 14486. [Google Scholar] [CrossRef]
- Bajpai, S.; Shreyash, N.; Singh, S.; Memon, A.R.; Sonker, M.; Kr Tiwary, S.; Biswas, S. Opportunities, challenges and the way ahead for carbon capture, utilization and sequestration (CCUS) by the hydrocarbon industry: Towards a sustainable future. Energ. Rep. 2022, 8, 15595–15616. [Google Scholar] [CrossRef]
- Langie, K.M.G.; Tak, K.; Kim, C.; Lee, H.W.; Park, K.; Kim, D.; Jung, W.; Lee, C.W.; Oh, H.S.; Lee, D.K.; et al. Toward economical application of carbon capture and utilization technology with near-zero carbon emission. Nat. Commun. 2022, 13, 7482. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilization technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Padhi, D.; Sen, R.; Nayak, M. Bio-inspired CO2 capture and utilization by microalgae for bioenergy feedstock production: A greener approach for environmental protection. Bioresour. Technol. Rep. 2022, 19, 101116. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Qv, M.; Dai, D.; Liu, D.; Wang, W.; Tang, C.; Zhu, L. Microalgal cultivation for the upgraded biogas by removing CO2, coupled with the treatment of slurry from anaerobic digestion: A review. Bioresour. Technol. 2022, 364, 128118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Kratzer, R.; Murkovic, M. Food ingredients and nutraceuticals from microalgae: Main product classes and biotechnological production. Foods 2021, 10, 1626. [Google Scholar] [CrossRef]
- Casanova, L.M.; Mendes, L.B.B.; Corrêa, T.d.S.; da Silva, R.B.; Joao, R.R.; Macrae, A.; Vermelho, A.B. Development of microalgae biodiesel: Current status and perspectives. Microorganisms 2023, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Almeida, F.; Rocha, F.A.; Ferreira, A. Improving CO2 mass transfer in microalgal cultures using an oscillatory flow reactor with smooth periodic constrictions. J. Environ. Chem. Eng. 2021, 9, 106505. [Google Scholar] [CrossRef]
- Becker, E.W. (Ed.) Culture media. In Microalgae. Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; pp. 9–42. [Google Scholar]
- Cents, A.; Brilman, D.; Versteeg, G. CO2 absorption in carbonate/bicarbonate solutions: The Danckwerts-criterion revisited. Chem. Eng. Sci. 2005, 60, 5830–5835. [Google Scholar] [CrossRef]
- La Plante, E.; Wang, J.; Alturki, A.; Simonetti, D.; Jassby, D.; Sant, G. Saline water-based mineralization pathway for gigatonne-scale CO2 management. ACS Sustain. Chem. Eng. 2021, 9, 1073–1089. [Google Scholar] [CrossRef]
- Ochoa-Alfaro, A.E.; Gaytán-Luna, D.E.; González-Ortega, O.; Zavala-Arias, K.G.; Paz-Maldonado, L.M.T.; Rocha-Uribe, A. pH effects on the lipid and fatty acids accumulation in Chlamydomonas reinhardtii. Biotechnol. Prog. 2019, 35, e2891. [Google Scholar] [CrossRef] [PubMed]
- Andeden, E.E.; Ozturk, S.; Aslim, B. Effect of alkaline pH and nitrogen starvation on the triacylglycerol (TAG) content, growth, biochemical composition, and fatty acid profile of Auxenochlorella protothecoides KP7. J. Appl. Phycol. 2021, 33, 211–225. [Google Scholar] [CrossRef]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Ogawa, T. Studies on the growth of Spirulina platensis: (I) On the pure culture of Spirulina platensis. J. Ferment. Technol. 1970, 48, 361–367. [Google Scholar]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol:chloroform. Cold Springs Harbor Protoc. 2006, 2006, pdb-prot4455. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–522. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Shiozaki, K.; Okumura, Y.; Ishikawa, M.; Waqalevu, V.; Hayasaka, O.; Honda, A.; Kotani, T. Effects of phosphorous deficiency of a microalga Nannochloropsis oculata on its fatty acid profiles and intracellular structure and the effectiveness in rotifer nutrition. Algal Res. 2020, 49, 101905. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–78. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Toi, K. Pressure dependence of diffusion coefficient for CO2 in glassy polymers. Polym. Eng. Sci. 1980, 20, 30–35. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kurihara, I.; Kawano, S. Late type of daughter cell wall synthesis in one of the Chlorellaceae, Parachlorella kessleri (Chlorophyta, Trebouxiophyceae). Planta 2005, 221, 766–775. [Google Scholar] [CrossRef]
- Hadi, S.I.I.A.; Santana, H.; Brunale, P.P.M.; Gomes, T.G.; Oliveira, M.D.; Matthiensen, A.; Oliveira, M.E.C.; Silva, F.C.P.; Brasil, B.S.A.F. DNA barcoding green microalgae isolated from neotropical inland waters. PLoS ONE 2016, 11, e0149284. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F. Biology and industrial applications of Chlorella: Advances and prospects. In Microalgae Biotechnology; Posten, C., Chen, S.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 153, pp. 1–35. [Google Scholar]
- Bell, T.A.; Prithiviraj, B.; Wahlen, B.D.; Fields, M.W.; Peyton, B.M. A lipid-accumulating alga maintains growth in outdoor, alkaliphilic raceway pond with mixed microbial communities. Front. Microbiol. 2015, 6, 1480. [Google Scholar] [CrossRef]
- Vadlamani, A.; Pendyala, B.; Viamajala, S.; Varanasi, S. High productivity cultivation of microalgae without concentrated CO2 input. ACS Sustain. Chem. Eng. 2019, 7, 1933–1943. [Google Scholar] [CrossRef]
- Sung, K.D.; Lee, J.S.; Shin, C.S.; Park, S.C.; Cho, M.J. CO2 fixation by Chlorella sp. KR-1 and its cultural characteristics. Bioresour. Technol. 1999, 68, 269–273. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Pan, K.; Zhu, B.; Li, Y.; Ma, X.; Zhao, Y. The regulating mechanisms of CO2 fixation and carbon allocations of two Chlorella sp. strains in response to high CO2 levels. Chemosphere 2020, 247, 125814. [Google Scholar] [CrossRef]
- Sergeenko, T.V.; Muradyan, E.A.; Pronina, N.A.; Klyachko-Gurvich, G.L.; Mishina, I.M.; Tsoglin, L.N. The effect of extremely high CO2 concentration on the growth and biochemical composition of microalgae. Russ. J. Plant Physiol. 2000, 47, 632–638. [Google Scholar]
- China, S.; Fujii, K. Isolation of high-CO2-acclimated Micractinium sp. strains from eutrophic reservoir water. Algal Res. 2018, 34, 126–133. [Google Scholar] [CrossRef]
- Cao, B.; Hu, S.; Zhu, K.; Pan, C.; Marrakchi, F.; Ni, J.; Yuan, C.; Qian, L.; Chen, H.; Yuan, J.; et al. Response surface optimization of product yields and biofuel quality during fast hydrothermal liquefaction of a highly CO2-tolerant microalgae. Sci. Total Environ. 2023, 860, 160541. [Google Scholar] [CrossRef]
- Jothibasu, K.; Muniraj, I.; Jayakumar, T.; Ray, B.; Dhar, D.W.; Karthikeyan, S.; Rakesh, S. Impact of microalgal cell wall biology on downstream processing and nutrient removal for fuels and value-added products. Biochem. Eng. J. 2022, 187, 108642. [Google Scholar] [CrossRef]
- Kiani, H.; Azimi, Y.; Li, Y.; Mousavi, M.; Cara, F.; Mulcahy, S.; McDonnell, H.; Blanco, A.; Halim, R. Nitrogen and phosphate removal from dairy processing side-streams by monocultures or consortium of microalgae. J. Biotechnol. 2023, 361, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sust. Energ. Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquacult. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- de Almeida Moreira, B.R.; de Almeida Viana, C.R.; Cruz, V.H.; Lopes, P.R.M.; da Silva Viana, R.; Ramos, R.A.V. Meta-analytic review on third-generation biodiesel. Bioenerg. Res. 2022, 15, 27–45. [Google Scholar] [CrossRef]
- Gardner, R.; Peters, P.; Peyton, B.; Cooksey, K.E. Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta. J. Appl. Phycol. 2011, 23, 1005–1016. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Chen, F. High throughput screening of CO2-tolerating microalgae using GasPak bags. Aquat. Biosyst. 2013, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, M.J.; Retotar, A.; Rismani-Yazdi, H.; Peccia, J. Using carbon dioxide to maintain an elevated oleaginous microalga concentration in mixed-culture photo-bioreactors. Bioresour. Technol. 2015, 185, 178–184. [Google Scholar] [CrossRef]
- Scherbakov, P.; Ismagulova, T.; Chernov, T.; Gorelova, O.; Selyakh, I.; Semenova, L.; Baulina, O.; Chivkunova, O.; Lobakova, E.; Solovchenko, A. A new subarctic strain of Tetradesmus obliquus. Part II: Comparative studies of CO2-stress tolerance. J. Appl. Phycol. 2018, 30, 2751–2761. [Google Scholar] [CrossRef]
- Kumar, R.; Goswami, G.; Debnath, D.; Sinha, A.; Das, D. Screening and evaluation of novel microalga Desmodesmus pannonicus CT01 for CO2 sequestration potential and aqua feed application. Biomass Conv. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Sinha, A.; Goswami, G.; Kumar, R.; Das, D. A microalgal biorefinery approach for bioactive molecules, biofuel, and biofertilizer using a novel carbon dioxide-tolerant strain Tetradesmus obliquus CT02. Biomass Conv. Biorefin. 2022, 13, 12605–12618. [Google Scholar] [CrossRef]
- Debs-Louka, E.; Louka, N.; Abraham, G.; Chabot, V.; Allaf, K. Effect of compressed carbon dioxide on microbial cell viability. Appl. Environ. Microbiol. 1999, 65, 626–631. [Google Scholar] [CrossRef]
- Kon, A.; Omata, S.; Hayakawa, Y.; Aburai, N.; Fujii, K. Microflora communities which can convert digested sludge to biogas. Environ. Technol. 2022, 43, 2391–2403. [Google Scholar] [CrossRef]
- Gao, S.; Hu, C.; Sun, S.; Xu, J.; Zhao, Y.; Zhang, H. Performance of piggery wastewater treatment and biogas upgrading by three microalgal cultivation technologies under different initial chemical oxygen demand concentration. Energy 2018, 165, 360–369. [Google Scholar] [CrossRef]
- Herold, C.; Ishika, T.; Nwoba, E.G.; Tait, S.; Ward, A.; Moheimani, N.R. Biomass production of marine microalga Tetraselmis suecica using biogas and wastewater as nutrients. Biomass Bioenerg. 2021, 145, 105945. [Google Scholar] [CrossRef]
- Rodero, M.D.R.; Lebrero, R.; Serrano, E.; Lara, E.; Arbib, Z.; García-Encina, P.A.; Muñoz, R. Technology validation of photosynthetic biogas upgrading in a semi-industrial scale algal-bacterial photobioreactor. Bioresour. Technol. 2019, 279, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Guenka Scarcelli, P.; Ruas, G.; Lopez-Serna, R.; Leite Serejo, M.; Blanco, S.; Árpád Boncz, M.; Muñoz, R. Integration of algae-based sewage treatment with anaerobic digestion of the bacterial-algal biomass and biogas upgrading. Bioresour. Technol. 2021, 340, 125552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, C.; Sun, S.; Zhao, Y.; Liu, J. Performance of different microalgae based technologies in nutrient removal and biogas upgrading in response to various GR24 concentrations. Int. Biodeter. Biodegr. 2021, 158, 105166. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, B.; Zhao, C.; Liu, J.; Zhao, Y.; Sun, S.; Wei, J. Simultaneous biogas upgrading and biogas slurry treatment by different microalgae-based technologies under various strigolactone analog (GR24) concentrations. Bioresour. Technol. 2022, 351, 127033. [Google Scholar] [CrossRef]
- Méndez, L.; García, D.; Perez, E.; Blanco, S.; Muñoz, R. Photosynthetic upgrading of biogas from anaerobic digestion of mixed sludge in an outdoors algal-bacterial photobioreactor at pilot scale. J. Water. Proce. Eng. 2022, 48, 102891. [Google Scholar] [CrossRef]
- Marín, D.; Méndez, L.; Suero, I.; Díaz, I.; Blanco, S.; Fdz-Polanco, M.; Muñoz, R. Anaerobic digestion of food waste coupled with biogas upgrading in an outdoors algal-bacterial photobioreactor at pilot scale. Fuel 2022, 324, 124554. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview 1, 2. J. Nut. 2002, 132, 518S–524S. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Ievina, B.; Romagnoli, F. Potential of Chlorella species as feedstock for bioenergy production: A review. Environ. Clim. Technol. 2020, 24, 203–220. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Chen, J.; Wang, T.; Huang, X.; Chen, G. The extraction of β-carotene from microalgae for testing their health benefits. Food 2022, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef]
- Levasseur, W.; Perre, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D. Free amino acids in human health and pathologies. I. Arginine. Biomed. Pharmacother. 2002, 56, 439–445. [Google Scholar] [CrossRef]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine supplementation and wound healing. Nut. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effect. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef]


| Sample Nos. | Sampling Location | Isolated Algal Strain |
|---|---|---|
| 1 | Ooyori, the midstream of the Kanna river, Gumma Pref. (36°13′ N, 138°94′ E) | OY |
| 2 | Furuta, the midstream of the Kanna river, Gumma Pref. (36°10′ N, 138°87′ E) | FR |
| 3 | Ueno, the upstream of the Kanna river, Gumma Pref. (36°08′ N, 138°79′ E) | KU |
| 4 | Shimokubo, the downstream of the Kanna river, Gumma Pref. (36°13′ N, 139°03′ E) | − |
| 5 | Fountain pond in Kogakuin University, Hachioji city (35°68′ N, 139°32′ E) | UF |
| 6 | Ornamental pond in Kogakuin University, Hachioji city (35°68′ N, 139°32′ E) | UP |
| 7 | Ornamental pond in Shimizu park, Hachioji city (35°68′ N, 139°31′ E) | SH |
| 8 | Ornamental pond in Ishikawa-higashi park, Hachioji city (35°68′ N, 139°37′ E) | IS |
| 9 | Gotanda, the downstream of the Shiroyama river, Hachioji city (35°67′ N, 139°30′ E) | SG |
| 10 | Yokogawa, the downstream of the Minami-asakawa river, Hachioji city (35°67′ N, 139°31′ E) | MA |
| 11 | Akishima, the midstream of the Tama river, Hachioji city (35°69′ N, 139°37′ E) | TM |
| Strain | Initial pH of the Culture Medium | CO2 Concentration of Gas Phase | pH of Grown Culture | Strain | Initial pH of the Culture Medium | CO2 Concentration of Gas Phase | pH of Grown Culture | ||
|---|---|---|---|---|---|---|---|---|---|
| Average | Stdev | Average | Stdev | ||||||
| Strain OY | 12.0 | 5% | 7.34 | 0.02 | Strain SH | 9.0 | 5% † | 10.85 | 0.58 |
| 10% | 10.16 | 1.58 | 10% | 10.53 | 0.70 | ||||
| 20% † | 11.96 | 0.01 | 20% | 5.68 | 0.07 | ||||
| 30% | 6.55 | 0.13 | 30% | 5.38 | 0.02 | ||||
| 50% | 6.33 | 0.07 | 50% | 5.33 | 0.05 | ||||
| Strain FR | 9.0 | 5% | 11.20 | 0.05 | Strain SG | 9.0 | 5% | 7.30 | 0.19 |
| 10% † | 11.25 | 0.04 | 10% | 6.64 | 0.14 | ||||
| 20% | 6.26 | 0.13 | 20% † | 11.11 | 0.03 | ||||
| 30% | 6.02 | 0.17 | 30% | 11.14 | 0.07 | ||||
| 50% | 5.37 | 0.06 | 50% | 5.47 | 0.02 | ||||
| Strain KU | 8.0 | 5% | 6.90 | 0.18 | Strain MA | 12.0 | 5% | 9.45 | 0.85 |
| 10% | 11.34 | 0.03 | 10% | 11.46 | 0.02 | ||||
| 20% † | 11.22 | 0.10 | 20% † | 11.43 | 0.05 | ||||
| 30% | 6.05 | 0.37 | 30% | 6.99 | 0.07 | ||||
| 50% | 5.58 | 0.06 | 50% | 6.27 | 0.12 | ||||
| Strain UF | 9.0 | 5% | 6.77 | 0.22 | Strain IS | 8.0 | 5% | 11.56 | 0.01 |
| 10% † | 6.66 | 0.03 | 10% † | 11.64 | 0.02 | ||||
| 20% | 5.78 | 0.05 | 20% | 5.89 | 0.08 | ||||
| 30% | 5.48 | 0.02 | 30% | 5.62 | 0.02 | ||||
| 50% | 5.24 | 0.02 | 50% | 5.54 | 0.06 | ||||
| Strain UP | 10.0 | 5% | 11.12 | 0.02 | Strain TM | 10.0 | 5% | 6.24 | 0.09 |
| 10% | 11.11 | 0.03 | 10% | 7.63 | 0.53 | ||||
| 20% † | 10.22 | 0.78 | 20% † | 11.19 | 0.07 | ||||
| 30% | 5.74 | 0.17 | 30% | 11.23 | 0.05 | ||||
| 50% | 5.46 | 0.07 | 50% | 5.49 | 0.09 | ||||
| Amino Acid | Strain OY | Strain FR | Strain KU | Strain UF | Strain UP | Strain SH | Strain SG | Strain MA | Strain IS | Strain TM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | |
| His * | 2.17 a | 0.06 | 1.83 be | 0.04 | 2.06 c | 0.04 | 1.87 b | 0.04 | 2.17 af | 0.03 | 2.63 d | 0.13 | 2.15 a | 0.01 | 1.73 e | 0.08 | 2.24 f | 0.03 | 2.21 f | 0.04 |
| Ser | 3.70 a | 0.01 | 4.04 b | 0.04 | 5.03 c | 0.05 | 4.63 d | 0.03 | 3.38 e | 0.03 | 4.12 b | 0.09 | 4.62 d | 0.01 | 4.27 e | 0.06 | 3.54 f | 0.05 | 4.82 g | 0.07 |
| Arg | 5.49 a | 0.06 | 4.99 b | 0.07 | 5.01 b | 0.08 | 6.48 c | 0.03 | 6.63 c | 0.07 | 12.20 d | 0.25 | 5.02 b | 0.01 | 5.06 b | 0.12 | 5.20 e | 0.04 | 5.05 b | 0.03 |
| Gly | 13.72 a | 0.27 | 13.12 b | 0.09 | 12.67 c | 0.12 | 12.02 d | 0.14 | 12.74 c | 0.02 | 13.50 a | 0.18 | 13.07 b | 0.11 | 12.70 c | 0.31 | 13.63 a | 0.06 | 13.13 b | 0.07 |
| Asp+Asn | 4.97 a | 0.18 | 5.33 b | 0.13 | 5.41 b | 0.09 | 5.75 c | 0.21 | 6.13 d | 0.09 | 4.64 e | 0.15 | 5.13 f | 0.07 | 5.79 c | 0.35 | 4.84 ae | 0.23 | 4.64 e | 0.17 |
| Glu+Gln | 7.81 a | 0.29 | 8.38 b | 0.12 | 7.90 c | 0.11 | 12.14 e | 0.14 | 9.24 f | 0.14 | 9.45 f | 0.26 | 8.34 b | 0.12 | 9.21 f | 0.42 | 7.63 a | 0.23 | 7.83 a | 0.19 |
| Thr * | 4.97 a | 0.01 | 4.65 b | 0.07 | 4.92 c | 0.06 | 4.91 c | 0.06 | 4.69 b | 0.04 | 5.16 d | 0.15 | 4.63 e | 0.03 | 4.77 b | 0.08 | 4.75 b | 0.10 | 4.94 ac | 0.10 |
| Ala | 11.51 a | 0.25 | 11.04 b | 0.11 | 11.08 b | 0.12 | 10.97 bc | 0.15 | 10.80 c | 0.08 | 8.73 d | 0.33 | 11.02 b | 0.05 | 10.86 c | 0.39 | 9.84 e | 0.12 | 10.59 f | 0.05 |
| Pro | 6.18 a | 0.01 | 6.27 b | 0.02 | 6.22 c | 0.04 | 5.84 d | 0.04 | 5.95 e | 0.08 | 5.12 f | 0.16 | 6.13 a | 0.07 | 6.01 e | 0.08 | 6.36 f | 0.08 | 6.23 g | 0.08 |
| Cys | 0.25 a | 0.01 | 0.08 b | 0.00 | 0.10 c | 0.00 | 0.13 d | 0.00 | 0.20 e | 0.00 | 0.01 f | 0.03 | 0.08 b | 0.00 | 0.07 g | 0.00 | 0.08 b | 0.00 | 0.09 h | 0.00 |
| Lys * | 4.15 ab | 0.36 | 3.91 a | 0.08 | 4.33 b | 0.11 | 4.14 ab | 0.19 | 4.48 b | 0.11 | 2.34 c | 0.36 | 4.25 ab | 0.08 | 4.46 b | 0.42 | 4.92 d | 0.18 | 4.05 a | 0.08 |
| Tyr | 2.98 a | 0.14 | 2.87 b | 0.03 | 2.98 a | 0.03 | 2.35 c | 0.04 | 2.85 b | 0.01 | 2.96 a | 0.16 | 2.72 d | 0.05 | 2.89 a | 0.17 | 3.13 e | 0.08 | 2.85 b | 0.04 |
| Met * | 2.93 a | 0.09 | 2.74 b | 0.02 | 2.69 c | 0.02 | 2.51 d | 0.02 | 2.73 b | 0.01 | 2.85 a | 0.10 | 2.78 e | 0.02 | 2.66 f | 0.10 | 2.70 e | 0.03 | 2.79 e | 0.01 |
| Val * | 7.47 a | 0.02 | 7.98 b | 0.05 | 7.47 a | 0.03 | 7.17 c | 0.02 | 7.40 d | 0.08 | 6.75 e | 0.07 | 7.61 f | 0.01 | 7.62 f | 0.10 | 7.86 g | 0.09 | 7.65 f | 0.05 |
| Ile * | 4.98 a | 0.03 | 5.16 b | 0.03 | 4.90 c | 0.01 | 4.46 d | 0.00 | 4.85 e | 0.05 | 4.30 f | 0.03 | 5.00 g | 0.03 | 5.00 g | 0.08 | 5.25 h | 0.05 | 5.06 g | 0.04 |
| Leu * | 10.18 a | 0.12 | 11.12 b | 0.09 | 10.79 cd | 0.00 | 9.35 e | 0.02 | 9.83 f | 0.09 | 8.79 g | 0.08 | 10.93 h | 0.04 | 10.71 d | 0.13 | 10.99 ch | 0.12 | 11.14 b | 0.07 |
| Phe * | 6.53 a | 0.32 | 6.51 a | 0.08 | 6.42 a | 0.08 | 5.28 b | 0.12 | 5.94 c | 0.05 | 6.46 a | 0.36 | 6.54 a | 0.16 | 6.18 ac | 0.42 | 7.05 d | 0.19 | 6.94 d | 0.12 |
| Trp * | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||
| Essential amino acids | 43.39 | 1.02 | 43.89 | 0.47 | 43.58 | 0.35 | 39.69 | 0.47 | 42.09 | 0.46 | 39.27 | 1.28 | 43.89 | 0.38 | 43.13 | 1.42 | 45.76 | 0.80 | 44.76 | 0.49 |
| Fatty Acid | Strain OY | Strain FR | Strain KU | Strain UF | Strain UP | Strain SH | Strain SG | Strain MA | Strain IS | Strain TM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | Average | Stdev | |
| Myristic acid (C14:0) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.27 | 0.02 | |||||||||
| Palmitic acid (C16:0) | 28.62 a | 3.19 | 16.65 b | 0.13 | 15.48 c | 0.53 | 25.83 d | 2.67 | 26.46 d | 3.15 | 21.39 e | 6.12 | 17.31 f | 0.57 | 14.89 g | 0.13 | 22.63 e | 0.85 | 15.71 c | 0.09 |
| Palmitoleic acid (C16:1) | 6.50 a | 0.53 | 17.33 b | 0.05 | 12.24 c | 2.86 | 9.45 d | 0.85 | 11.46 c | 1.79 | 12.15 c | 2.72 | 16.54 e | 0.50 | 16.83 e | 1.16 | 7.07 f | 0.24 | 15.76 g | 0.18 |
| Hexadecaenoic acid (C16:2) | 5.72 a | 0.58 | 6.75 b | 0.19 | 6.38 c | 0.48 | 4.15 d | 0.23 | 3.66 d | 0.79 | 4.15 d | 1.43 | 7.06 be | 0.87 | 6.28 c | 0.42 | 7.17 be | 0.63 | 7.01 e | 0.12 |
| Stearic acid (C18:0) | 18.83 a | 0.78 | 1.46 b | 0.03 | 0.92 be | 0.53 | 15.28 c | 0.34 | 10.09 d | 0.86 | 11.75 d | 9.36 | 1.36 b | 0.42 | 0.86 e | 0.07 | 4.03 f | 0.08 | 1.68 g | 0.02 |
| Oleic acid (C18:1) | 4.47 a | 0.38 | 15.98 b | 0.09 | 24.72 c | 2.01 | 13.43 d | 0.50 | 9.31 e | 0.20 | 16.98 bdf | 4.78 | 18.02 f | 0.63 | 21.61 g | 0.50 | 30.92 h | 0.11 | 22.38 i | 0.27 |
| Linoleic acid (C18:2) * | 30.34 a | 0.93 | 32.45 b | 0.19 | 20.41 c | 1.61 | 25.13 d | 1.19 | 32.97 e | 0.37 | 26.54 d | 2.40 | 28.28 f | 0.72 | 28.11 f | 0.68 | 9.11 g | 0.04 | 26.68 d | 0.25 |
| Linolenic acid (C18:3) * | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | ||||||||||
| Unidentified fatty acid | 5.52 | 9.38 | 19.48 | 6.37 | 6.05 | 7.03 | 11.43 | 11.42 | 19.06 | 10.50 | ||||||||||
| Unsaturated fatty acids | 47.03 | 2.42 | 72.51 | 0.51 | 63.76 | 6.96 | 52.16 | 2.78 | 57.39 | 3.15 | 59.83 | 11.33 | 69.90 | 2.72 | 72.83 | 2.76 | 54.28 | 1.02 | 71.83 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, Y.; Kanai, D.; Sugiyama, K.; Fujii, K. Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology. Fermentation 2024, 10, 134. https://doi.org/10.3390/fermentation10030134
Kikuchi Y, Kanai D, Sugiyama K, Fujii K. Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology. Fermentation. 2024; 10(3):134. https://doi.org/10.3390/fermentation10030134
Chicago/Turabian StyleKikuchi, Yuri, Daichi Kanai, Kenjiro Sugiyama, and Katsuhiko Fujii. 2024. "Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology" Fermentation 10, no. 3: 134. https://doi.org/10.3390/fermentation10030134
APA StyleKikuchi, Y., Kanai, D., Sugiyama, K., & Fujii, K. (2024). Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology. Fermentation, 10(3), 134. https://doi.org/10.3390/fermentation10030134





