Methane Production Reduced by Lignin Derivatives in Pulping Wastewater: Inhibition of Free Hydrolase
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Chemical Analysis
2.3. Granule Disintegration
2.4. EPS Extraction and Characterization
2.5. Zeta Potential
2.6. Total Organic Carbon of Substrate
2.7. Stepwise Inhibition of Metabolism in AnGS under HA Stress
2.8. Key Enzymatic Activity Tests
2.8.1. Alkaline Protease
2.8.2. α-glucosidase
3. Results and Discussion
3.1. Methanogenic Potential Inhibition
3.2. Substrate and Enzyme
3.2.1. Substrate Aggregation
3.2.2. Enzymatic Activity
3.3. Assessment of Potential Impacts on Microbial Aggregation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Wu, P.; Liu, Y.; Wong, J.W.; Yong, X.; Wu, X.; Zhou, J. Enhanced biogas production and biodegradation of phenanthrene in wastewater sludge treated anaerobic digestion reactors fitted with a bioelectrode system. Chem. Eng. J. 2019, 365, 1–9. [Google Scholar] [CrossRef]
- Singh, A.K.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef]
- Lappalainen, J.; Baudouin, D.; Hornung, U.; Schuler, J.; Melin, K.; Bjelić, S.; Joronen, T. Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies 2020, 13, 3309. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T. Treatment of pulp and paper mill wastewater—A review. Sci. Total Environ. 2004, 333, 37–58. [Google Scholar] [CrossRef]
- Martin, N.; Anglani, N.; Einstein, D.; Khrushch, M.; Worrell, E.; Price, L.K. Opportunities to Improve Energy Efficiency and Reduce Greenhouse Gas Emissions in the U.S. Pulp and Paper Industry. 2000. 56p. Available online: https://escholarship.org/content/qt31b2f7bd/qt31b2f7bd.pdf (accessed on 7 April 2024).
- Kumar, V.; Thakur, I.S.; Shah, M.P. Bioremediation Approaches for Treatment of Pulp and Paper Industry Wastewater: Recent Advances and Challenges. In Microbial Bioremediation & Biodegradation; Shah, M.P., Ed.; Springer: Singapore, 2020; pp. 1–48. [Google Scholar]
- Hermosilla, D.; Merayo, N.; Gascó, A.; Blanco, Á. The application of advanced oxidation technologies to the treatment of effluents from the pulp and paper industry: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 168–191. [Google Scholar] [CrossRef]
- Zainith, S.; Chowdhary, P.; Bharagava, R.N. Recent Advances in Physico-chemical and Biological Techniques for the Management of Pulp and Paper Mill Waste. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R.N., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 271–297. [Google Scholar]
- Ramesh, S.; Chaurasia, A.S.; Mahalingam, H.; Rao, N.J. Kinetics of Devolatilization of Black Liquor Droplets in Chemical Recovery Boilers—Pyrolysis of Dry Black Liquor Solids. Int. J. Chem. Eng. Appl. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Jian, Z.; Yuan-Fang, P.; Wan-Li, W.; Qin, W.; Gong-Nan, X.; Hong-Fei, L.; Tian, X.; Shuang-Fei, W. Black liquor increases methane production from excess pulp and paper industry sludge. Chemosphere 2021, 280, 130665. [Google Scholar] [CrossRef]
- Welander, T. An Anaerobic Process for Treatment of CTMP Effluent. Water Sci. Technol. 1988, 20, 143–147. [Google Scholar] [CrossRef]
- Kudo, A.; Kennedy, K.; Andras, E. Anaerobic (UASB) Treatment of Pulp (CTMP) Wastewater and the Toxicity on Granules. Water Sci. Technol. 1991, 23, 1919–1928. [Google Scholar] [CrossRef]
- Pathak, P.; Sharma, C. Processes and problems of pulp and paper industry: An overview. Phys. Sci. Rev. 2023, 8, 299–325. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef]
- Kumar, S.; Haq, I.; Yadav, A.; Prakash, J.; Raj, A. Immobilization and Biochemical Properties of Purified Xylanase from Bacillus amyloliquefaciens SK-3 and Its Application in Kraft Pulp Biobleaching. J. Clin. Microbiol. Biochem. Technol. 2016, 2, 26–34. [Google Scholar]
- Fang, X.; Li, Q.; Lin, Y.; Lin, X.; Dai, Y.; Guo, Z.; Pan, D. Screening of a microbial consortium for selective degradation of lignin from tree trimmings. Bioresour. Technol. 2018, 254, 247–255. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Xiao, L.-P.; Guo, X.; Fang, Y.; Sun, R.-C.; Song, G. Fragmentation of Woody Lignocellulose into Primary Monolignols and Their Derivatives. ACS Sustain. Chem. Eng. 2019, 7, 4666–4674. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Sun, L. The pyrolysis of lignin: Pathway and interaction studies. Fuel 2021, 290, 120078. [Google Scholar] [CrossRef]
- Balasundaram, G.; Banu, R.; Varjani, S.; Kazmi, A.; Tyagi, V.K. Recalcitrant compounds formation, their toxicity, and mitigation: Key issues in biomass pretreatment and anaerobic digestion. Chemosphere 2022, 291 Pt 3, 132930. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Shad, M.; Nazir, A.; Usman, M.; Akhtar, M.W.; Sajjad, M. Investigating the effect of SUMO fusion on solubility and stability of amylase-catalytic domain from Pyrococcus abyssi. Int. J. Biol. Macromol. 2024, 266 Pt 2, 131310. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.; Sbardelotto, A.F.; Ziegler DD, R.; Pinto LM, N.; de Souza Ramos, R.C.; Marczak LD, F.; Tessaro, I.C. Obtaining and purification of a highly soluble hydrolyzed rice endosperm protein. Sep. Purif. Technol. 2017, 183, 279–292. [Google Scholar] [CrossRef]
- Li, B.; Xie, Y.; Guo, Q. Thermal acid hydrolysis modulates the solubility of quinoa protein: The formation of different types of protein aggregates. Food Hydrocoll. 2024, 151, 109825. [Google Scholar] [CrossRef]
- Priya; Gogate, P.R. Ultrasound-Assisted Intensification of Activity of Free and Immobilized Enzymes: A Review. Ind. Eng. Chem. Res. 2021, 60, 9650–9668. [Google Scholar] [CrossRef]
- Bernardes, A.; Pellegrini, V.; Curtolo, F.; Camilo, C.; Mello, B.; Johns, M.; Scott, J.; Guimaraes, F.; Polikarpov, I. Carbohydrate binding modules enhance cellulose enzymatic hydrolysis by increasing access of cellulases to the substrate. Carbohydr. Polym. 2019, 211, 57–68. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; van Loosdrecht, M.C.; Liu, R. Relieving the inhibition of humic acid on anaerobic digestion of excess sludge by metal ions. Water Res. 2021, 188, 116541. [Google Scholar] [CrossRef] [PubMed]
- El Ouaqoudi, F.Z.; Meddich, A.; Lemée, L.; Amblès, A.; Hafidi, M. Assessment of Compost-Derived Humic Acids Structure from Ligno-Cellulose Waste by TMAH-Thermochemolysis. Waste Biomass Valorization 2018, 10, 2661–2672. [Google Scholar] [CrossRef]
- Norrman, J.; Narbuvold, R. Anaerobic Treatability of Waste Waters from Pulp and Paper Industries. Biotechnol. Adv. 1984, 2, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Noike, T.; Endo, G.; Chang, J.; Yaguchi, J.; Matsumoto, J. Characteristics of Carbohydrate Degradation and the Rate-limiting Step in Anaerobic Digestion. Biotechnol. Bioeng. 1985, 27, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y.; Zheng, L.; Wang, Z.; Dai, X. Effects of humic matter on the anaerobic digestion of sewage sludge: New insights from sludge structure. Chemosphere 2020, 243, 125421. [Google Scholar] [CrossRef] [PubMed]
- Azman, S.; Khadem, A.F.; Plugge, C.M.; Stams, A.J.M.; Bec, S.; Zeeman, G. Effect of humic acid on anaerobic digestion of cellulose and xylan in completely stirred tank reactors: Inhibitory effect, mitigation of the inhibition and the dynamics of the microbial communities. Appl. Microbiol. Biotechnol. 2017, 101, 889–901. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; van Loosdrecht, M.C.; Yu, J.; Liu, R. Adaptation of semi-continuous anaerobic sludge digestion to humic acids. Water Res. 2019, 161, 329–334. [Google Scholar] [CrossRef]
- Batstone, D.; Landelli, J.; Saunders, A.; Webb, R.; Blackall, L.; Keller, J. The influence of calcium on granular sludge in a full-scale UASB treating paper mill wastewater. Water Sci. Technol. 2002, 45, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Vegunta, V.; Sevastyanova, O.; Deshpande, R.; Lindén, P.A.; Garcia, A.; Björk, M.; Jansson, U.; Henriksson, G.; Lindström, M.E. Addition of green and black liquor in kraft pulping of Eucalyptus dunnii wood: Possible solutions for the problems with kraft pulping caused by high calcium content. Cellulose 2023, 31, 1223–1236. [Google Scholar] [CrossRef]
- Schneider, T.; Roffael, E.; Dix, B. Einfluß von Holzaufschlußverfahren (TMP-, CTMP-Verfahren) und Aufschlußbedingungen auf die physikalisch-technologischen Eigenschaften von mitteldichten Faserplatten (MDF). Eur. J. Wood Wood Prod. 2000, 58, 123–124. [Google Scholar] [CrossRef]
- Hering, J.G.; Morel, F.M. Humic acid complexation of calciun and copper. Environ. Sci. Technol. 1988, 22, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Christl, I. Ionic strength- and pH-dependence of calcium binding by terrestrial humic acids. Environ. Chem. 2012, 9, 89–96. [Google Scholar] [CrossRef]
- Lin, L.; Ishida, K.; Zhang, Y.; Usui, N.; Miyake, A.; Abe, N.; Li, Y.-Y. Improving the biomass retention and system stability of the anammox EGSB reactor by adding a calcium silicate hydrate functional material. Sci. Total Environ. 2023, 857 Pt 3, 159719. [Google Scholar] [CrossRef] [PubMed]
- CJ/T 221-2005; Municipal Wastewater Treatment Plant Sludge Inspection Methods. Technical Committee for the Standardisation of Water Supply and Drainage Products of the Ministry of Construction: Beijing, China, 2005.
- Wang, X.; Zhang, M.; Zhou, Z.; Qu, T.; Ran, J.; Zhang, J.; Li, X.; Zhang, L.; Zhang, A. Effect of extracellular polymeric substances removal and re-addition on anaerobic digestion of waste activated sludge. J. Water Process Eng. 2024, 57, 108069. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Goel, R.; Mino, T.; Satoh, H.; Matsuo, T. Enzyme Activities under Anaerobic and Aerobic Conditions in Activated Sludge Sequencing Batch Reactor. Water Res. A J. Int. Water Assoc. 1988, 32, 2081–2088. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Gao, J.; Wan, J.; Ding, J.; Li, T. Enhancing enzyme activity via low-intensity ultrasound for protein extraction from excess sludge. Chemosphere 2022, 303, 134936. [Google Scholar] [CrossRef]
- Shi, M.; Liu, H.; Zhang, X.; Li, Y.; Huang, F.; Zhao, C.; Liu, H. A neglected contributor of thermal hydrolysis to sludge anaerobic digestion: Fulvic acids release and their influences. J. Environ. Manag. 2023, 343, 118217. [Google Scholar] [CrossRef]
- Dong, H.; Lo, I.M. Influence of calcium ions on the colloidal stability of surface-modified nano zero-valent iron in the absence or presence of humic acid. Water Res. 2013, 47, 2489–2496. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Sung, S.; Raskin, L.; Khanal, S.K. Anaerobic co-digestion of various organic wastes: Kinetic modeling and synergistic impact evaluation. Bioresour. Technol. 2022, 343, 126063. [Google Scholar] [CrossRef]
- Allen, E.; Wall, D.; Herrmann, C.; Xia, A.; Murphy, J.D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.; Sung, S.; Raskin, L.; Khanal, S.K. Biogas production from residual marine macroalgae biomass: Kinetic modelling approach. Bioresour. Technol. 2022, 359, 127473. [Google Scholar]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Catalytic Activity in Organic Solvents and Stability of Immobilized Enzymes Depend on the Pore Size and Surface Characteristics of Mesoporous Silica. Chem. Mater. 2000, 12, 3301–3305. [Google Scholar] [CrossRef]
- Huron, M.; Hudebine, D.; Ferreira, N.L.; Lachenal, D. Mechanistic modeling of enzymatic hydrolysis of cellulose integrating substrate morphology and cocktail composition. Biotechnol. Bioeng. 2016, 113, 1011–1023. [Google Scholar] [CrossRef]
- Kumar, R.; Wyman, C.E. Physical and Chemical Features of Pretreated Biomass that Influence Macro-/Micro-Accessibility and Biological Processing. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Wiley: Hoboken, NJ, USA, 2013; pp. 281–310. [Google Scholar]
- Xu, E.; Li, D.; Cheng, H.; Zhao, H.; Tian, J.; Wu, Z.; Chen, S.; Ye, X.; Liu, D. Effect of anion type on enzymatic hydrolysis of starch-(thermostable alpha-amylase)-calcium system in a low-moisture solid microenvironment of bioextrusion. Carbohydr. Polym. 2020, 240, 116331. [Google Scholar] [CrossRef]
- Kraithong, S.; Junejo, S.A.; Jiang, Y.; Zhang, B.; Huang, Q. Effects of pectin-calcium matrices on controlling in vitro digestion of normal maize starch. Food Hydrocoll. 2023, 140, 108575. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Yang, H.; Zhang, J.; Liu, X.; Regenstein, J.M.; Zhou, P. Effect of calcium sequestration by ion-exchange treatment on the dissociation of casein micelles in model milk protein concentrates. Food Hydrocoll. 2016, 60, 59–66. [Google Scholar] [CrossRef]
- Sentís-Moré, P.; Ortega-Olivé, N.; Mas-Capdevila, A.; Romero-Fabregat, M.-P. Impact of centrifugation and vacuum filtration step on the yield and molecular weight distribution of protein hydrolysates from rapeseed and sunflower meals. LWT 2022, 165, 113741. [Google Scholar] [CrossRef]
- Yap, S.; Astals, S.; Lu, Y.; Peces, M.; Jensen, P.; Batstone, D.; Tait, S. Humic acid inhibition of hydrolysis and methanogenesis with different anaerobic inocula. Waste Manag. 2018, 80, 130–136. [Google Scholar] [CrossRef]
- Ayol, A.; Filibeli, A.; Sir, D.; Kuzyaka, E. Aerobic and anaerobic bioprocessing of activated sludge: Floc disintegration by enzymes. J. Environ. Sci. Health Part A 2008, 43, 1528–1535. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, W.; Putnis, C.V.; Wang, L. Direct observation of humic acid-promoted hydrolysis of phytate through stabilizing a conserved catalytic domain in phytase. Environ. Sci. Process. Impacts 2022, 24, 1082–1093. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, S.; Huang, D.; Tao, X.; Li, Y. Inhibition of starch digestion by phenolic acids with a cinnamic acid backbone: Structural requirements for the inhibition of α-amylase and α-glucosidase. Food Chem. 2024, 435, 137499. [Google Scholar] [CrossRef]
- Zhang, Y.; Hess, H. Enhanced Diffusion of Catalytically Active Enzymes. ACS Cent. Sci. 2019, 5, 939–948. [Google Scholar] [CrossRef]
- Chu, C.P.; Tsai, D.G.; Lee, D.J.; Tay, J.H. Size-dependent anaerobic digestion rates of flocculated activated sludge: Role of intrafloc mass transfer resistance. J. Environ. Manag. 2005, 76, 239–244. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, Y.; Liu, Z.; Zhang, G.; Rao, J.; Li, X. Optimization of low-intensity ultrasonic irradiation for low-strength sewage treatment in anaerobic baffled reactor. J. Environ. Chem. Eng. 2022, 10, 108022. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Zou, X.; Zhong, Y.; Pan, X.; Pang, H.; Zhang, J.; Cui, X.; Wu, X.; Li, B.; et al. Impact of divalent cations on lysozyme-induced solubilisation of waste-activated sludge: Perspectives of extracellular polymeric substances and surface electronegativity. Chemosphere 2022, 302, 134841. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Wei, Y. A model-based approach for evaluating the effects of sludge rheology on methane production during high solid anaerobic digestion: Focusing on mass transfer resistance. Biochem. Eng. J. 2024, 201, 109147. [Google Scholar] [CrossRef]
- Li, Z.; Stenstrom, M.K. Impacts of SRT on Particle Size Distribution and Reactor Performance in Activated Sludge Processes. Water Environ. Res. 2018, 90, 48–56. [Google Scholar] [CrossRef]
- Ward, B.; Nguyen, M.; Sam, S.; Korir, N.; Niwagaba, C.; Morgenroth, E.; Strande, L. Particle size as a driver of dewatering performance and its relationship to stabilization in fecal sludge. J. Environ. Manag. 2023, 326, 116801. [Google Scholar] [CrossRef]
- Abeshu, G.W.; Li, H.-Y.; Zhu, Z.; Tan, Z.; Leung, L.R. Median bed-material sediment particle size across rivers in the contiguous US. Earth Syst. Sci. Data 2022, 14, 929–942. [Google Scholar] [CrossRef]
- Chen, W.; Gao, X.; Xu, H.; Cai, Y.; Cui, J. Influence of extracellular polymeric substances (EPS) treated by combined ultrasound pretreatment and chemical re-flocculation on water treatment sludge settling performance. Chemosphere 2017, 170, 196–206. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Yang, F.; Liu, X.; Wang, D.; Xu, Q.; Zhang, Y.; Yang, Q. Understanding the mechanism of how anaerobic fermentation deteriorates sludge dewaterability. Chem. Eng. J. 2021, 404, 127026. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Z.; Chen, L.; Chen, G.; Zhang, Y.; Jiang, L.; Qiu, Z.; He, K.; Wu, Z. Conditioning for excess sludge and ozonized sludge by ferric salt and polyacrylamide: Orthogonal optimization, rheological characteristics and floc properties. Chem. Eng. J. 2019, 373, 1081–1090. [Google Scholar] [CrossRef]
- El Hagrasy, A.S.; Hennenkamp, J.R.; Burke, M.D.; Cartwright, J.J.; Litster, J.D. Twin screw wet granulation: Influence of formulation parameters on granule properties and growth behavior. Powder Technol. 2013, 238, 108–115. [Google Scholar] [CrossRef]
- Mendez Torrecillas, C.; Halbert, G.W.; Lamprou, D.A. A novel methodology to study polymodal particle size distributions produced during continuous wet granulation. Int. J. Pharm. 2017, 519, 230–239. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Huang, J.-L.; Alam, F. Fast granulation of halophilic activated sludge treating low-strength organic saline wastewater via addition of divalent cations. Chemosphere 2021, 264, 128396. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.; Xu, C.; Lu, W.; Li, D.; Zhao, C.; Liu, B.; Li, X.; Khan, S.; Zheng, H.; et al. Better understanding the polymerization kinetics of ultrasonic-template method and new insight on sludge floc characteristics research. Sci. Total Environ. 2019, 689, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yan, X.; Xu, J.; Jiang, L.; Wu, W. Sorption of organic compounds by pyrolyzed humic acids. Sci. Total Environ. 2021, 781, 146646. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303 Pt 2, 135172. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, T.; Lv, L.; Chen, Y.; Tang, W.; Tang, S. Destroying the structure of extracellular polymeric substance to improve the dewatering performance of waste activated sludge by ionic liquid. Water Res. 2021, 199, 117161. [Google Scholar] [CrossRef]
- Zheng, Y.; Xing, M.; Cai, L.; Xiao, T.; Lu, Y.; Jiang, J. Interaction of earthworms-microbe facilitating biofilm dewaterability performance during wasted activated sludge reduction and stabilization. Sci. Total Environ. 2017, 581–582, 573–581. [Google Scholar] [CrossRef]
- Ding, W.; Jin, W.; Zhou, X.; Yang, Q.; Chen, C.; Wang, Q. Role of extracellular polymeric substances in anaerobic granular sludge: Assessing dewaterability during Fe(II)-peroxydisulfate conditioning and granulation processes. J. Clean. Prod. 2021, 286, 124968. [Google Scholar] [CrossRef]
- Yousefi, S.A.; Nasser, M.S.; Hussein, I.A.; Benamor, A. Enhancement of flocculation and dewaterability of a highly stable activated sludge using a hybrid system of organic coagulants and polyelectrolytes. J. Water Process Eng. 2020, 35, 101237. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Das, S. Bacterial extracellular polymeric substances: Biosynthesis and interaction with environmental pollutants. Chemosphere 2023, 332, 138876. [Google Scholar]
- An, Q.; Chen, Y.; Tang, M.; Zhao, B.; Deng, S.; Li, Z. The mechanism of extracellular polymeric substances in the formation of activated sludge flocs. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131009. [Google Scholar] [CrossRef]
- Wu, S.; Hua, X.; Miao, R.; Ma, B.; Hu, C.; Liu, H.; Qu, J. Influence of floc charge and related distribution mechanisms of humic substances on ultrafiltration membrane behavior. J. Membr. Sci. 2020, 609, 118260. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Z.; Yu, Z.; Dai, X.; Xu, X.; Alvarez, P.J.; Zhu, L. Evolution and functional analysis of extracellular polymeric substances during the granulation of aerobic sludge used to treat p-chloroaniline wastewater. Chem. Eng. J. 2017, 330, 596–604. [Google Scholar] [CrossRef]
- Wang, B.-B.; Liu, X.-T.; Chen, J.-M.; Peng, D.-C.; He, F. Composition and functional group characterization of extracellular polymeric substances (EPS) in activated sludge: The impacts of polymerization degree of proteinaceous substrates. Water Res. 2018, 129, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Deng, D.; Li, R.; Guo, C.; Ma, J.; Chen, M. Investigation of extracellular polymeric substances (EPS) in four types of sludge: Factors influencing EPS properties and sludge granulation. J. Water Process Eng. 2021, 40, 101924. [Google Scholar] [CrossRef]
- Wan, P.; Liu, Y.; Zhang, Q.; Jiang, L.; Chen, H.; Lv, W. Enhanced degradation of extracellular polymeric substances by yeast in activated sludge to achieve sludge reduction. Bioresour. Technol. 2023, 377, 128915. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-C.; Zhang, X.; He, Z.-W.; Tian, Y.; Wang, X.C. Role of extracellular polymeric substances on nutrients storage and transfer in algal-bacteria symbiosis sludge system treating wastewater. Bioresour. Technol. 2021, 331, 125010. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Lin, W.; Chen, R.; Ngo, H.H.; Zhang, R.; He, X.; Ma, J. Improvement of sludge dewaterability by energy uncoupling combined with chemical re-flocculation: Reconstruction of floc, distribution of extracellular polymeric substances, and structure change of proteins. Sci. Total Environ. 2022, 816, 151646. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zeng, Q.; Liu, Q.; Chai, H.; Xiang, J.; Li, H.; Shi, S.; Yang, A.; Chen, Z.; Cui, Y.; et al. Impacts of electric field-magnetic powder coupled membrane bioreactor on phenol wastewater treatment: Performance, synergistic mechanism, antibiotic resistance genes, and eco-environmental benefit evaluation. Sci. Total Environ. 2024, 909, 168607. [Google Scholar] [CrossRef]
- Deng, S.; Wang, L.; Su, H. Role and influence of extracellular polymeric substances on the preparation of aerobic granular sludge. J. Environ. Manag. 2016, 173, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Xu, J.; Li, W.-H.; Yu, H.-Q. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge. Chemosphere 2013, 93, 1436–1441. [Google Scholar] [CrossRef]



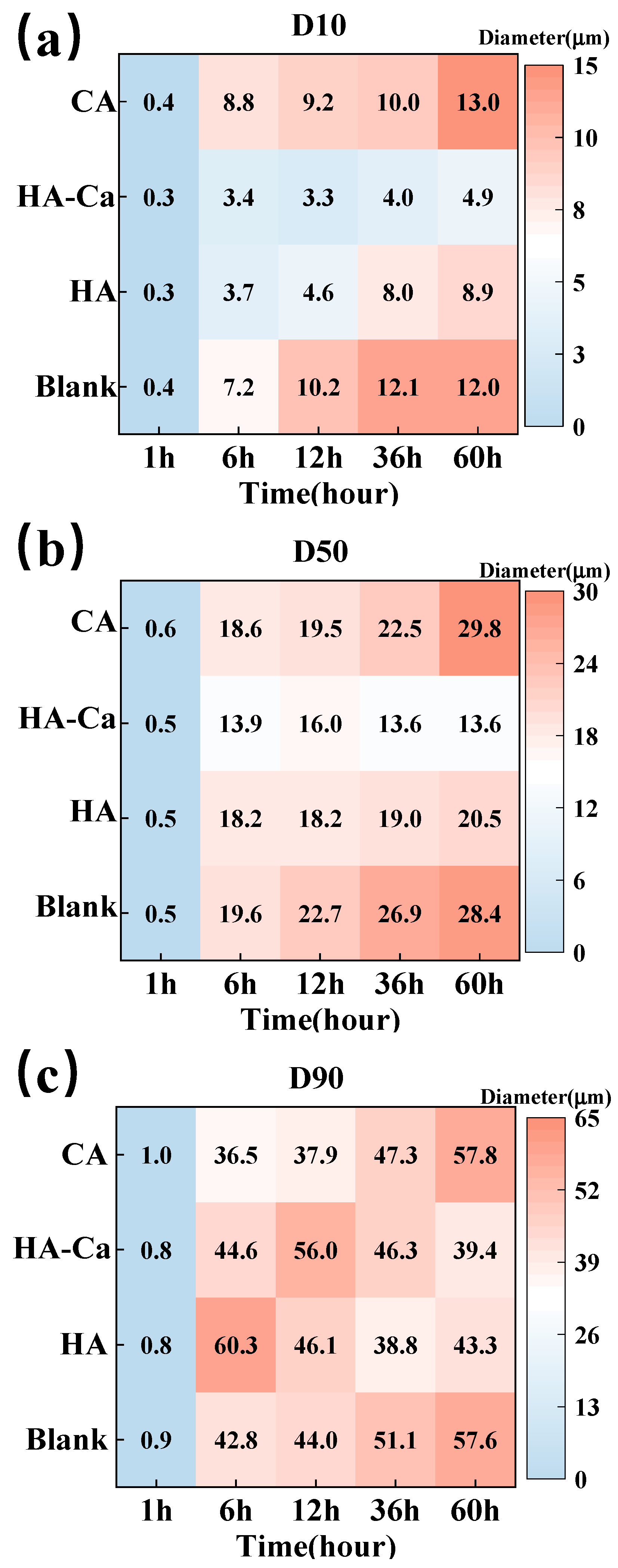
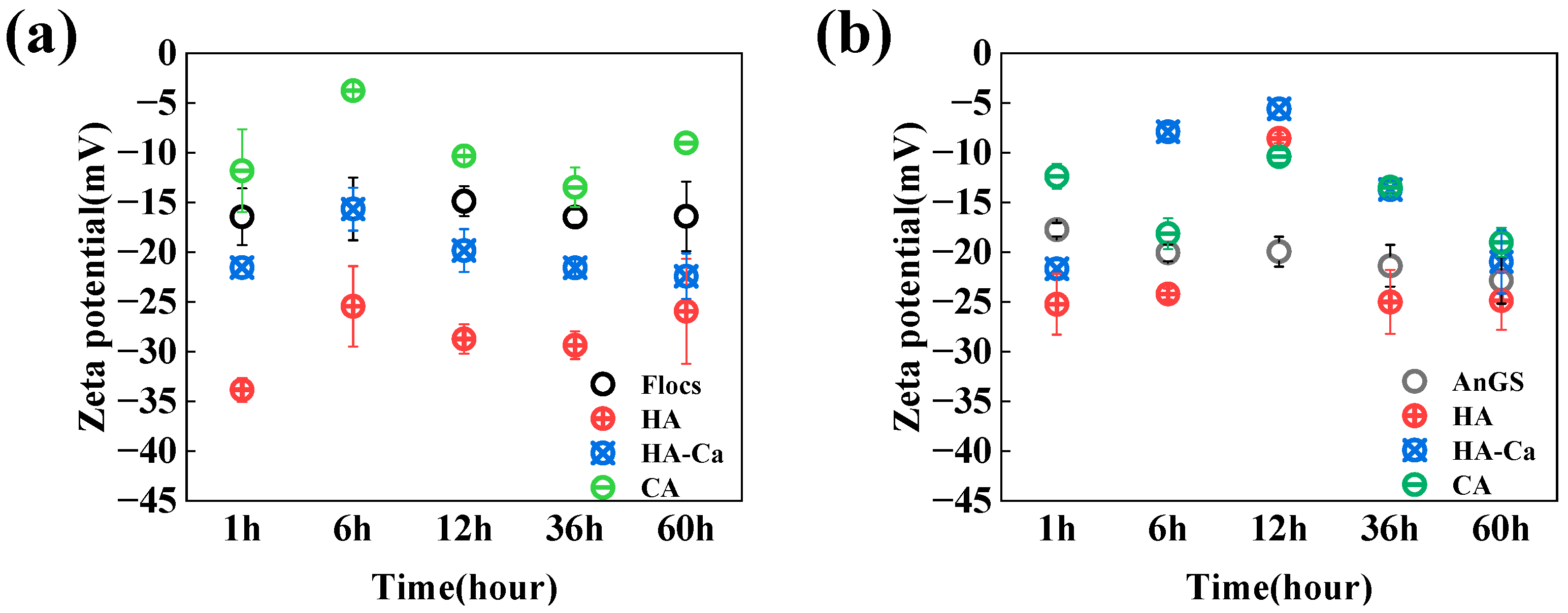

| Substrate Type | Group | M0 (mL/g VSS) | Rm (mL/g VSS/h) | λ (h) | R2 |
|---|---|---|---|---|---|
| Starch | S-Blank | 36.53 ± 0.10 | 0.97 ± 0.01 | 23.05 ± 0.12 | 0.993 |
| S-Ca | 35.21 ± 0.10 | 1.17 ± 0.02 | 23.53 ± 0.12 | 0.992 | |
| S-HAs | 32.54 ± 0.15 | 0.75 ± 0.01 | 19.40 ± 0.20 | 0.981 | |
| S-HA–Ca | 34.32 ± 0.13 | 1.22 ± 0.03 | 17.59 ± 0.18 | 0.978 | |
| Casein | C-Blank | 42.33 ± 0.10 | 2.12 ± 0.03 | 14.79 ± 0.09 | 0.992 |
| C-Ca | 41.30 ± 0.11 | 1.60 ± 0.02 | 16.55 ± 0.10 | 0.992 | |
| C-HAs | 30.74 ± 0.04 | 1.11 ± 0.01 | 16.49 ± 0.05 | 0.998 | |
| C-HA–Ca | 35.53 ± 0.08 | 1.19 ± 0.01 | 11.26 ± 0.10 | 0.992 | |
| Glucose | G-Blank | 57.46 ± 0.17 | 3.48 ± 0.09 | 1.45 ± 0.12 | 0.969 |
| G-Ca | 56.70 ± 0.15 | 3.28 ± 0.07 | 1.43 ± 0.12 | 0.974 | |
| G-HAs | 56.92 ± 0.18 | 2.76 ± 0.06 | 1.97 ± 0.13 | 0.975 | |
| G-HA–Ca | 55.83 ± 0.16 | 2.96 ± 0.07 | 1.08 ± 0.13 | 0.972 | |
| Sodium acetate | SA-Blank | 41.29 ± 0.17 | 2.91 ± 0.04 | 4.43 ± 0.06 | 0.995 |
| SA-Ca | 43.52 ± 0.18 | 2.89 ± 0.04 | 4.45 ± 0.05 | 0.995 | |
| SA-HAs | 41.46 ± 0.17 | 2.67 ± 0.04 | 4.59 ± 0.05 | 0.996 | |
| SA-HA–Ca | 41.87 ± 0.13 | 3.07 ± 0.04 | 4.36 ± 0.04 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Xu, Z.; Chen, Y.; Yu, G.; Liu, Z.; Wang, S.; Zhang, J.; Li, K.; Xie, L. Methane Production Reduced by Lignin Derivatives in Pulping Wastewater: Inhibition of Free Hydrolase. Fermentation 2024, 10, 247. https://doi.org/10.3390/fermentation10050247
Lei J, Xu Z, Chen Y, Yu G, Liu Z, Wang S, Zhang J, Li K, Xie L. Methane Production Reduced by Lignin Derivatives in Pulping Wastewater: Inhibition of Free Hydrolase. Fermentation. 2024; 10(5):247. https://doi.org/10.3390/fermentation10050247
Chicago/Turabian StyleLei, Jinxu, Zhihong Xu, Yong Chen, Guo Yu, Zexiang Liu, Shuangfei Wang, Jian Zhang, Kelin Li, and Li Xie. 2024. "Methane Production Reduced by Lignin Derivatives in Pulping Wastewater: Inhibition of Free Hydrolase" Fermentation 10, no. 5: 247. https://doi.org/10.3390/fermentation10050247
APA StyleLei, J., Xu, Z., Chen, Y., Yu, G., Liu, Z., Wang, S., Zhang, J., Li, K., & Xie, L. (2024). Methane Production Reduced by Lignin Derivatives in Pulping Wastewater: Inhibition of Free Hydrolase. Fermentation, 10(5), 247. https://doi.org/10.3390/fermentation10050247







