The Biosynthesis of the Monoterpene Tricyclene in E. coli through the Appropriate Truncation of Plant Transit Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Plasmids Construction and Bacterial Strains
2.3. Shake-Flask Fermentation
2.4. Identification and Quantification Analysis of Products
2.5. Protein Analysis of Cell Lysate by SDS-PAGE
3. Results
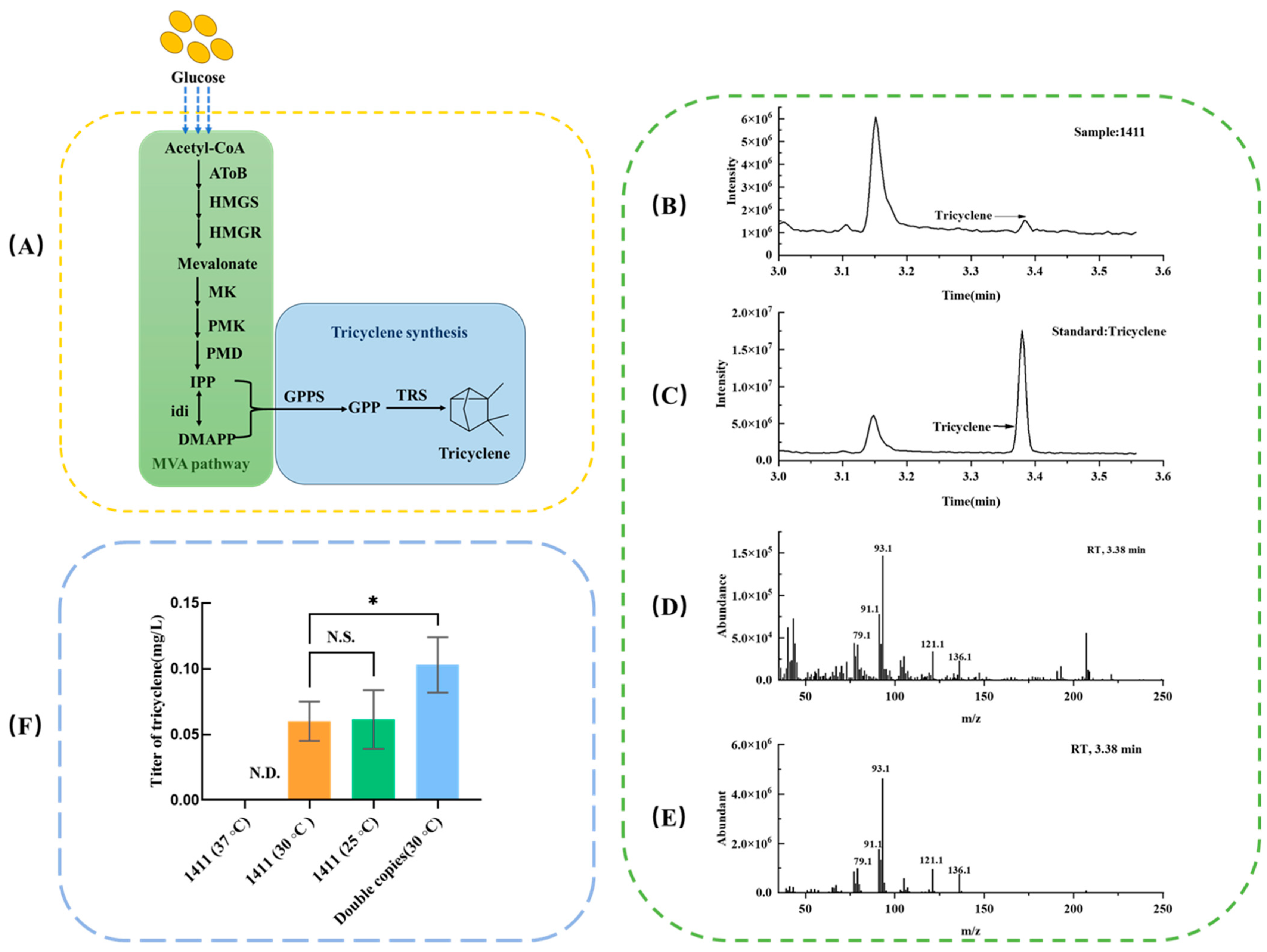
3.1. Characterization of Tricyclene by GC-MS
3.2. Effect of Induction Temperature on Tricyclene Production
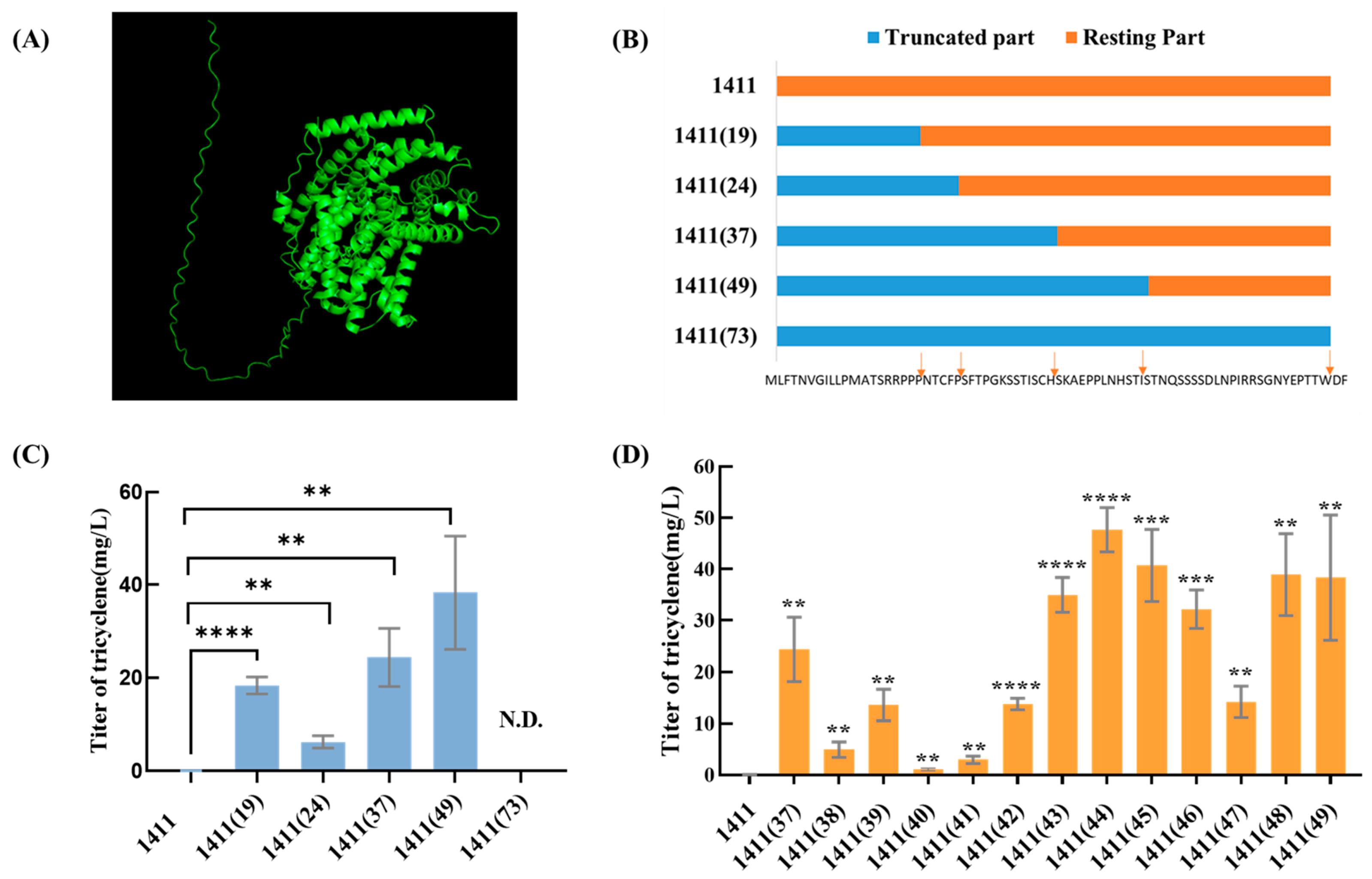
3.3. Truncation of TS 1411
3.4. SDS-PAGE Analysis of TS 1411
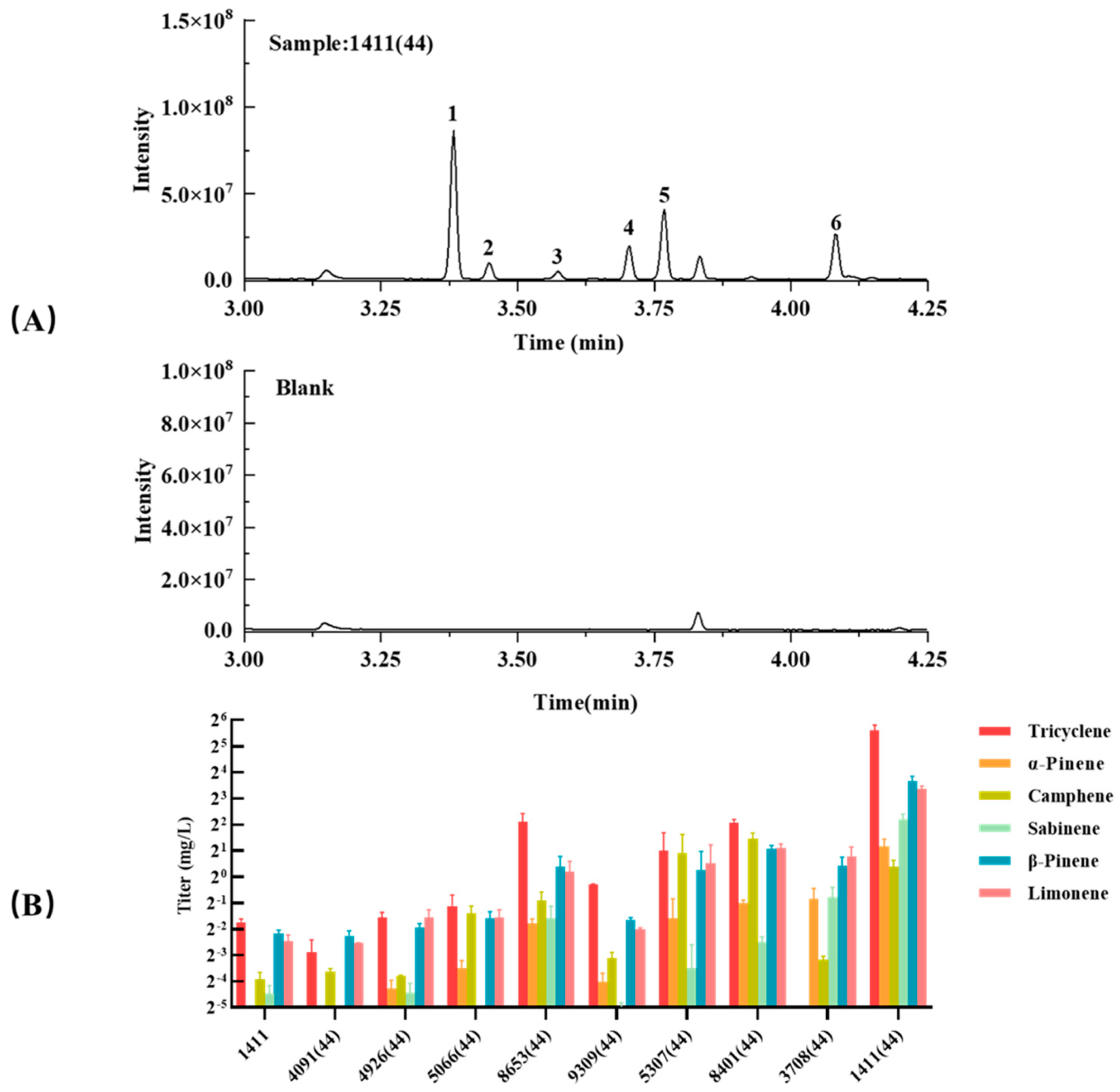
3.5. Truncations of the Other TSs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Meylemans, H.A.; Quintana, R.L.; Harvey, B.G. Efficient Conversion of Pure and Mixed Terpene Feedstocks to High Density Fuels. Fuel 2012, 97, 560–568. [Google Scholar] [CrossRef]
- Smaili, T.; Bendif, H.; Zedam, A.; Flamini, G.; Maggi, F. A New Chemotype with High Tricyclene Content from the Essential Oil of Salvia aegyptiaca L. Growing in Algerian Pre-Sahara. Nat. Prod. Res. 2022, 36, 5364–5369. [Google Scholar] [CrossRef] [PubMed]
- The, S.N.; Le Tuan, A.; Thu, T.D.T.; Dinh, L.N.; Thi, T.T. Essential Oils of Polyalthia suberosa Leaf and Twig and Their Cytotoxic and Antimicrobial Activities. Chem. Biodivers. 2021, 18, e2100020. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Ray, A.; Sahoo, A.; Champati, B.B.; Padhiari, B.M.; Dash, B.; Nayak, S.; Panda, P.C. Chemical Composition and Antioxidant Activities of Essential Oil from Leaf and Stem of Elettaria cardamomum from Eastern India. J. Essent. Oil Bear. Plants 2021, 24, 538–546. [Google Scholar] [CrossRef]
- Yates, D.I.; Ownley, B.H.; Labbé, N.; Bozell, J.J.; Klingeman, W.E.; Batson, E.K.; Gwinn, K.D. Sciadopitys Verticillata Resin: Volatile Components and Impact on Plant Pathogenic and Foodborne Bacteria. Molecules 2019, 24, 3767. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.S.; Shawky, E.; El Naggar, E.M.B.; Hammoda, H.M.; Harraz, F.M. Evaluation of the Effect of Seasonal Variation and Organ Selection on the Chemical Composition and Antimicrobial Activity of the Essential Oil of Oriental-Cedar (Platyclaudus orientalis (L.) Franco). J. Essent. Oil Res. 2021, 33, 69–79. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Tawfeek, N.; Elbaramawi, S.S.; Fikry, E. Agathis Robusta Bark Essential Oil Effectiveness against COVID-19: Chemical Composition, In Silico and In Vitro Approaches. Plants 2022, 11, 663. [Google Scholar] [CrossRef]
- Madavi, T.B.; Chauhan, S.; Keshri, A.; Alavilli, H.; Choi, K.; Pamidimarri, S.D.V.N. Whole-cell Biocatalysis: Advancements toward the Biosynthesis of Fuels. Biofuels Bioprod. Bioref. 2022, 16, 859–876. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Wang, X.; Wang, F.; Li, X. Efficient Production of α-Pinene through Identifying the Rate-Limiting Enzymes and Tailoring Inactive Terminal of Pinene Synthase in Escherichia coli. Fuel 2023, 343, 127872. [Google Scholar] [CrossRef]
- Harvey, B.G.; Wright, M.E.; Quintana, R.L. High-Density Renewable Fuels Based on the Selective Dimerization of Pinenes. Energy Fuels 2010, 24, 267–273. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid Drugs, Biofuels, and Chemicals—Artemisinin, Farnesene, and Beyond. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2015; Volume 148, pp. 355–389. ISBN 978-3-319-20106-1. [Google Scholar]
- Zhang, J.; Zhao, C. A New Approach for Bio-Jet Fuel Generation from Palm Oil and Limonene in the Absence of Hydrogen. Chem. Commun. 2015, 51, 17249–17252. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.H.; Rapp, V.H.; Broeckelmann, M.; Lee, T.S.; Dibble, R.W. Investigation of Biofuels from Microorganism Metabolism for Use as Anti-Knock Additives. Fuel 2014, 117, 939–943. [Google Scholar] [CrossRef]
- Geron, C.; Rasmussen, R.; Arnts, R.R.; Guenther, A. A Review and Synthesis of Monoterpene Speciation from Forests in the United States. Atmos. Environ. 2000, 34, 1761–1781. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, L.; Qiu, X.; Zhu, C.; Zhu, L. Advances in the Metabolic Engineering of Escherichia coli for the Manufacture of Monoterpenes. Catalysts 2019, 9, 433. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Lu, Y.; Lan, H.; Tian, L.; Zhang, Z.; Xie, C.; Jiang, L. Pathway Engineering of Saccharomyces Cerevisiae for Efficient Lycopene Production. Bioprocess Biosyst. Eng. 2021, 44, 1033–1047. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, W.-H.; Wang, Y.; Yao, M.-D.; Jiang, G.-Z.; Zeng, B.-X.; Zhang, R.-S.; Yuan, Y.-J. Chassis and Key Enzymes Engineering for Monoterpenes Production. Biotechnol. Adv. 2017, 35, 1022–1031. [Google Scholar] [CrossRef]
- Wang, C.; Pfleger, B.F.; Kim, S.-W. Reassessing Escherichia coli as a Cell Factory for Biofuel Production. Curr. Opin. Biotechnol. 2017, 45, 92–103. [Google Scholar] [CrossRef]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming Yeast Peroxisomes into Microfactories for the Efficient Production of High-Value Isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef]
- Rolf, J.; Julsing, M.K.; Rosenthal, K.; Lütz, S. A Gram-Scale Limonene Production Process with Engineered Escherichia coli. Molecules 2020, 25, 1881. [Google Scholar] [CrossRef]
- Mcdaniel, R.; Meshulam-Simon, G.; Alvizo, O.; Zhang, X. Production of Monoterpenes. U.S. Patent No. 20130143291, 30 March 2011. [Google Scholar]
- Mendez-Perez, D.; Alonso-Gutierrez, J.; Hu, Q.; Molinas, M.; Baidoo, K.; Wang, G.; Chan, L.J.G.; Adams, P.D.; Keasling, J.D.; Lee, T.S. Production of Jet Fuel Precursor Monoterpenoids from Engineered Escherichia coli. Biotechnol. Bioeng. 2017, 114, 1703–1712. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a Mevalonate Pathway in Escherichia coli for Production of Terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Q.; Ren, M.; Feng, H.; Jiang, X.; Zheng, Y.; Liu, M.; Zhang, H.; Xian, M. Metabolic Engineering of Escherichia coli for the Biosynthesis of Alpha-Pinene. Biotechnol. Biofuels 2013, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, S.; Cao, J.; Qiao, J.; Zhao, G.-R. Systematic Optimization of Limonene Production in Engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q.; Cao, Y.; Feng, X.; Zheng, Y.; Zou, H.; Liu, H.; Yang, J.; Xian, M. Microbial Production of Sabinene—A New Terpene-Based Precursor of Advanced Biofuel. Microb. Cell Fact. 2014, 13, 20. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, H.; Li, W.; Li, X.; Xiang, H.; Zhang, G.; Liu, H.; Wang, Q.; Wang, Y.; Xian, M.; et al. Production of γ-Terpinene by Metabolically Engineered Escherichia coli Using Glycerol as Feedstock. RSC Adv. 2018, 8, 30851–30859. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.; Gershenzon, J.; Nielson, E.E.; Froehlich, J.E.; Croteau, R. Limonene Synthase, the Enzyme Responsible for Monoterpene Biosynthesis in Peppermint, Is Localized to Leucoplasts of Oil Gland Secretory Cells. Plant Physiol. 1999, 120, 879–886. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in Biosynthesis, Regulation, and Metabolic Engineering of Plant Specialized Terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Jiang, G.-Z.; Yao, M.-D.; Wang, Y.; Zhou, L.; Song, T.-Q.; Liu, H.; Xiao, W.-H.; Yuan, Y.-J. Manipulation of GES and ERG20 for Geraniol Overproduction in Saccharomyces Cerevisiae. Metab. Eng. 2017, 41, 57–66. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sakai, H.; Shimada, T.; Omura, M.; Kumazawa, S.; Nakayama, T. Characterization of Γ-terpinene Synthase from Citrus unshiu (Satsuma Mandarin). BioFactors 2004, 21, 79–82. [Google Scholar] [CrossRef]
- Bao, S.-H.; Zhang, D.-Y.; Meng, E. Improving Biosynthetic Production of Pinene through Plasmid Recombination Elimination and Pathway Optimization. Plasmid 2019, 105, 102431. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.-H.; Jiang, H.; Zhu, L.-Y.; Yao, G.; Han, P.-G.; Wan, X.-K.; Wang, K.; Song, T.-Y.; Liu, C.-J.; Wang, S.; et al. A Dynamic and Multilocus Metabolic Regulation Strategy Using Quorum-Sensing-Controlled Bacterial Small RNA. Cell Rep. 2021, 36, 109413. [Google Scholar] [CrossRef] [PubMed]
- Hunke, S.; Betton, J. Temperature Effect on Inclusion Body Formation and Stress Response in the Periplasm of Escherichia coli. Mol. Microbiol. 2003, 50, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- de Groot, N.S.; Ventura, S. Effect of Temperature on Protein Quality in Bacterial Inclusion Bodies. FEBS Lett. 2006, 580, 6471–6476. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, J.; Yao, G.; Bao, S.; Wan, X.; Wang, F.; Wang, K.; Song, T.; Han, P.; Liu, T.; et al. A Novel, Genetically Encoded Whole-Cell Biosensor for Directed Evolution of Myrcene Synthase in Escherichia coli. Biosens. Bioelectron. 2023, 228, 115176. [Google Scholar] [CrossRef]
- Schepmann, H.G.; Pang, J.; Matsuda, S.P.T. Cloning and Characterization of Ginkgo Biloba Levopimaradiene Synthase, Which Catalyzes the First Committed Step in Ginkgolide Biosynthesis. Arch. Biochem. Biophys. 2001, 392, 263–269. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Chen, W.; Shen, F.; Bi, H.; Ke, G.; Zhang, L.; Technology, D. Uni-Fold: An Open-Source Platform for Developing Protein Folding Models beyond AlphaFold. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tran, H.T.D.; Nguyen, H.T.T.; Huynh, T.B.; Nguyen, H.N.; Nguyen, L.T.; Tran, N.U.; Pham, B.T.M.; Nguyen, D.H.; Tran, T.; Nguyen, T.T.H. Functional Characterization of a Bark-Specific Monoterpene Synthase Potentially Involved in Wounding- and Methyl Jasmonate-Induced Linalool Emission in Rubber (Hevea brasiliensis). J. Plant Physiol. 2023, 282, 153942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Bao, S.; Liu, J.; Wang, F.; Yao, G.; Han, P.; Wan, X.; Chen, C.; Jiang, H.; Zhang, X.; et al. The Biosynthesis of the Monoterpene Tricyclene in E. coli through the Appropriate Truncation of Plant Transit Peptides. Fermentation 2024, 10, 173. https://doi.org/10.3390/fermentation10030173
Zhao M, Bao S, Liu J, Wang F, Yao G, Han P, Wan X, Chen C, Jiang H, Zhang X, et al. The Biosynthesis of the Monoterpene Tricyclene in E. coli through the Appropriate Truncation of Plant Transit Peptides. Fermentation. 2024; 10(3):173. https://doi.org/10.3390/fermentation10030173
Chicago/Turabian StyleZhao, Meijia, Shaoheng Bao, Jiajia Liu, Fuli Wang, Ge Yao, Penggang Han, Xiukun Wan, Chang Chen, Hui Jiang, Xinghua Zhang, and et al. 2024. "The Biosynthesis of the Monoterpene Tricyclene in E. coli through the Appropriate Truncation of Plant Transit Peptides" Fermentation 10, no. 3: 173. https://doi.org/10.3390/fermentation10030173
APA StyleZhao, M., Bao, S., Liu, J., Wang, F., Yao, G., Han, P., Wan, X., Chen, C., Jiang, H., Zhang, X., & Zhu, W. (2024). The Biosynthesis of the Monoterpene Tricyclene in E. coli through the Appropriate Truncation of Plant Transit Peptides. Fermentation, 10(3), 173. https://doi.org/10.3390/fermentation10030173




