1. Introduction
In the quest for sustainable solutions to contemporary environmental challenges, the potential of algae as a multifaceted resource has garnered increasing attention. The escalating global demand for energy and chemicals, driven by population growth and development, underscores the need to explore alternative resources that can be accomplished by the potential of microalgae and cyanobacteria as a promising solution [
1,
2]. Notably, microalgae and cyanobacteria offer the potential to efficiently produce biomass for various applications, including food, feed, fuels, and chemicals, while addressing challenges in wastewater treatment and carbon capture [
3]. The economic significance of algae in marine biotechnology, coupled with their higher energy yields and faster photosynthesis, has attracted attention from economists [
4,
5]. Cyanobacterium, particularly
Arthrospira platensis, known as spirulina, stands out for its diverse applications in health, cosmetics, nutrition, and bioremediation [
6,
7]. Despite the immense potential of microalgae and cyanobacteria, the commercialization of related technologies faces a significant hurdle: the high cost of microalgal biomass production, emphasizing the importance of careful species selection and cultivation practices [
8].
Moreover, the utilization of algae in producing high-value compounds presents an avenue for climate change mitigation, reducing reliance on traditional energy-intensive production methods [
9,
10]. Algae-based biofuels hold promise in reducing greenhouse gas emissions and contributing to renewable energy alternatives[
11,
12,
13]. Onyeaka et al. (2021) highlight the carbon sequestration potential of algae, emphasizing their role in carbon capture and storage initiatives [
10].
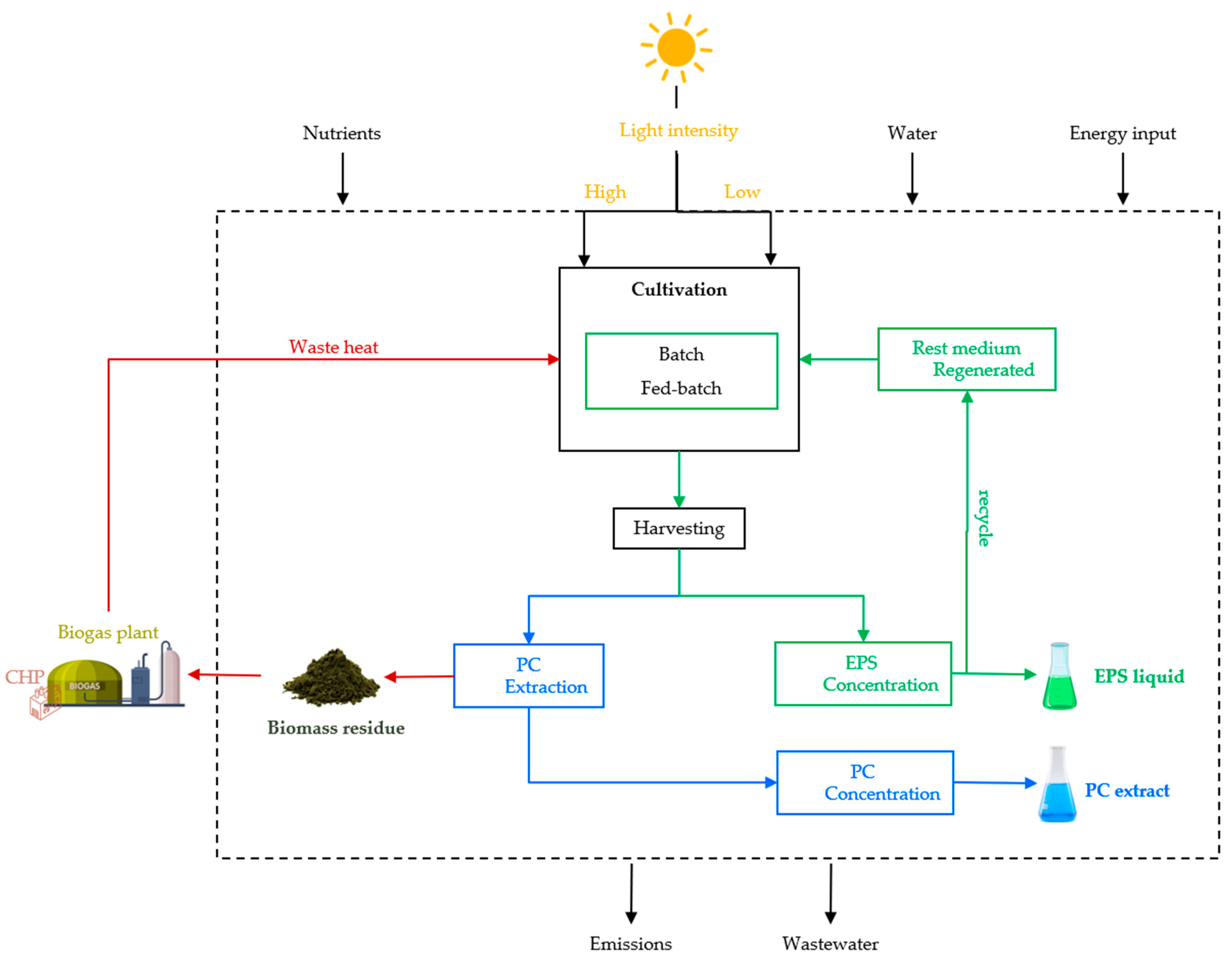
In this respect, this study delves into the development of a comprehensive life cycle analysis (LCA) framework to produce antiviral exopolysaccharides (EPS) and phycocyanin (PC) with A. platensis (spirulina).
Phycocyanin, renowned as the most valuable component of
A. platensis, stands out as a valuable blue pigment [
14]. Regarding exopolysaccharides, studies research underscores the potential antiviral efficacy of EPS in preventing KHV infection in carp [
15,
16,
17]. The cultivation growth occurred in a pilot-scale photobioreactor (PBR).
Prompted by the Antiviral Substances and Pigments project, supported by the Agency for Renewable Resources (Federal Ministry of Food and Agriculture of Germany), the primary objective is to contribute to the growing field of algae extraction and residue biomass valorization. The LCA will serve as a baseline structure applicable to diverse processes, technologies, and end products, aiming to improve the environmental performance of the project and facilitate widespread replication and scalability.
Hence, it holds significance to assess and gain a comprehensive understanding of each stage involved in a process. Implementing the LCA is a fitting method to analyze product impacts throughout their life cycles. The ISO 14044/14040 standard guides LCA analysis, emphasizing precise definition of process boundaries [
18]. This involves delineating limits, which can range from cradle to grave, covering the entire production system cycle, or gate to gate, focusing solely on manufacturing. Adhering to these standards, the study evaluates cradle-to-gate boundaries, from cyanobacteria supply to value-added product production at the laboratory’s gate.
Furthermore, this research integrates environmental impact methodologies to aid in interpreting LCA studies. It achieves this by translating system emissions and resource extractions into concise environmental impact scores [
19]. This is done using characterization factors (CFs), which measure the environmental impact per unit of stressor, such as per kilogram of resource used or emission released. These factors are crucial for impact assessment [
20]. Two primary approaches, midpoint and endpoint, are employed in this process. Midpoint factors are strategically positioned along the impact pathway, establishing robust connections to environmental flows and minimizing uncertainty [
21]. For instance, in the water consumption category, it represents the number of cubic meters of water consumed per cubic meter of water extracted, reflecting relative water loss through evaporation or incorporation in products [
20]. Conversely, endpoint factors cover human health, ecosystem quality, and resource scarcity, offering valuable insights into environmental relevance with higher uncertainty. Using the water consumption example, the endpoint perspective quantifies damage to human health based on malnutrition potentially caused by water scarcity or vulnerability to water shortages.
Together, these two approaches complement each other in the comprehensive assessment of environmental impacts [
20]. Given the diversity of methodologies, the models will vary in terms of the substances considered in the calculations and the characterization factors. These factors can significantly influence the choice of life cycle impact assessment (LCIA) methodology, making it an important decision point [
22].
Based on prevailing literature, a predominant focus has been observed in LCA studies, with the majority concentrating solely on reporting global warming potential as part of the climate change impact category [
23,
24]. In contrast, our present study takes a different approach by selecting indicators relevant to the specific system under analysis. For the midpoint category, we consider marine eutrophication, climate change aspects, and cumulative energy demand associated with non-renewable resources. Some of these classifications have been used in other microalgae biomass studies [
25,
26] . Meanwhile, within the endpoint category, our focus extends to water consumption and its impact on aquatic ecosystems and human health. These chosen environmental impacts result from the applied methodology and normalization process, enabling the identification of the most significant impacts by converting diverse units into single scores [
22,
27].
In this context, specific impact categories such as abiotic depletion potential, acidification, freshwater and terrestrial ecotoxicity, and human toxicity may not be considered pertinent for the present study. The investigation of microalgae reveals that some of these categories are not representative [
28]. This decision could be influenced by specific processes and materials flows of system that render these impact categories less significant or negligible. Despite being part of established methodologies, their exclusion may stem from a focus on more critical or pertinent environmental aspects within the context of the study, allowing for a streamlined and targeted LCA performance. The LCIA result will generate a profile offering valuable insights into environmental aspects, highlighting both favorable (potentials) and unfavorable (hot spots) performance within a product’s life cycle.
In the pursuit of a holistic understanding, the research factors in realistic environments are subject to seasonal variations. These variations encompass diverse elements, including fluctuations in temperature and sunlight intensity. To elucidate the nature of real-life scenarios, the study introduces extreme scenarios, depicting high sunlight conditions akin to sunny days and low light scenarios indicative of overcast conditions or environments in different geographic locations. Incorporating such boundaries strives to provide a comprehensive understanding of the impact of varying environmental conditions on algae cultivation, extending to different harvesting schedules.
Therefore, this study aims to explore two fundamental factors. Firstly, the impact of different harvesting cycles on productivity and on the environment is examined. Secondly, an assessment is conducted on how variations in light supply quality, influenced by factors such as weather and location, impact productivity and environmental consequences. Furthermore, this line intends to encompass a broad spectrum of outcomes and gain insights into how diverse environmental conditions may shape results.
This nuanced approach contributes to a thorough evaluation of the environmental impact, and it helps establish clear boundaries and constraints for the analysis, empowering informed decision-making in sustainable algae cultivation practice.
Furthermore, the study seeks to evaluate the potential independence of LCA outcomes across different harvesting cycles in varied light scenarios. If confirmed, this observation could extend beyond countries with high sunlight intensity, such as Spain, to those in diverse conditions, including Denmark. This assumption implies that statistical annual light intensity is likely to align with the spectrum defined by the study’s optimal and suboptimal scenarios. This discourse establishes the foundation for a nuanced exploration of the intricate interplay between environmental conditions, harvesting cycles, and LCA outcomes in the subsequent sections of the research.
3. Results
The emissions and resource flows underwent mapping during the inventory analysis (2.4), aligning seamlessly with the specific impact methodologies (2.5), marking the culmination of the life cycle assessment step. The calculations for algae productivity, mass, energy, and water consumption were executed, accounting for the two scenarios. Subsequently, these data were transposed into the OpenLCA software, enabling the detailed assessment of environmental impacts.
Thus, the study findings unveil insights into the environmental impact of various circumstances. For this purpose, the light intensity variables are devised to establish boundary conditions, ensuring a representation of realistic environments that are subject to seasonal variations throughout the year. These variations encompass diverse factors such as temperature and sunlight intensity fluctuations. Consequently, the research presents extreme scenarios, with one depicting high sunlight conditions representing sunny days, while the low light scenario indicates overcast conditions or environments in different countries with distinct geographic locations. By incorporating such boundaries, the study provides a comprehensive understanding of the impact of varying environmental conditions on algae cultivation, offering insights that align with the dynamic nature of real-life scenarios.
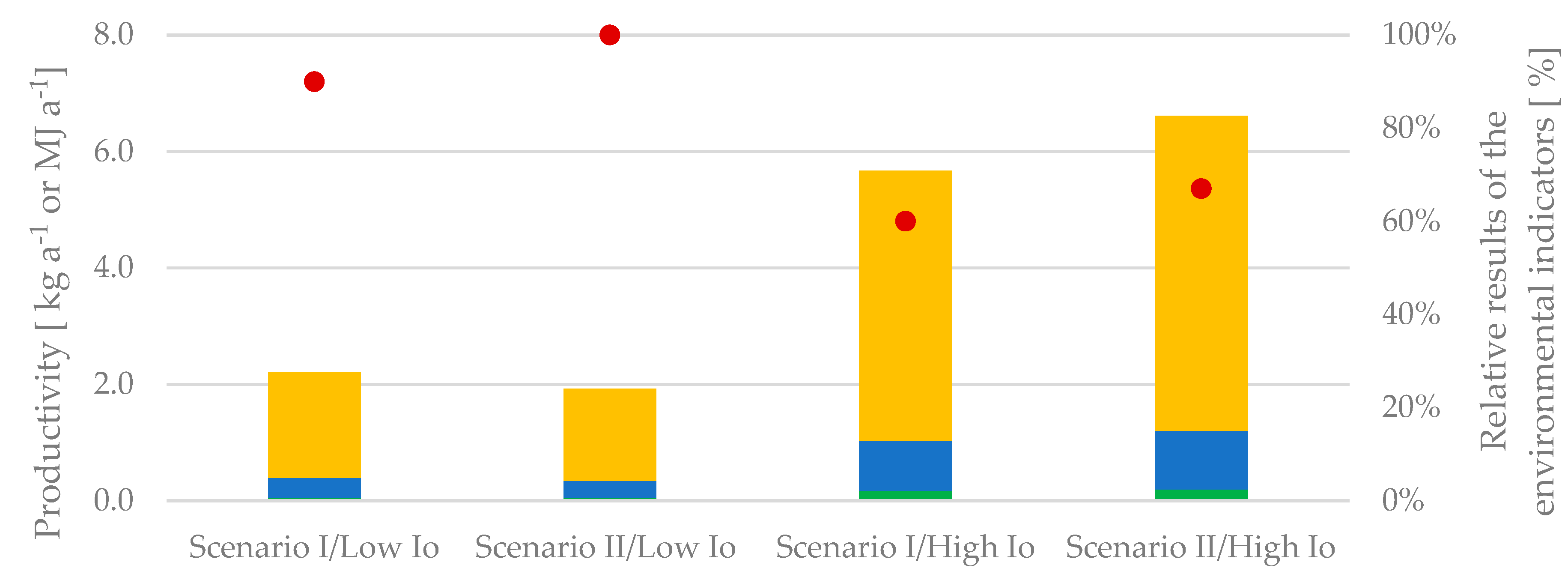
Considering the entirety and proportional averages of environmental impact outcomes, the scenarios with the most significant impact are those characterized by receiving the least amount of sunlight (low I
o) and the one operating the system with the highest volume of culture harvested (Scenario II), as seen in
Figure 3. The most favorable situation is found in Scenario I under high light intensity, in both regards: achieving higher productivity when comparing conditions within the same scenario and minimizing environmental harm.
3.1. Environmental Impact Analysis for Exopolysaccharide Extraction
Overall, for producing 1 kg of EPS, the electricity demand of the system varies from 0.69 to 1.28 MJ a−1, wherein the energy consumption is assigned to the operation of the machinery. Within this process, the study highlights that the predominant portion of electricity usage occurs during upstream processes. Furthermore, the regenerated medium process, integrated into the system, involves the calculation of energy flow using negative values due to its recycling and loop closure.
The extraction process stands out as the major driver of water consumption, representing a share of 69.78%, with harvesting contributing 30.22%. Additionally, the fed-batch stage accounts for 6.97% of the total. The operation of dynamic cross-flow filtration, in this process step (2.3.4), demands a significant volume of water, leading to this result. The water impact unfolds similarly, with the extraction phase accounting for nearly the entirety of the environmental impact, succeeded by a minor contribution from the upstream process.
Table 6 displays the environmental impact results and the consistency in absolute deviation across various impacts. Regardless of the specific categories, a 12% deviation is consistently observed in all four scenarios.
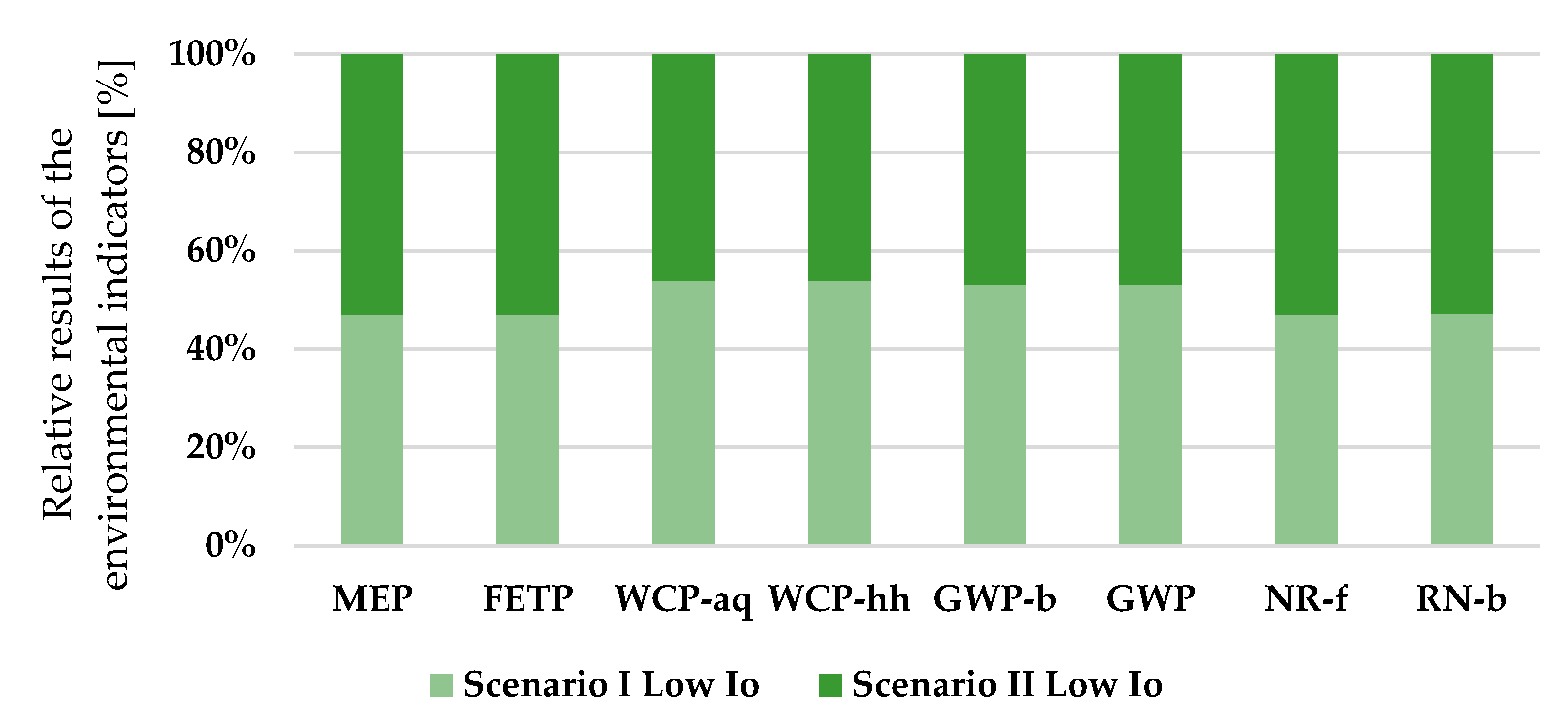
Despite the overarching tendency shown previously (
Figure 3), which indicates that Scenario II causes a greater environmental impact, the analysis of EPS output (
Figure 4) yields different results, specifically for the categories of water consumption related to aquatic ecosystems and human health, as well as for impacts associated to biogenic carbon in climate change and the GWP 100a of fossil fuels. These disparities highlight the complexity of the variables and system parameterization between harvesting volume and duration, light intensity, and its peculiarities within the entire extraction process.
3.2. Environmental Impact Analysis for Phycocyanin Extraction
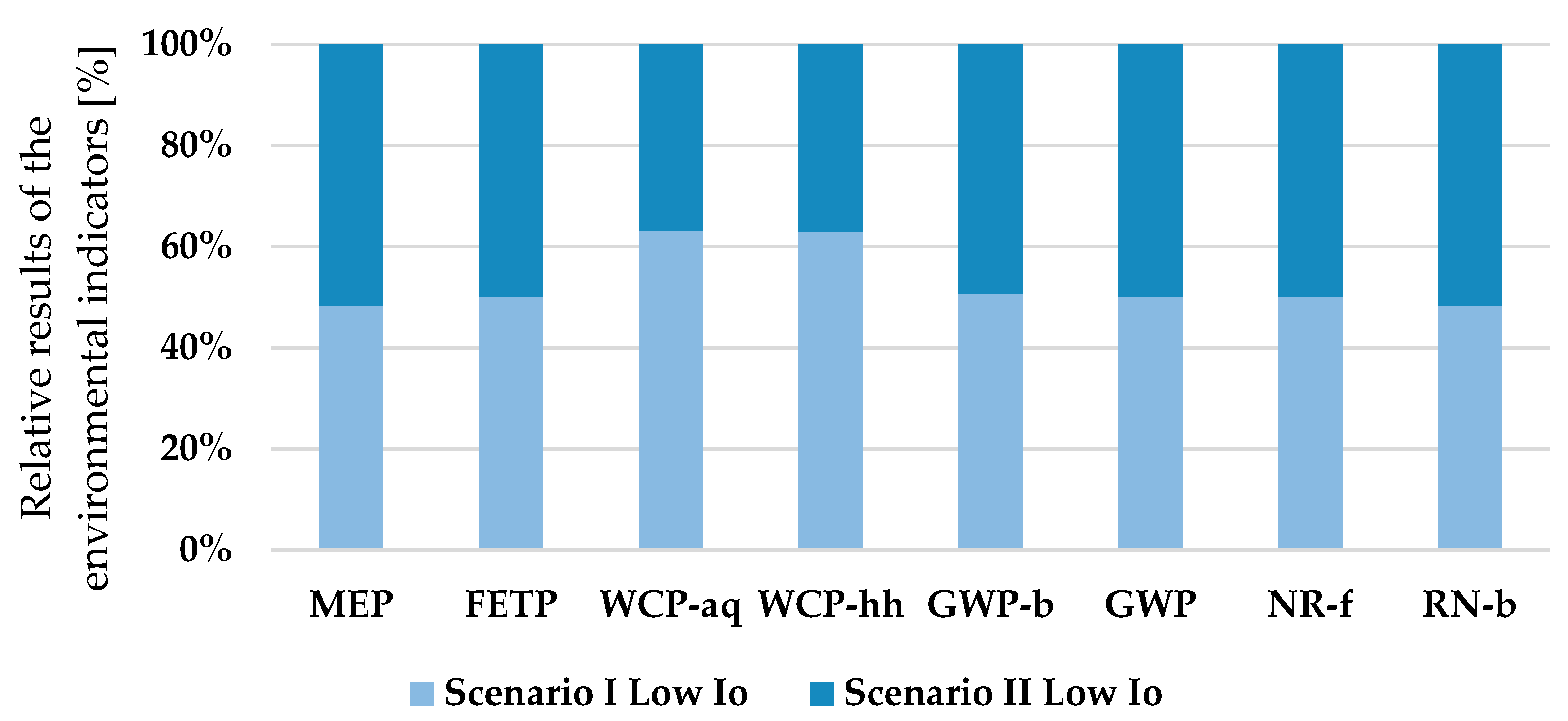
Environmental impact categories were likewise scrutinized in the assessment for the extraction of 1 kg of PC. The relative contributions of each life cycle scenario to ecological impacts are presented in
Figure 5 and
Table 7.
Despite the array of equipment utilized in PC extraction and concentration, the focal point of energy demand lies in the initial processes. To delve into specifics, within the batch and fed-batch steps, energy demands are primarily driven by the electricity required for algae culture circulation and the embedded energy within the cultivation process. This is warranted as the compressor and chiller operate daily throughout the year, showcasing a consistent pattern in EPS production.
The results show significant variations in the water consumption impact category, with Scenario II exhibiting a 36.2% reduction compared to Scenario I, both under low light intensity. Additionally, water-related impacts on aquatic ecosystems and human health represent more than 40% in deviation. This evidence points to an intensified harvesting schedule in the scenario where each cycle harvests 20 L—the total duration extends to approximately 91 d·a−1, while opting for 70 L per cycle reduces this timeframe to 23 d—leading to increased water demand, which is correlated with increased environmental repercussions.
In the realm of other impacts, the deviation remains consistent at 12%. Therefore, under high sunlight conditions, water consumption, marine eutrophication, and overall climate change impacts increase at the same rate in Scenario II. The environmental impact performance in high light intensity scenarios exhibits a similar correlation between deviations, albeit with elevated figures.
4. Discussion
This environmental assessment reveals that scenarios with lower light intensity and higher water volume demonstrate greater environmental impacts, emphasizing the relationship between harvesting volume, duration, and environmental repercussions. Despite higher environmental impacts in Scenario II, a nuanced analysis of EPS and PC extraction shows variations, highlighting the complexity of system parameters. As per the findings by Wenzel et al. [
50] study, the data quality can be classified as “very high” in terms of specificity, as they are either directly measured at the specific process site or accurately scaled from measurements. Henceforth, a systematic identification and quantification of material and energy flows took place for each of the eight process steps.
The project’s environmental impact is notably influenced by sunlight intensity and harvesting practices. Moreover, the corresponding impacts and resource demand are closely tied to the productivity levels of the high-value products considered within this project’s scope. In examining the overall environmental impacts, encompassing both midpoint and endpoint analyses, the study underscores critical environmental effects induced by the system. The research reveals that the primary environmental impacts stem from marine ecotoxicity, fossil resource depletion, and climate change-fossil. Significantly, the stages exerting the most substantial influence on these environmental aspects are upstreaming and harvesting. This correlation can be traced back to the considerable energy requirements and nutrient consumption intrinsic to algae cultivation.
Pierre et al. (2019) highlight the connection between microalgae biomass production and factors such as light intensity and aeration levels, underscoring a direct correlation with photosynthesis activity[
37,
51,
52,
53,
54]. Notably, the enhanced biomass production of diverse microalgae species, including
Cyanospira capsulata,
Porphyridium, and
Synechococcus, as well as the researched cyanobacteria
A. platensis, is particularly notable under conditions of high continuous light irradiance [
25,
26,
27,
28]. This becomes apparent in the study setups when simulated for a situation characterized by increased solar exposure. The PC content at the light intensities of 450 µmol·m
−2s
−1 showed a significant rise relative to that at intensities of 100 µmol·m
−2s
−1. This fact is likewise noted in the studies from[
55]. Soni et al. (2019) further indicate that the greatest biomass productivity occurred in the summer months, coinciding with the highest recorded chlorophyll content [
56].
The highest PC yield was 16.52 g per cycle under 70 L harvesting volume, while the lowest PC content was 3.68 g per cycle at low light intensity and 20 L of harvested water. Similarly, this holds true for EPS, showcasing peak values (3.28 g) in simulations conducted in regions with abundant sunlight and Scenario II configuration. In contrast, the lowest value is observed in the contrasting scenario, registering 0.62 g. Regarding biogas production, Scenario II exhibits the highest value at high light intensities, followed by Scenario II with low light intensities. Irrespective of the harvesting scenarios, processes under high light intensities demonstrate a higher yearly yield, correlating with the elevated growth kinetics. However, when comparing the yield of a single batch, harvesting 70 L yields a superior outcome. This discrepancy arises from the comparison between yield (per batch) and space-time yield (per year)
In the present study, the environmental impact of PC extraction concerning climate change impact, the authors report lower values with water as the solvent (1.25 × 103 kg CO2 eq) compared to ethanol (3.26 × 105 kg CO2 eq). In contrast, the current investigation, conducted under varying light intensities, revealed divergent GWP100 values. In low light settings, it was 3.09 × 107 kg CO2 eq, while scenarios of high light intensity demonstrated higher GWP100 values, ranging from 1.20 × 108 kg CO2 eq in Scenario I to 1.36 E8 kg CO2 eq in Scenario II.
Consistent with this observation, the LCA results reaffirm that sodium bicarbonate, representing the bulk of the SOT compound, emerges as the primary stressor to this environmental impact. Furthermore, within the ReCiPe midpoint, this impact has the utmost magnitude after applying the normalization.
For the majority of the impacts analyzed, the cultivation process exerts a considerable environmental footprint. Therefore, the system is designed to recycle the regenerated media from the output of the EPS concentration process, lowering the need for additional resources. This approach enhances supply chain management, contributing to a reduction in the depletion of natural resources and minimizing environmental impacts throughout nutrient production and utilization [
6,
57,
58].
The outcomes obtained from the IPCC (GWP 100a) methodology highlight the most significant environmental impact in the climate change categories, specifically climate change-fossil for the former and non-renewable fossil for the latter. This outcome primarily stems from the electricity consumption required for machinery operations. Potential improvements to mitigate this impact involve exploring alternatives, such as shifting to renewable energy sources in lieu of grid electricity.
Conversely, employing microalgae and cyanobacteria yields positive outcomes for carbon mitigation and encompasses the capacity to extract nutrients from wastewater and various gaseous emissions. Studies have shown that the production of 1 kg of dry biomass requires approximately 1.8 kg of CO
2. Combined with their fast growth, their CO
2 fixation efficiency is 10–50 times greater compared to terrestrial plants [
37,
51,
52,
55,
58]. Likewise, phototrophic organisms have the capability to capture around 1.3 kg of carbon dioxide in the process of generating 1 kg of biomass. However, it is essential to consider the overall carbon balance, including not just the fixation of CO
2 during growth but also the subsequent release of CO2 upon product utilization or degradation. For this reason, in this study, the boundaries were chosen so that the fixation of CO
2 and the subsequent release are net zero.
Looking at it from an endpoint methodology standpoint and considering the factors contributing to the process, the primary stressor impacting both water resources and human health is focused on the media components. These components are once again associated with the production of sodium bicarbonate. Water consumption during manufacturing poses challenges to water availability and quality, contributing to environmental stress and potential contamination. This is in line with the concerns raised by the World Health Organization regarding the implications of industrial activities on water quality and accessibility [
59]. Additionally, the discharge of industrial by-products may introduce pollutants into water bodies, further compromising water quality. The compromised water quality resulting poses risks to human health, consistent with studies highlighting the correlation between water quality and public health [
60,
61,
62]. Therefore, the management of material flows and impact factors stands out as a key area that demands attention in future technological advancements.
Responsible water management practices are fundamental to mitigate adverse effects on aquatic environments. Implementing efficient water recirculation systems and employing nutrient recovery techniques in microalgae and cyanobacteria cultivation is essential for responsible water management, helping minimize environmental impact by reducing water consumption and nutrient runoff [
7,
60,
61,
63]. Moreover, adopting precision technologies and optimizing cultivation parameters can enhance the sustainability of microalgae and cyanobacteria production, ensuring a balance between resource utilization and environmental conservation. Integrating microalgae and cyanobacteria production with photovoltaic panels presents numerous benefits, with the primary advantage being the utilization of surplus solar energy to fulfill the substantial energy requirements of biodiesel production [
62]. Alternatively, research indicates that optimizing algal biomass production could be achieved by combining open ponds and photobioreactors, creating a hybrid system and ensuring that the unique advantages of each methodology are appropriately utilized and valued [
64,
65].
Recognizing the intricacy of the situation emphasizes the importance of a dual approach utilizing both midpoint and endpoint indicators. This comprehensive method is essential for capturing the diverse aspects of environmental impacts, considering their implications on ecosystems and human well-being, and providing globally representative characterization factors [
21,
47,
66].
The economic outlook of exploring algae cultivation for economic benefits reveals significant promise, particularly in addressing environmental and human health concerns. Nevertheless, the hurdles to achieving project scalability while ensuring economic viability persist. Therefore, a key objective of this project is to overcome these challenges by concurrently generating two high-value products for the market.
Moreover, generating high-value products, such as PC at market price, market prices at 170–280 USD·kg
−1 [
67], and EPS at 300 USD·kg
−1 [
37], distinguishes this initiative from conventional bioenergy-oriented projects. The strategic emphasis on products with significant market value aims to offer a unique approach to mitigating costs. Moreover, findings reported by Chalermthai et al. (2023) indicate that the simultaneous production of spirulina powder and bioplastic represents a promising endeavor, demonstrating a payback time (PBT) as brief as 2.6 years and a return on investment (ROI) reaching a noteworthy 38.5% [
68].
The project’s potential is clarified by examining the energy demand relationship between the processes of extracting both products compared to the overall energy consumption for cultivation and harvesting. In essence, when we allocate the cultivation and extraction stages as the system’s core elements, the extraction processes for by-products exert minimal influence on the total energy demand. To emphasize, even if EPS and PC were neglected from the system, their absence would not significantly affect the overall impact. This resilience highlights the potential economic viability of the system. As a result, the project’s dual focus on environmental sustainability and the production of high-value goods positions it as a promising avenue for economic progress and resource optimization.
Finally, performing a comprehensive LCA is paramount when evaluating cyanobacteria cultivation projects’ environmental impacts. However, it is essential to acknowledge the inherent limitations of comparing studies in this field. Numerous variables come into play, including the specific species of microorganisms being cultivated, technology employed, cultivation conditions, and geographical factors. Each of these variables can significantly influence the outcomes and make direct comparisons challenging. Moreover, the diversity in methodologies used for analysis across studies introduces an additional layer of complexity. Recognizing these limitations underscores the importance of cautiously approaching the assessment and advocating for standardized methods to enhance the reliability and comparability of results in the evolving field of algae cultivation.