Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste
Abstract
1. Introduction
2. Materials and Methods
Adopted Strategy for Literature Search: Methodological Approach and Selection Criteria
3. Results and Discussion
3.1. PRISMA Flow Diagram of the Literature Search
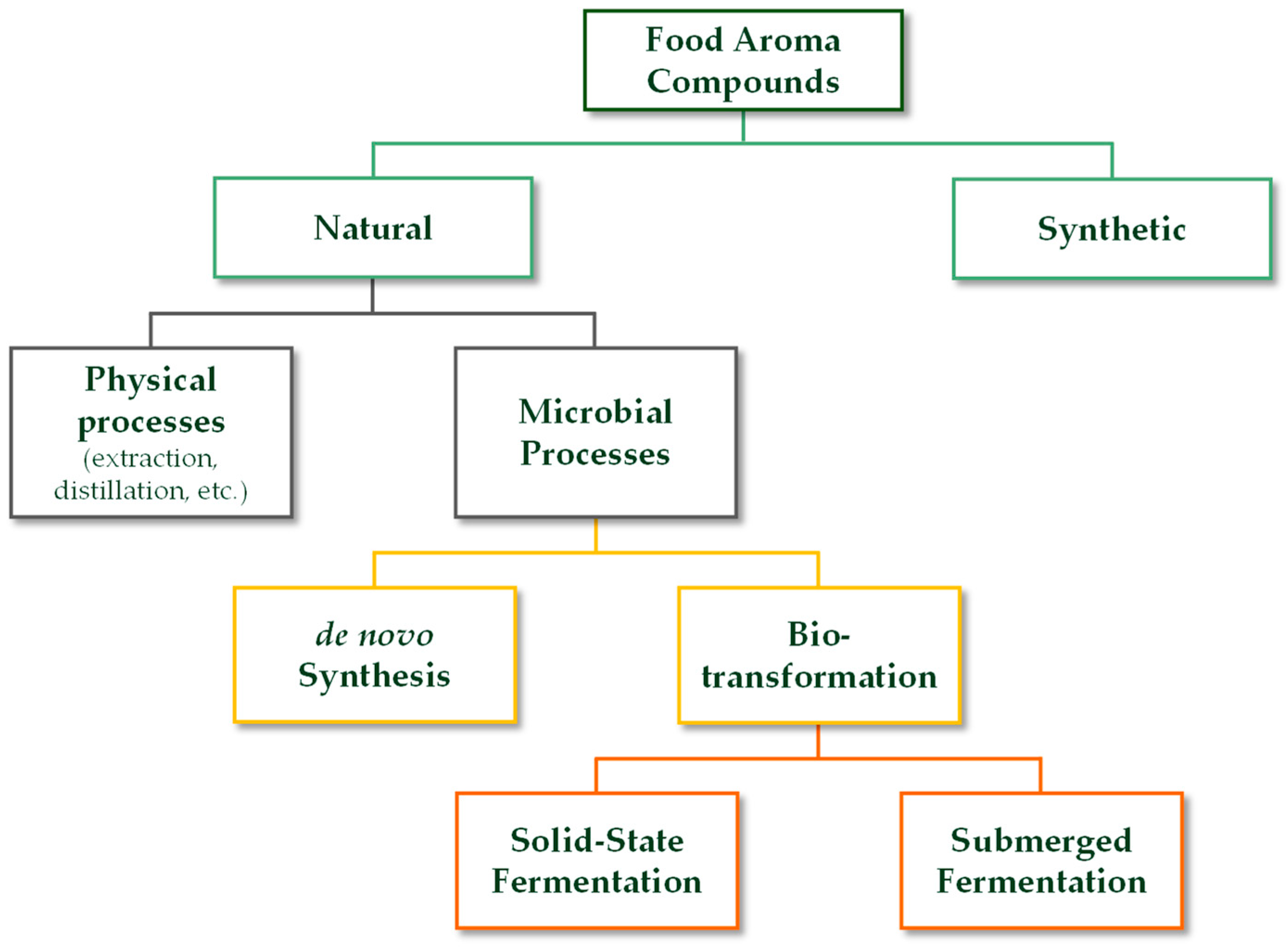
3.2. Microbial Processes in the Bio-Production of Aroma Compounds: Focus on Solid-State Fermentation and Submerged Fermentation
3.2.1. Solid-State Fermentation in the Bio-Production of Aroma Compounds
3.2.2. Submerged Fermentation in the Bio-Production of Aroma Compounds
3.3. Evidence of Aroma Compounds Bio-Production from Agri-Food Waste
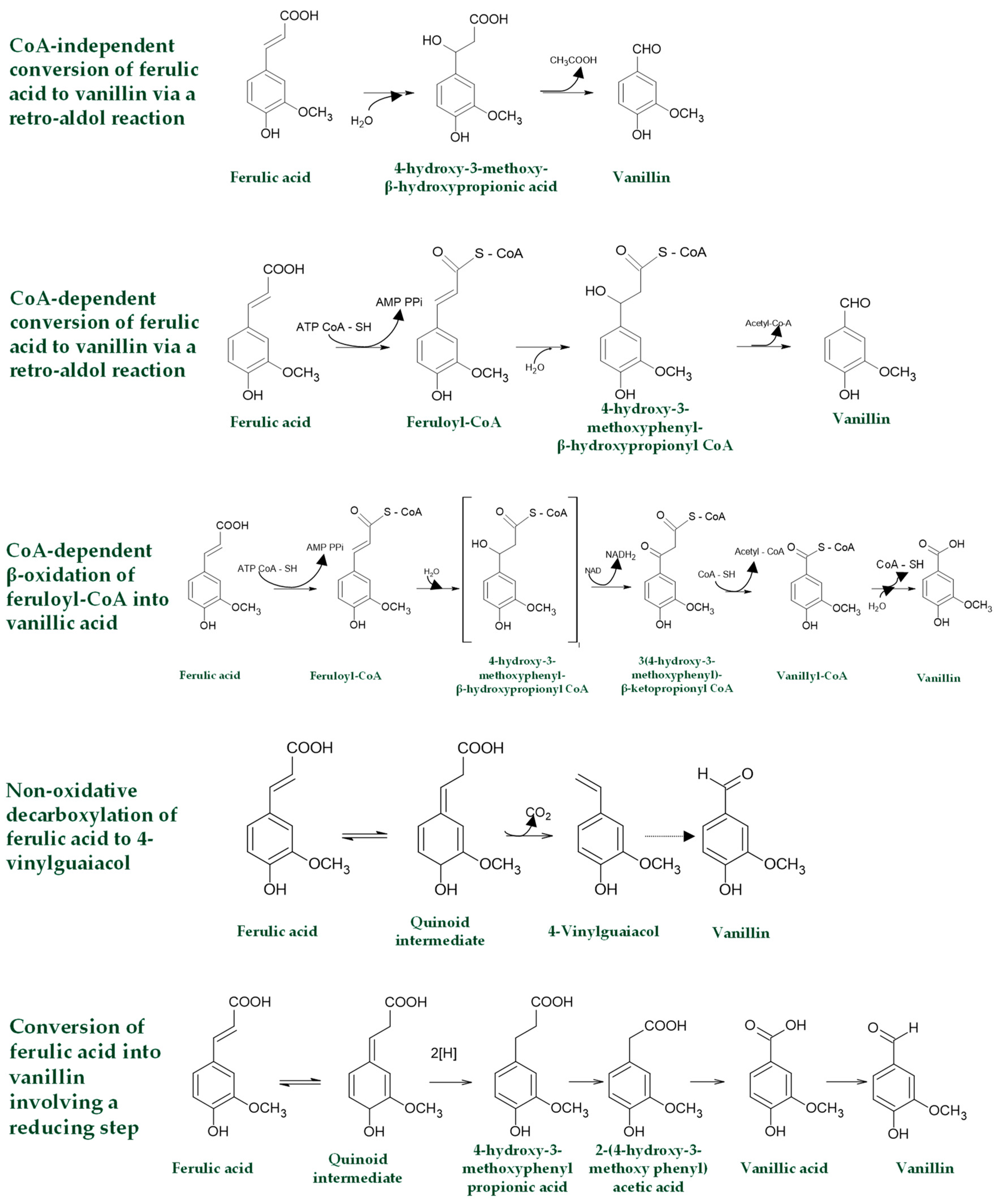
3.3.1. Bio-Production of Aroma Compounds with a Sweet Note
3.3.2. Bio-Production of Aroma Compounds with a Fruity Note
3.3.3. Bio-Production of Aroma Compounds with a Floral Note
3.3.4. Bio-Production of Mixtures of Aroma Compounds
4. Strengths and Limitations of This Study
5. Conclusions and Future Trends for the Bioproduction of Aroma Compounds
Author Contributions
Funding
Conflicts of Interest
References
- Voilley, A.; Etievant, P. Flavour in Food; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–451. [Google Scholar] [CrossRef]
- Salles, C. Odour-Taste Interactions in Flavour Perception. In Flavour in Food; Andrée, V., Etiévant, P., Eds.; Woodhead Publishing Limited: Sawston, UK, 2006; pp. 345–368. [Google Scholar]
- Sánchez-Rodríguez, L.; Syd Ali, N.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Sendra, E. Flavors and Aromas; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar]
- Bojanowski, V.; Hummel, T. Retronasal Perception of Odors. Physiol. Behav. 2012, 107, 484–487. [Google Scholar] [CrossRef]
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M.T. Food Aroma Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128052570. [Google Scholar]
- Paulino, B.N.; Sales, A.; Felipe, L.; Pastore, G.M.; Molina, G.; Bicas, J.L. Recent Advances in the Microbial and Enzymatic Production of Aroma Compounds. Curr. Opin. Food Sci. 2021, 37, 98–106. [Google Scholar] [CrossRef]
- Dinu, V.; Kilic, A.; Wang, Q.; Ayed, C.; Fadel, A.; Harding, S.E.; Yakubov, G.E.; Fisk, I.D. Policy, Toxicology and Physicochemical Considerations on the Inhalation of High Concentrations of Food Flavour. npj Sci. Food 2020, 4, 15. [Google Scholar] [CrossRef]
- Martău, G.A.; Călinoiu, L.F.; Vodnar, D.C. Bio-Vanillin: Towards a Sustainable Industrial Production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- Karaalioğlu, O.; Yüceer, Y.K. Nonconventional Yeasts to Produce Aroma Compounds by Using Agri-Food Waste Materials. FEMS Yeast Res. 2021, 21, 63. [Google Scholar] [CrossRef]
- Braga, A.; Faria, N. Biotechnological Production of Specialty Aromatic and Aromatic-Derivative Compounds. World J. Microbiol. Biotechnol. 2022, 38, 80. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chen, H.S. Understanding Consumers’ Intentions to Purchase Clean Label Products: Evidence from Taiwan. Nutrients 2022, 14, 3684. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional Ingredients from Agri-Food Waste: Effect of Inclusion Thereof on Phenolic Compound Content and Bioaccessibility in Bakery Products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in Solid State Fermentation Technology: Design, Applications and Engineering Aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Di Fraia, S.; Godvin Sharmila, V.; Rajesh Banu, J.; Massarotti, N. A Comprehensive Review on Upscaling of Food Waste into Value Added Products towards a Circular Economy: Holistic Approaches and Life Cycle Assessments. Trends Food Sci. Technol. 2024, 143, 104288. [Google Scholar] [CrossRef]
- Gomathi, S.; Rameshpathy, M. Valorization of Agro-Waste Residues into Bio-Vanillin a Comprehensive Review. Ind. Crops Prod. 2023, 205, 117522. [Google Scholar] [CrossRef]
- Hosoglu, M.I.; Guneser, O.; Yuceer, Y.K. Different Bioengineering Approaches on Production of Bioflavor Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128115008. [Google Scholar]
- Kumar Verma, D.; Thyab Gddoa Al-Sahlany, S.; Kareem Niamah, A.; Thakur, M.; Shah, N.; Singh, S.; Baranwal, D.; Patel, A.R.; Lara Utama, G.; Noe Aguilar, C. Recent Trends in Microbial Flavour Compounds: A Review on Chemistry, Synthesis Mechanism and Their Application in Food. Saudi J. Biol. Sci. 2022, 29, 1565–1576. [Google Scholar] [CrossRef]
- Yazid, N.A.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-state fermentation as a novel paradigm for organic waste valorization: A review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Ben Akacha, N.; Gargouri, M. Microbial and Enzymatic Technologies Used for the Production of Natural Aroma Compounds: Synthesis, Recovery Modeling, and Bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Sales, A.; Paulino, B.N.; Pastore, G.M.; Bicas, J.L. Biogeneration of Aroma Compounds. Curr. Opin. Food Sci. 2018, 19, 77–84. [Google Scholar] [CrossRef]
- Yafetto, L. Application of Solid-State Fermentation by Microbial Biotechnology for Bioprocessing of Agro-Industrial Wastes from 1970 to 2020: A Review and Bibliometric Analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef] [PubMed]
- Try, S.; Voilley, A.; Chunhieng, T.; De-Coninck, J.; Waché, Y. Aroma Compounds Production by Solid State Fermentation, Importance of in Situ Gas-Phase Recovery Systems. Appl. Microbiol. Biotechnol. 2018, 102, 7239–7255. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast Flavour Production by Solid State Fermentation of Orange Peel Waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Ramos, O.L.; Xavier Malcata, F. Food-Grade Enzymes. Compr. Biotechnol. 2017, 3, 587–603. [Google Scholar] [CrossRef]
- Ouedraogo, J.P.; Tsang, A. Production of Native and Recombinant Enzymes by Fungi for IndustrialApplications. Encycl. Mycol. 2021, 2, 222–232. [Google Scholar] [CrossRef]
- Saeed, S.; Ur, U.; Baig, R.; Tayyab, M.; Altaf, I.; Irfan, M.; Qasim Raza, S.; Nadeem, F.; Mehmood, T. Valorization of Banana Peels Waste into Biovanillin and Optimization of Process Parameters Using Submerged Fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 1878–8181. [Google Scholar] [CrossRef]
- Saeed, S.; Raza, S.Q.; Zafar, S.S.; Mujahid, H.; Irfan, M.; Mehmood, T. Microbial Conversion of Pomegranate Peels to Biovanillin Using Submerged Fermentation and Process Optimization through Statistical Design. Biomass Convers. Biorefinery 2022, 14, 679–688. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Levante, A.; Ferrillo, A.; Trapani, F.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Exploring the Potential of Lactic Acid Fermentation for the Recovery of Exhausted Vanilla Beans. Front. Nutr. 2022, 9, 858716. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Saleem, F.; Javed, S.; Nawaz, S.; Sultan, A.; Safdar, A.; Ullah, A.; Waseem, R.; Saeed, S.; Abbas, M.; et al. Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM). Fermentation 2022, 8, 206. [Google Scholar] [CrossRef]
- Sandes, R.D.D.; De Jesus, M.S.; Araujo, H.C.S.; Dos Santos, R.A.R.; Nogueira, J.P.; Leite Neta, M.T.S.; Narain, N. The Production of Bioaroma by Auriporia Aurulenta Using Agroindustrial Waste as a Substrate in Submerged Cultures. Fermentation 2023, 9, 593. [Google Scholar] [CrossRef]
- Lindsay, M.A.; Granucci, N.; Greenwood, D.R.; Villas-Boas, S.G. Fermentative Production of Volatile Metabolites Using Brettanomyces Bruxellensis from Fruit and Vegetable By-Products. Fermentation 2022, 8, 457. [Google Scholar] [CrossRef]
- Astuti, R.D.; Fibri, D.L.N.; Handoko, D.D.; David, W.; Budijanto, S.; Shirakawa, H. Ardiansyah the Volatile Compounds and Aroma Description in Various Rhizopus Oligosporus Solid-State Fermented and Nonfermented Rice Bran. Fermentation 2022, 8, 120. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Ricci, A.; Cirlini, M.; Bancalari, E.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Production and Recovery of Volatile Compounds from Fermented Fruit By-Products with Lacticaseibacillus Rhamnosus. Food Bioprod. Process. 2021, 128, 215–226. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia Kudriavzevii through Solid-State Fermentation. Process. Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Muñoz-Torrero, P.; Sánchez, A.; Font, X.; Barrena, R. Valorization of Agro-Industrial Wastes by Producing 2-Phenylethanol via Solid-State Fermentation: Influence of Substrate Selection on the Process. Waste Manag. 2021, 121, 403–411. [Google Scholar] [CrossRef]
- Parker, J.K. Introduction to Aroma Compounds in Foods. In Flavour Development, Analysis and Perception in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–30. ISBN 9781782421030. [Google Scholar]
- Rao, S.R.; Ravishankar, G.A. Vanilla Flavour: Production by Conventional and Biotechnological Routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Hofmann, E.; Degot, P.; Touraud, D.; König, B.; Kunz, W. Novel Green Production of Natural-like Vanilla Extract from Curcuminoids. Food Chem. 2023, 417, 135944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Cheng, Y.; Sun, H.; Bai, S.; Li, C. Identifying Environmental Hotspots and Improvement Strategies of Vanillin Production with Life Cycle Assessment. Sci. Total Environ. 2021, 769, 144771. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Parliament Regulation (EC) No 1334/2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off. J. Eur. Union 2008, L 354/34, 34–50. [Google Scholar]
- Gallage, N.J.; Møller, B.L. Vanillin-Bioconversion and Bioengineering of the Most Popular Plant Flavor and Its de Novo Biosynthesis in the Vanilla Orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Dos, E.; Barbosa, S.; Perrone, D.; Lúcia, A.; Vendramini, A.; Gomes, S.; Leite, F. Vanillin from Coconut Husk. BioResources 2008, 3, 1042–1050. [Google Scholar]
- Stentelaire, C.; Lesage-Meessen, L.; Oddou, J.; Bernard, O.; Bastin, G.; Colonna Ceccaldi, B.; Asther, M. Design of a Fungal Bioprocess for Vanillin Production from Vanillic Acid at Scalable Level by Pycnoporus Cinnabarinus. J. Biosci. Bioeng. 2000, 89, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Yadav, G.D. Comparative Studies of White-Rot Fungal Strains (Trametes Hirsuta MTCC-1171 and Phanerochaete Chrysosporium NCIM-1106) for Effective Degradation and Bioconversion of Ferulic Acid. ACS Omega 2018, 3, 14858–14868. [Google Scholar] [CrossRef]
- Galadima, A.I.; Salleh, M.M.; Hussin, H.; Shiong, C.C.; Yahaya, A.; Mohamad, S.E.; Aziz, S.A.; Yusof, N.N.M.; Al-Junid, A.F.M. Improvement of Biovanillin Production with Two-Stage PH Control Strategy from Lemongrass Leaves Hydrolysates Using Phanerochaete Chrysosporium ATCC 24725 in Batch Culture. Biomass Convers. Biorefinery 2022, 12, 2727–2736. [Google Scholar] [CrossRef]
- Fraatz, M.A.; Riemer, S.J.L.; Stöber, R.; Kaspera, R.; Nimtz, M.; Berger, R.G.; Zorn, H. A Novel Oxygenase from Pleurotus Sapidus Transforms Valencene to Nootkatone. J. Mol. Catal. B Enzym. 2009, 61, 202–207. [Google Scholar] [CrossRef]
- Krügener, S.; Krings, U.; Zorn, H.; Berger, R.G. A Dioxygenase of Pleurotus Sapidus Transforms (+)-Valencene Regio-Specifically to (+)-Nootkatone via a Stereo-Specific Allylic Hydroperoxidation. Bioresour. Technol. 2010, 101, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Alchihab, M.; Aldric, J.M.; Aguedo, M.; Destain, J.; Wathelet, J.P.; Thonart, P. The Use of Macronet Resins to Recover Gamma-Decalactone Produced by Rhodotorula Aurantiaca from the Culture Broth. J. Ind. Microbiol. Biotechnol. 2010, 37, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Bouws, H.; Wattenberg, A.; Zorn, H. Fungal Secretomes—Nature’s Toolbox for White Biotechnology. Appl. Microbiol. Biotechnol. 2008, 80, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-Phenylethanol and 2-Phenylethyl Acetate Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Jawaid, M.; Chandrasekar, M.; Senthilkumar, K.; Yadav, B.; Saba, N.; Siengchin, S. Sugarcane Wastes into Commercial Products: Processing Methods, Production Optimization and Challenges. J. Clean. Prod. 2021, 328, 129453. [Google Scholar] [CrossRef]
- Moon, S.H.; Chang, H.C. Rice Bran Fermentation Using Lactiplantibacillus Plantarum Em as a Starter and the Potential of the Fermented Rice Bran as a Functional Food. Foods 2021, 10, 978. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Chan, L.C. Rice Bran: From Waste to Nutritious Food Ingredients. Nutrients 2023, 15, 2503. [Google Scholar] [CrossRef]
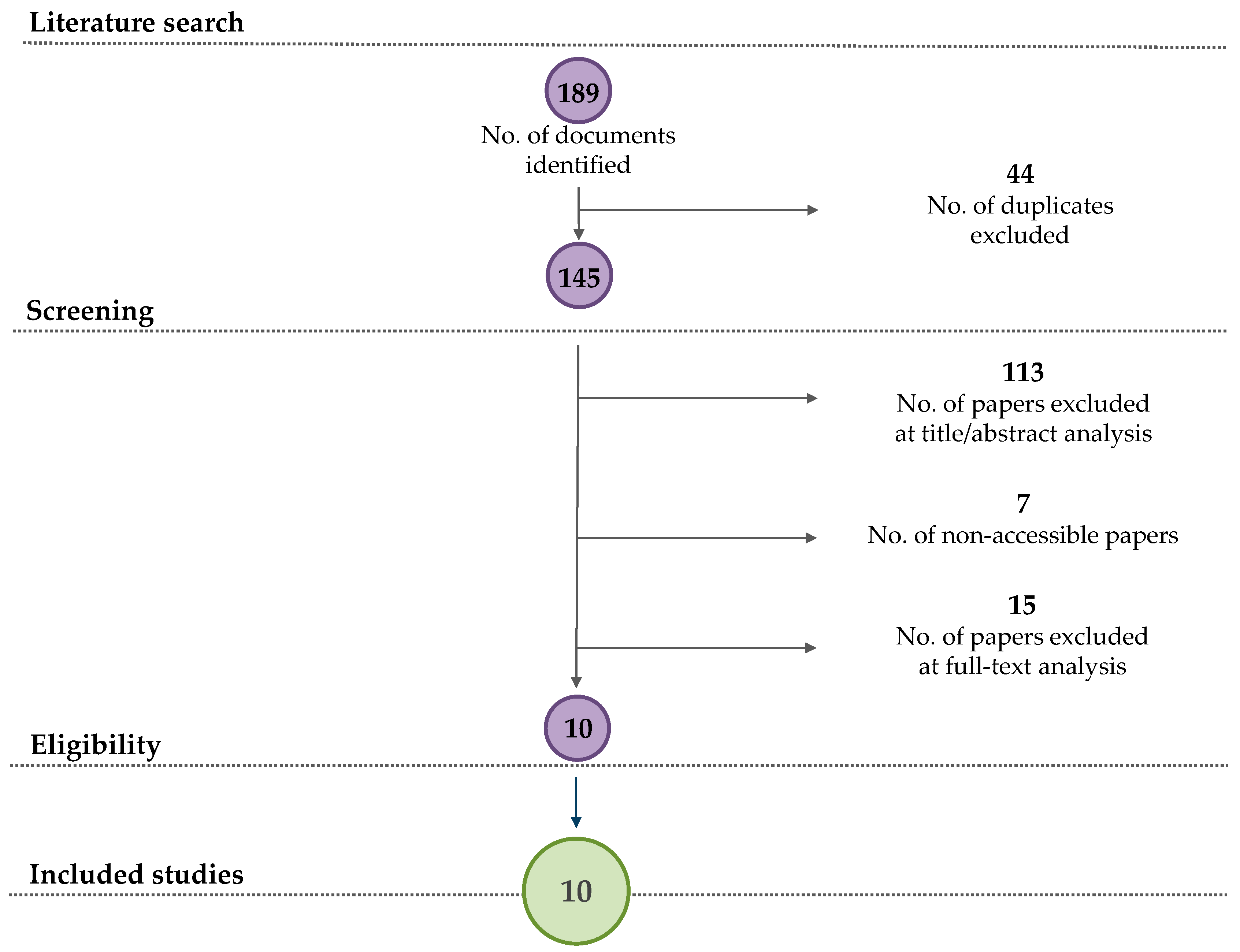
| Search (No.) | Scopus Queries | Results (No. of Documents) |
|---|---|---|
| #1 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (waste)) | 41 |
| #2 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (peeling)) | 1 |
| #3 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (pomace)) | 14 |
| #4 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (seed)) | 49 |
| #5 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (straw)) | 4 |
| #6 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (bran)) | 3 |
| #7 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (bagasse)) | 3 |
| #8 | (TITLE-ABS-KEY (aroma AND compound) AND TITLE-ABS-KEY (fermentation) AND TITLE-ABS-KEY (by-products)) | 49 |
| #9 | (TITLE-ABS-KEY (food and waste) AND TITLE-ABS-KEY (solid-state AND fermentation) AND TITLE-ABS-KEY (aroma OR aroma AND compounds)) | 5 |
| #10 | (TITLE-ABS-KEY (submerged AND fermentation) AND TITLE-ABS-KEY (aroma AND compounds)) | 11 |
| Total | 189 |
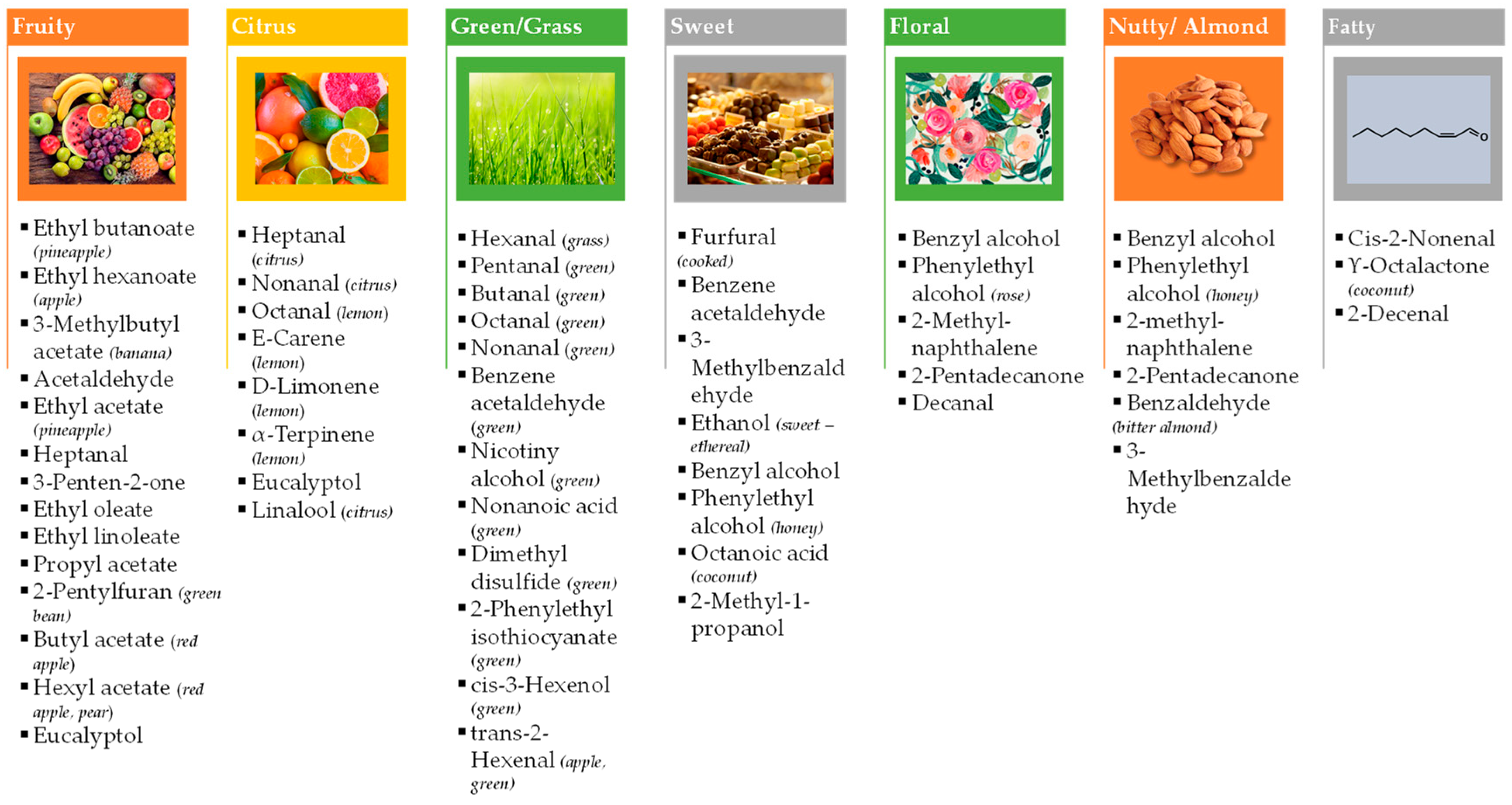
| Odor Descriptor | Main Aroma Compounds | Substrate | Strain | Fermentative Process | Reference |
|---|---|---|---|---|---|
| Main notes | |||||
| Sweet | vanillin | banana peels | Enterobacter hormaechei KT385666 | SmF | [27] |
| vanillin | pomegranate peels | E. hormaechei KT385666 | SmF | [28] | |
| vanillin, guaiacol, hexanoic acid | vanilla pods | LAB co-cultures | lactic acid fermentation | [29] | |
| vanillin | sugarcane bagasse | E. hormaechei KT385666 | SSF | [30] | |
| 3-phenylpropanol, methyl cinnamate | cajá residues | Auriporia aurulenta sp. | SmF | [31] | |
| γ-octalactone | umbu residues | A. aurulenta sp. | SmF | [31] | |
| phenylmethanol | apple pomace | Brettanomyces bruxellensis CCT 3469 | SmF | [32] | |
| ethyl dodecanoate | orange pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| ethanol | rice bran | Rhizopus oligosporus sp. | SSF | [33] | |
| Fruity | 2-methyl-1-propanol, 3-methyl-1-butanol, 2-methylpropyl acetate, isoamyl acetate, 2-methylbutanol acetate, (Z)-3-hexenyl acetate, 2-phenylethanol, 2-phenethyl acetate | umbu residues | A. aurulenta sp. | SmF | [31] |
| benzaldehyde, hexyl acetate, benzyl alcohol | cajá residues | A. aurulenta sp. | SmF | [31] | |
| myrtenyl acetate | persimmon residues | A. aurulenta sp. | SmF | [31] | |
| prenyl acetate, 1-octen-3-ol | plum residues | A. aurulenta sp. | SmF | [31] | |
| ethyl acetate | apple pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| 3-methylbutyl acetate | apple pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| ethyl nonanoate | apple pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| ethyl 2-methylbutanoate | orange pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| ethyl heptanoate | orange pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| methyl hexanoate | carrot pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| 3-methylbutyl hexanoate | carrot pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| methyl pentanoate | carrot pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| butanoic acid-ethyl ester and butanoic acid, 2-methyl-, ethyl ester | melon by-products | Lacticaseibacillus rhamnosus 1473 | - | [34] | |
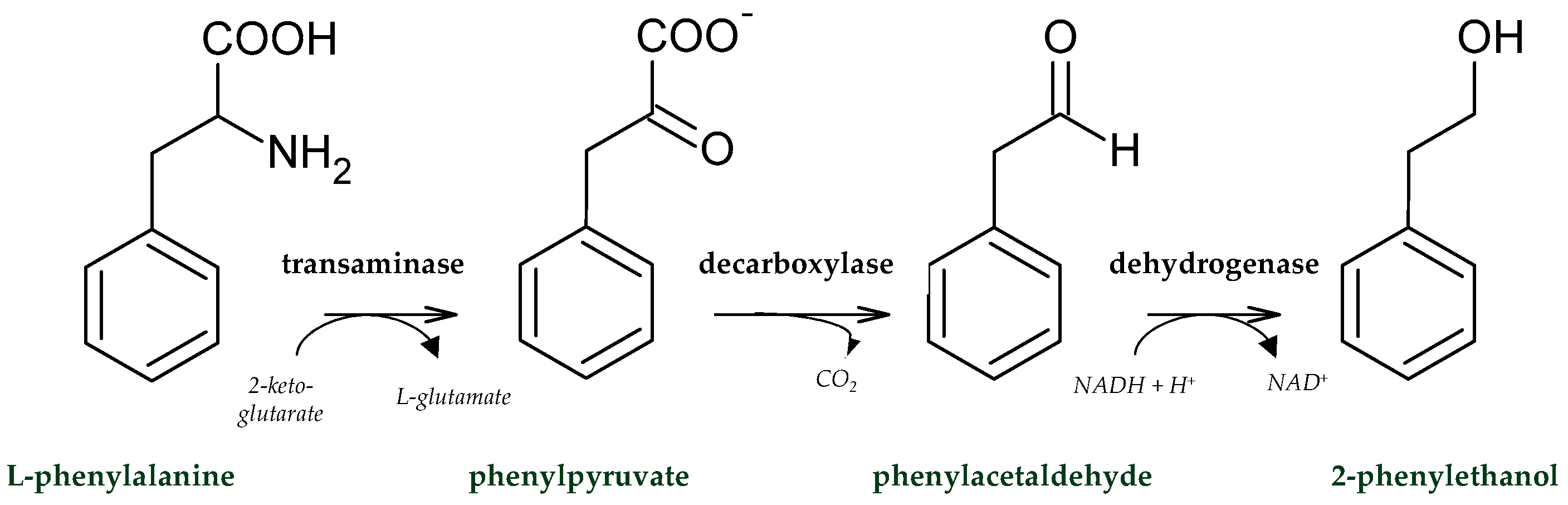
| Floral | 2-Phenylethanol | sugarcane bagasse | Pichia kudriavzevii sp. | batch-SSF | [35] |
| 2-Phenylethanol | nine agro-industrial wastes (supplemented with l-phenylalanine) | Pichia kudriavzevii CECT 13184. | SSF | [36] | |
| 2-Phenylethyl acetate | apple/orange pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| Phenylethyl alcohol | orange pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| Phenylethyl alcohol | carrot pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| octyl acetate, cinnamil acetate | cajá residues | A. aurulenta sp. | SmF | [31] | |
| (cis)-rose oxide, β-linalool, 1-nonanol, citronellyl formate, trans- and cis-geraniol, nerolidol | orange pomace | L. rhamnosus 1473 | SSF | [34] | |
| β-damascenone | melon by-products | L. rhamnosus 1473 | SSF | [34] | |
| Other notes | |||||
| Cheesy/buttery | butanoic acid | rice bran | R. oligosporus | SSF | [33] |
| Citrus | (E)-2-octenol, 1-octanol | persimmon | A. aurulenta sp. | SmF | [31] |
| limonene, citral, and valencene | orange pomace | L. rhamnosus 1473 | SSF | [34] | |
| Citrus/Lemon | D-limonene | rice bran | R. oligosporus sp. | SSF | [33] |
| Creamy | 2,3-butanediol | rice bran | R. oligosporus sp. | SSF | [33] |
| Fatty | ethyl oleat, 9,12-octadecadienoic acid (Z,Z), ethyl linoleate | rice bran | R. oligosporus sp. | SSF | [33] |
| Floral/Green | (3S)-7-hydroxy-3,7-dimethyloctanal | apple pomace | B. bruxellensis CCT 3469 | SmF | [32] |
| Grass | hexanal | rice bran | R. oligosporus sp. | SSF | [33] |
| Green | 2-hexenal | melon by-products | L. rhamnosus 1473 | SSF | [34] |
| Herbal | 1-hexanol | orange pomace | L. rhamnosus 1473 | SSF | [34] |
| Mouldy | 3-octanone, 3-octanol | cajá residues | A. aurulenta sp. | SmF | [31] |
| Spicy | γ-terpinene and α-terpineol, 4-terpineol, carveol, cis-carveol, and eugenol | orange pomace | L. rhamnosus 1473 | SSF | [34] |
| Sweet/fruity | ethyl decanoate | apple pomace | B. bruxellensis CCT 3469 | SmF | [32] |
| ethyl hexanoate | orange pomace | B. bruxellensis CCT 3469 | SmF | [32] | |
| Waxy/fatty | 1-octanol, (E)-2-decenal | melon by-products | L. rhamnosus 1473 | SSF | [34] |
| Fermentative Process | Substrate | Substrate Pre-Treatment | Strain(s) | Inoculum | Fermentation Conditions | Reference |
|---|---|---|---|---|---|---|
| SSF (plates) | Exhausted vanilla pods without seeds (10 g for each experiment) | Autoclaving (121 °C, 21 min) |
| 7 Log CFU/g | RSM conditions: Temp: 25, 30, 35 °C Time: 30, 75, 120 h Glucose: 0, 2.5, 5% | [29] |
| SSF (plates) | Exhausted vanilla pods without seeds (10 g for each experiment) | Autoclaving (121 °C, 21 min) |
| 7 Log CFU/g | RSM conditions: Temp: 25, 32 °C Time: 30 h Glucose: 0–0.45% | [29] |
| SSF (plates) | Exhausted vanilla pods with seeds (10 g for each experiment) | Autoclaving (121 °C, 21 min) |
| 7 Log CFU/g | RSM conditions: Temp: 32, 37, 42 °C Time: 30, 75, 120 h Glucose: 0, 2.5, 5% | [29] |
| SSF (plates) | Exhausted vanilla pods with seeds (10 g for each experiment) | Autoclaving (121 °C, 21 min) |
| 7 Log CFU/g | RSM conditions: Temp: 25, 30, 35 °C Time: 30, 75, 120 h Glucose: 0, 2.5, 5% | [29] |
| SSF (plates) | Exhausted vanilla pods with seeds (10 g for each experiment) | Autoclaving (121 °C, 21 min) |
| 7 Log CFU/g | RSM conditions: Temp: 25, 32 °C Time: 30 h Glucose: 0% | [29] |
| SSF (Erlenmeyer flasks) | Sugarcane bagasse, rice straw, wheat straw, rice bran, corn cob |
|
| OD: 0.6 | Incubation temperature: 30 °C Incubation time: 48 h pH: 7.0 | [30] |
| SSF (Erlenmeyer flasks) | Sugarcane bagasse |
|
| OD: 0.6 Volume: 2 mL | RSM conditions: Moisture content: 40, 50, 60, 70, 80% pH: 5, 6.5, 7.5, 9, 10 Inoculum: 1, 2, 3, 4, 5 mL Temp: 25, 30, 37.5, 45, 50 °C Incubation time: 12, 24, 32, 48, 60 min | [30] |
| SmF (shaking flasks) | banana peels |
|
| OD: 0.6 Volume: 1 mL | OFAT conditions: Incubation time: 8, 16, 24, 32, 40 h pH: 5, 6, 7, 8, 9 Temp: 20, 30, 40, 50, 60 °C Agitation speed: 110, 130, 150, 170, 190 rpm | [27] |
| SSF (shaking flasks) | pomegranate peels |
|
| OD: 0.6 Volume: 2 mL | RSM conditions: Ferulic acid concentration: 0.2–1.2% Incubation time: 8–56 h pH: 5–10 Temp: 20–50 °C Agitation speed: 100–200 rpm | [28] |
| Fermentative Process | Substrate | Substrate Pre-Treatment | Strain(s) | Inoculum | Fermentation Conditions | Reference |
|---|---|---|---|---|---|---|
| SmF (shaking flask experiments) | Powder of umbu, cajá, plum, and persimmon waste (6.25 g) + SNL minimum culture medium (125 mL) | Oven-dried (35 °C) until moisture content <5%, ground and sieved (20-mesh sieve) |
| 25 mL | Agitation speed: 150 rpm Incubation time: 7 days Temperature: 24 °C In the dark | [31] |
| SmF (shaking flask experiments) | apple, orange, and carrot pomaces (moisture content > 75%, acid pH) | wet substrate (150 g) was dissolved in 350 mL sterile, distilled water; sterilized by autoclavation (121 °C, 20 min) |
| OD600: 10 Volume: 10 mL Suspended in saline medium. | Incubation time: 72 h Temperature: 30 °C Agitation speed: 200 rpm | [32] |
| Fermentative Process | Substrate | Substrate Pre-Treatment | Strain(s) | Inoculum | Fermentation Conditions | Reference |
|---|---|---|---|---|---|---|
| SSF (glass reactors, 0.5 L volume) | Sugarcane bagasse supplemented with sugar beet molasses, and L-phe | Oven-dried (60 °C), ground, sieved to a particle size of 0.5–4.75 mm, sterilized |
| 5·107 CFU g−1 | pH: 4 ML: 7.5% (w/w dry basis) L-phe content: 3.8% (w/w dry basis) SAFR: 0.13 L h−1 g−1 MC0: 76% Temperature: 31 °C Time: 40 h | [35] |
| SSF (glass reactors, 0.5 L volume) | Rice husk, brewer’s spent grain, soy fibre, rice fibre, green and red apple pomace, asparagus tails, orange peels, banana peels | Oven-dried (60 °C), sterilized |
| 5·107 CFU g−1 dry substrate | Substrate: 95 ± 1 g Temperature- controlled water bath: 30 °C Time: 96 h SAFR: 0.13 L h−1 g−1 L-phe (4% dry basis) addition or de novo synthesis without L-phe. With molasses (10%) or without molasses | [36] |
| Fermentative Process | Substrate | Substrate Pre-Treatment | Strain(s) | Inoculum | Fermentation Conditions | Reference |
|---|---|---|---|---|---|---|
| SSF (Petri dishes) | Rice bran (9 g) + distilled water (20% v/w) | Milling, grinding, sieving, autoclaving (121 °C, 15 min) |
| 106 spore/mL Volume: 15% (v/w) | Incubation time: 72 h Incubation temperature: 30 °C | [33] |
| SSF (flasks) | Melon and orange by products | Autoclaving (121 °C for 20 min) |
| 7 Log CFU/g | Incubation time: 72 h Incubation temperature: 37 °C | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melini, F.; Melini, V. Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste. Fermentation 2024, 10, 132. https://doi.org/10.3390/fermentation10030132
Melini F, Melini V. Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste. Fermentation. 2024; 10(3):132. https://doi.org/10.3390/fermentation10030132
Chicago/Turabian StyleMelini, Francesca, and Valentina Melini. 2024. "Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste" Fermentation 10, no. 3: 132. https://doi.org/10.3390/fermentation10030132
APA StyleMelini, F., & Melini, V. (2024). Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste. Fermentation, 10(3), 132. https://doi.org/10.3390/fermentation10030132







