Research Progress on Bioaugmentation Technology for Improving Traditional Chinese Fermented Seasonings
Abstract
1. Introduction
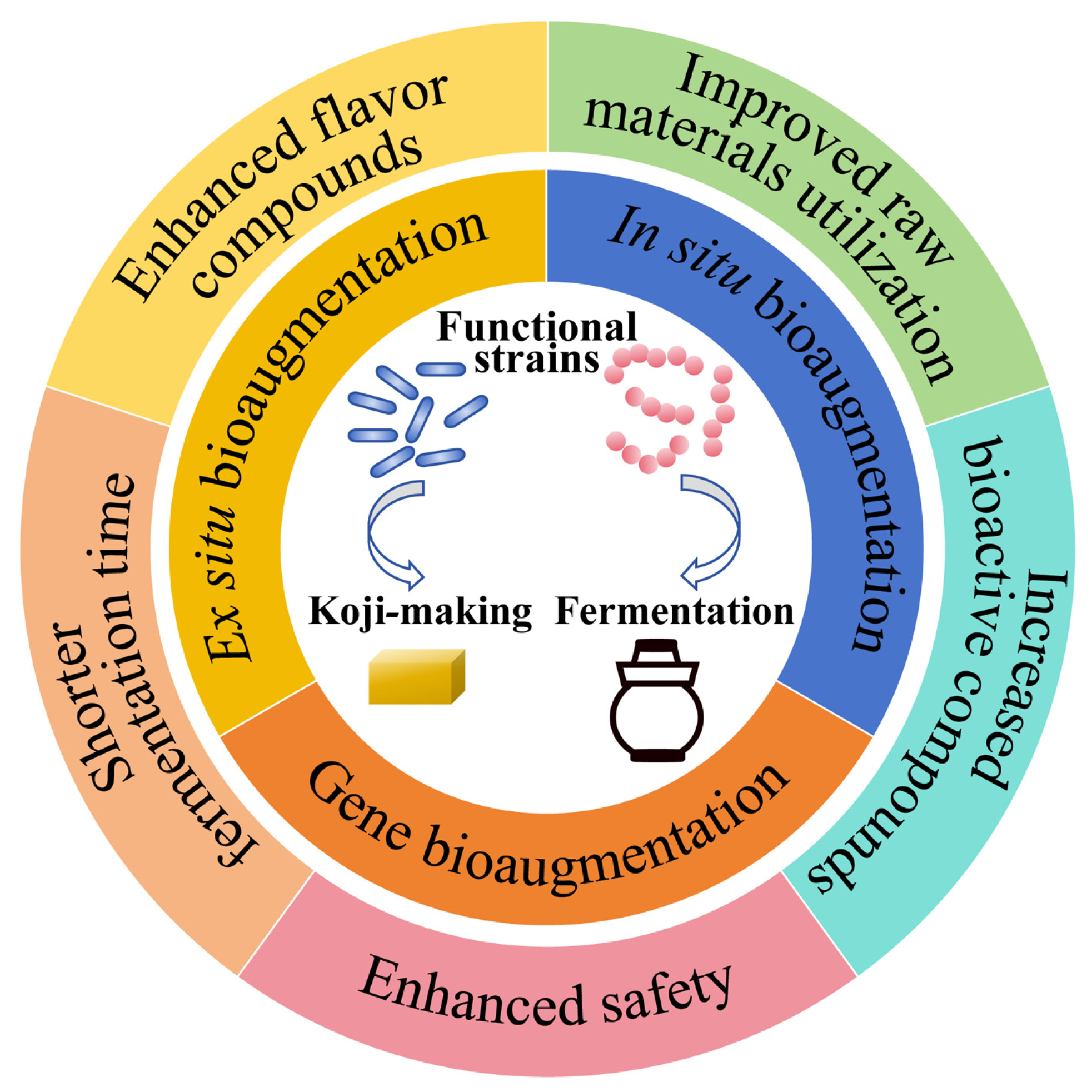
2. Bioaugmentation Technology
3. Traditional Fermented Seasonings and Their Associated Microorganisms
3.1. Vinegar
3.2. Soy Sauce
3.3. Sufu
3.4. Doubanjiang
3.5. Dajiang
3.6. Douchi
4. Effect of Bioaugmentation on Traditional Fermented Seasonings
4.1. Enhancement in the Key Flavor Substances
4.2. Improvement in Raw Material Utilization
4.3. Shorter Maturity Time
4.4. Producing Bioactive Compounds
4.5. Improving Safety
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Chen, W. Demystification of fermented foods by omics technologies. Curr. Opin. Food Sci. 2022, 46, 100845. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. The psychology of condiments: A review. Int. J. Gastron. Food Sci. 2018, 11, 41–48. [Google Scholar] [CrossRef]
- García Casal, M.N.; Peña Rosas, J.P.; Malavé, H.G. Sauces, spices, and condiments: Definitions, potential benefits, consumption patterns, and global markets. Ann. N. Y. Acad. Sci. 2016, 1379, 3–16. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Parkouda, C.; Adewumi, G.A.; Ouoba, L.I.I.; Jespersen, L. Technologically relevant Bacillus species and microbial safety of West African traditional alkaline fermented seed condiments. Crit. Rev. Food. Sci. Nutr. 2022, 62, 871–888. [Google Scholar] [CrossRef]
- El Fantroussi, S.; Agathos, S.N. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol. 2005, 8, 268–275. [Google Scholar] [CrossRef]
- Pu, S.; Zhang, Y.; Lu, N.; Shi, C.; Yan, S. Yeasts from Chinese strong flavour Daqu samples: Isolation and evaluation of their potential for fortified Daqu production. AMB Express 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xie, G.; Wu, D.; Li, X.; Lu, J. A Lactobacillus brevis strain with citrulline re-uptake activity for citrulline and ethyl carbamate control during Chinese rice wine fermentation. Food Biosci. 2020, 36, 100612. [Google Scholar] [CrossRef]
- Xu, B.; Xu, S.; Cai, J.; Sun, W.; Mu, D.; Wu, X.; Li, X. Analysis of the microbial community and the metabolic profile in medium-temperature Daqu after inoculation with Bacillus licheniformis and Bacillus velezensis. LWT 2022, 160, 113214. [Google Scholar] [CrossRef]
- Tang, J.; Chen, T.; Hu, Q.; Lei, D.; Sun, Q.; Zhang, S.; Zeng, C.; Zhang, Q. Improved protease activity of Pixian broad bean paste with cocultivation of Aspergillus oryzae QM-6 and Aspergillus niger QH-3. Electron. J. Biotechnol. 2020, 44, 33–40. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Xu, Y. Effects of Tetragenococcus halophilus and Candida versatilis on the production of aroma-active and umami-taste compounds during soy sauce fermentation. J. Sci. Food Agric. 2020, 100, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xu, Y.; Lan, X. Isolation, characterization, and application of biogenic amines-degrading strains from fermented food. J. Food Saf. 2020, 40, e12716. [Google Scholar] [CrossRef]
- Singer, A.C.; van der Gast, C.J.; Thompson, I.P. Perspectives and vision for strain selection in bioaugmentation. Trends Biotechnol. 2005, 23, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Mawang, C.; Azman, A.; Fuad, A.M.; Ahamad, M. Actinobacteria: An eco-friendly and promising technology for the bioaugmentation of contaminants. Biotechnol. Rep. 2021, 32, e679. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Zhang, L.; Xie, D.; Onwosi, C.O.; Muhammad, W.I.; Odoh, C.K.; Sam, K.; Idenyi, J.N. Bioaugmentation as a green technology for hydrocarbon pollution remediation. Problems and prospects. J. Environ. Manag. 2022, 304, 114313. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. Bioaugmentation as a strategy for tailor-made volatile fatty acid production. J. Environ. Manag. 2021, 295, 113093. [Google Scholar] [CrossRef]
- Kandylis, P.; Bekatorou, A.; Dimitrellou, D.; Plioni, I.; Giannopoulou, K. Health promoting properties of cereal vinegars. Foods 2021, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Pan, W.; Li, S.; Li, J.; Li, Q.; He, L.; Chen, S.; Hu, K.; Hu, X.; Han, G.; et al. Seasonal dynamics of microbiota and physicochemical indices in the industrial-scale fermentation of Sichuan Baoning vinegar. Food Chem. X 2022, 16, 100452. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zheng, Y.; Xie, S.; Zhang, X.; Song, J.; Xia, M.; Wang, M. Unraveling the correlation between microbiota succession and metabolite changes in traditional Shanxi aged vinegar. Sci. Rep. 2017, 7, 9240. [Google Scholar] [CrossRef]
- Chai, L.; Shen, M.; Sun, J.; Deng, Y.; Lu, Z.; Zhang, X.; Shi, J.; Xu, Z. Deciphering the d-/l-lactate-producing microbiota and manipulating their accumulation during solid-state fermentation of cereal vinegar. Food Microbiol. 2020, 92, 103559. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Zhou, R.; Wu, C. Evaluating the feasibility of fermentation starter inoculated with Bacillus amyloliquefaciens for improving acetoin and tetramethylpyrazine in Baoning bran vinegar. Int. J. Food Microbiol. 2017, 255, 42–50. [Google Scholar] [CrossRef]
- Ai, M.; Qiu, X.; Huang, J.; Wu, C.; Jin, Y.; Zhou, R. Characterizing the microbial diversity and major metabolites of Sichuan bran vinegar augmented by Monascus purpureus. Int. J. Food Microbiol. 2019, 292, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huo, N.; Wang, Y.; Zhang, Y.; Wang, R.; Hou, H. Aroma-enhancing role of Pichia manshurica isolated from Daqu in the brewing of Shanxi Aged Vinegar. Int. J. Food Prop. 2017, 20, 2169–2179. [Google Scholar] [CrossRef]
- Li, S.; Li, P.; Liu, X.; Luo, L.; Lin, W. Bacterial dynamics and metabolite changes in solid-state acetic acid fermentation of Shanxi aged vinegar. Appl. Microbiol. Biotechnol. 2016, 100, 4395–4411. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zheng, Y.; Wang, M.; Han, Y.; Wang, Y.; Luo, J.; Niu, D. Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresour. Technol. 2013, 148, 325–333. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Z.; Zhang, X.; Li, Q.; Lu, Z.; Shi, J.; Xu, Z.; Ma, Y. Monitoring the microbial community during solid-state acetic acid fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2011, 28, 1175–1181. [Google Scholar] [CrossRef]
- Nie, Z.; Zheng, Y.; Du, H.; Xie, S.; Wang, M. Dynamics and diversity of microbial community succession in traditional fermentation of Shanxi aged vinegar. Food Microbiol. 2015, 47, 62–68. [Google Scholar] [CrossRef]
- Li, W.; Tong, S.; Yang, Z.; Xiao, Y.; Lv, X.; Weng, Q.; Yu, K.; Liu, G.; Luo, X.; Wei, T.; et al. The dynamics of microbial community and flavor metabolites during the acetic acid fermentation of Hongqu aromatic vinegar. Curr. Res. Food Sci. 2022, 5, 1720–1731. [Google Scholar] [CrossRef]
- Yun, J.; Zhao, F.; Zhang, W.; Yan, H.; Zhao, F.; Ai, D. Monitoring the microbial community succession and diversity of Liangzhou fumigated vinegar during solid-state fermentation with next-generation sequencing. Ann. Microbiol. 2019, 69, 279–289. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, Z.; Luo, H.; Mao, X.; Yang, Y.; Tong, W.; Huang, D. Study of the phase characteristics of Sichuan bran vinegar fermentation based on flavor compounds and core bacteria. J. Am. Soc. Brew. Chem. 2021, 79, 201–211. [Google Scholar] [CrossRef]
- Fu, J.; Feng, J.; Zhang, G.; Liu, J.; Li, N.; Xu, H.; Zhang, Y.; Cao, R.; Li, L. Role of bacterial community succession in flavor formation during Sichuan sun vinegar grain (Cupei) fermentation. J. Biosci. Bioeng. 2023, 135, 109–117. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, R.; Cui, R.; Huang, J.; Wu, C. Characterizing soy sauce moromi manufactured by high-salt dilute-state and low-salt solid-state fermentation using multiphase analyzing methods. J. Food Sci. 2016, 81, C2639–C2646. [Google Scholar] [CrossRef]
- Sulaiman, J.; Gan, H.M.; Yin, W.; Chan, K. Microbial succession and the functional potential during the fermentation of Chinese soy sauce brine. Front. Microbiol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Li, K.; Tang, J.; Zhang, Z.; Wu, Z.; Zhong, A.; Li, Z.; Wang, Y. Correlation between flavor compounds and microorganisms of Chaling natural fermented red sufu. LWT 2022, 154, 112873. [Google Scholar] [CrossRef]
- Wei, G.; Regenstein, J.M.; Zhou, P. The aroma profile and microbiota structure in oil furu, a Chinese fermented soybean curd. Food Res. Int. 2021, 147, 110473. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Xu, L.; Wu, M.; Wang, X.; Wang, K.; Li, Z.; Zhang, D. Microbial community succession and metabolite changes during fermentation of BS Sufu, the fermented black soybean curd by Rhizopus microsporus, Rhizopus oryzae, and Actinomucor elegans. Front. Microbiol. 2021, 12, 665826. [Google Scholar] [CrossRef]
- He, W.; Chung, H.Y. Exploring core functional microbiota related with flavor compounds involved in the fermentation of a natural fermented plain sufu (Chinese fermented soybean curd). Food Microbiol. 2020, 90, 103408. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bi, X.; Zhou, B.; Fang, J.; Liu, P.; Ding, W.; Che, Z.; Wang, Q.; He, Q. Microbial communities succession and flavor substances changes during Pixian broad-bean paste fermentation. Food Biosci. 2021, 42, 101053. [Google Scholar] [CrossRef]
- Jia, Y.; Niu, C.; Zheng, F.; Liu, C.; Wang, J.; Lu, Z.; Xu, Z.; Li, Q. Development of a defined autochthonous starter through dissecting the seasonal microbiome of broad bean paste. Food Chem. 2021, 357, 129625. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Q.; Sun, W.; Wang, X.; Lin, J.; Che, Z.; Ma, P. Correlation between microbial communities and key flavors during post-fermentation of Pixian broad bean paste. Food Res. Int. 2020, 137, 109513. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, C.; Shan, W.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Physicochemical, flavor and microbial dynamic changes during low-salt doubanjiang (broad bean paste) fermentation. Food Chem. 2021, 351, 128454. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, T.; Jia, H.; Guo, C.; Wang, Z.; Yue, T.; Yuan, Y. Comparative evaluation of the effects of natural and artificial inoculation on soybean paste fermentation. LWT 2022, 155, 112936. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, B. Analysis of bacterial diversity and biogenic amines content during fermentation of farmhouse sauce from Northeast China. Food Control 2020, 108, 106861. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, F.; Zhang, Z.; Hou, Q.; Guo, Z. High-throughput sequencing-based analysis of fungal diversity and taste quality evaluation of Douchi, a traditional fermented food. Food Sci. Nutr. 2020, 8, 6612–6620. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Zhao, W.; Xiong, K.; Wen, H.; Yang, H.; Wang, X. Dynamic analysis of physicochemical characteristics and microbial communities of Aspergillus-type douchi during fermentation. Food Res. Int. 2022, 153, 110932. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, P.; He, W.; Liao, L.; Xia, B.; Jiang, L.; Liu, Y. Analysis of microbial community and the characterization of Aspergillus flavus in Liuyang Douchi during fermentation. LWT 2022, 154, 112567. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Ji, Y.; He, H.; Yang, H.; Tang, X.; Liu, Y. Characterization and correlation of dominant bacteria and volatile compounds in post-fermentation process of Ba-bao Douchi. Food Res. Int. 2022, 160, 111688. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.K. The role of microorganisms in soy sauce production. Adv. Appl. Microbiol. 2019, 108, 45–113. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.X.; Ferng, S.; Ting, C.; Huang, W.; Chiou, R.Y.; Hsu, C. Optimizing the initial moromi fermentation conditions to improve the quality of soy sauce. LWT 2016, 74, 242–250. [Google Scholar] [CrossRef]
- Sassi, S.; Wan Mohtar, W.A.A.Q.; Jamaludin, N.S.; Ilham, Z. Recent progress and advances in soy sauce production technologies: A review. J. Food Process Preserv. 2021, 45, e15799. [Google Scholar] [CrossRef]
- Yan, Y.; Qian, Y.; Ji, F.; Chen, J.; Han, B. Microbial composition during Chinese soy sauce koji-making based on culture dependent and independent methods. Food Microbiol. 2013, 34, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.H.; Huang, M.B.; Liu, F.Y.; Huang, W.; Tran, H.; Hsu, T.; Huang, C.; Chiang, T. Deciphering microbial community dynamics along the fermentation course of soy sauce under different temperatures using metagenomic analysis. Biosci. Microbiota Food Health 2023, 42, 104–113. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Liu, T.; Su, C.; Ji, X.; Wang, L.; Zhao, Y.; Wang, Z. Evaluation of bacterial and fungal communities during the fermentation of Baixi sufu, a traditional spicy fermented bean curd. J. Sci. Food Agric. 2020, 100, 1448–1457. [Google Scholar] [CrossRef]
- Xie, C.; Zeng, H.; Wang, C.; Xu, Z.; Qin, L. Volatile flavour components, microbiota and their correlations in different sufu, a Chinese fermented soybean food. J. Appl. Microbiol. 2018, 125, 1761–1773. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Ence Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Bao, W.; Huang, X.; Liu, J.; Han, B.; Chen, J. Influence of Lactobacillus brevis on metabolite changes in bacteria-fermented sufu. J. Food Sci. 2020, 85, 165–172. [Google Scholar] [CrossRef]
- Cai, H.; Dumba, T.; Sheng, Y.; Li, J.; Lu, Q.; Liu, C.; Cai, C.; Feng, F.; Zhao, M. Microbial diversity and chemical property analyses of sufu products with different producing regions and dressing flavors. LWT 2021, 144, 111245. [Google Scholar] [CrossRef]
- Huang, X.; Yu, S.; Han, B.; Chen, J. Bacterial community succession and metabolite changes during sufu fermentation. LWT 2018, 97, 537–545. [Google Scholar] [CrossRef]
- Zhao, S.; Niu, C.; Suo, J.; Zan, Y.; Wei, Y.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Unraveling the mystery of ‘bask in daytime and dewed at night’ technique in doubanjiang (broad bean paste) fermentation. LWT 2021, 149, 111723. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, W.; Xu, Y. Characterization of key aroma compounds in pixian broad bean paste through the molecular sensory science technique. LWT 2021, 148, 111743. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Jeon, J.; Kwon, S.Y.; Zhao, C.; Baek, H. Characterization and evaluation of aroma quality in Doubanjiang, a Chinese traditional fermented red pepper paste, using aroma extract dilution analysis and a sensory profile. Molecules 2019, 24, 3107. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Zheng, C.; Liu, J.; Vu, V.H.; Wang, X.; Sun, Q. Characteristics of microbial community and aroma compounds in traditional fermentation of Pixian broad bean paste as compared to industrial fermentation. Int. J. Food Prop. 2018, 20, S2520–S2531. [Google Scholar] [CrossRef]
- Ling, H.; Shi, H.; Chen, X.; Cheng, K. Detection of the microbial diversity and flavour components of northeastern Chinese soybean paste during storage. Food Chem. 2022, 374, 131686. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Kim, K.H.; Sang, E.J.; Che, O.J. The effect of salt concentrations on the fermentation of doenjang, a traditional Korean fermented soybean paste. Food Microbiol. 2020, 86, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Heo, S.; Lee, B.; Lee, H.; Jeong, K.; Her, J.Y.; Lee, K.G.; Lee, J.H. Effects of the predominant bacteria from meju and doenjang on the production of volatile compounds during soybean fermentation. Int. J. Food Microbiol. 2017, 262, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Wang, J.; Wang, S.; He, Q.; Wei, R.; Sun, Z.; Duan, S.; Li, Y. Correlation between microbial community succession and flavor substances during fermentation of Yongchuan Douchi. Food Biosci. 2023, 56, 103192. [Google Scholar] [CrossRef]
- Yang, L.; Yang, H.; Tu, Z.; Wang, X. High-throughput sequencing of microbial community diversity and dynamics during Douchi fermentation. PLoS ONE 2016, 11, e168166. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Wang, R.; Liu, X.; Peng, X.; Guo, S. Succession and diversity of microbial flora during the fermentation of Douchi and their effects on the formation of characteristic aroma. Foods 2023, 12, 329. [Google Scholar] [CrossRef]
- Li, J.; Peng, B.; Huang, L.; Zhong, B.; Yu, C.; Hu, X.; Wang, W.; Tu, Z. Association between flavors and microbial communities of traditional Aspergillus-Douchi produced by a typical industrial-scale factory. LWT 2023, 176, 114532. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, X.; Zhang, C.; Zheng, Y.; Zheng, B.; Duan, X.; Tian, Y. Microbial dynamics and flavor formation during the traditional brewing of Monascus vinegar. Food Res. Int. 2019, 125, 108531. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Xu, B.; Abughoush, M.; Li, L.; Sun, B. Processing technologies and flavor analysis of Chinese cereal vinegar: A Comprehensive Review. Food Anal. Meth. 2023, 16, 1–28. [Google Scholar] [CrossRef]
- Que, Z.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Flavor compounds of traditional fermented bean condiments: Classes, synthesis, and factors involved in flavor formation. Trends Food Sci. Technol. 2023, 133, 160–175. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Xie, N.; Huang, M.; Feng, Y. Community structure of yeast in fermented soy sauce and screening of functional yeast with potential to enhance the soy sauce flavor. Int. J. Food Microbiol. 2022, 370, 109652. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Qiu, T.; Lu, Z.; Deng, Y.; Zhang, X.; Shi, J.; Xu, Z. Modulating microbiota metabolism via bioaugmentation with Lactobacillus casei and Acetobacter pasteurianus to enhance acetoin accumulation during cereal vinegar fermentation. Food Res. Int. 2020, 138, 109737. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Cao, Z.; Wang, C. Effect of Tetragenococcus halophilus, Zygosaccharomyces rouxii, and Torulopsis versatilis addition sequence on soy sauce fermentation. Innov. Food Sci. Emerg. Technol. 2021, 69, 102662. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Linforth, R.; Onyeaka, H.; Gkatzionis, K. Effects of co-inoculation and sequential inoculation of Tetragenococcus halophilus and Zygosaccharomyces rouxii on soy sauce fermentation. Food Chem. 2018, 240, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, D.; Zhang, W.; Duan, M.; Ruan, Z.; Huang, S.; Zhou, S.; Fang, Q. Effect of aroma-producing yeasts in high-salt liquid-state fermentation soy sauce and the biosynthesis pathways of the dominant esters. Food Chem. 2021, 344, 128681. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Rao, J.W.; Meng, F.B.; Wang, Z.W.; Liu, D.Y.; Yu, H. Combination of mutagenesis and adaptive evolution to engineer salt-tolerant and aroma-producing yeast for soy sauce fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Zhou, R.; Qi, Q.; Yang, M.; Peng, C.; Wu, C.; Jin, Y. The effects of different coculture patterns with salt-tolerant yeast strains on the microbial community and metabolites of soy sauce moromi. Food Res. Int. 2021, 150, 110747. [Google Scholar] [CrossRef]
- Tian, M.; Lin, K.; Yang, L.; Jiang, B.; Zhang, B.; Zhu, X.; Ren, D.; Yu, H. Characterization of key aroma compounds in gray sufu fermented using Leuconostoc mesenteroides subsp. Mesenteroides F24 as a starter culture. Food Chem. X 2023, 20, 100881. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Ding, S.; Yang, L.; Pan, Y.; Suo, L.; Zhu, X.; Ren, D.; Yu, H. Weissella confusa M1 as an adjunct culture assists microbial succession and flavor formation in gray sufu. LWT 2023, 185, 115155. [Google Scholar] [CrossRef]
- Niu, C.; Yang, L.; Zheng, F.; Liu, C.; Wang, J.; Xu, X.; Li, Q. Systematic analysis of the aroma profiles produced by Zygosaccharomyces rouxii Y-8 in different environmental conditions and its contribution to doubanjiang (broad bean paste) fermentation with different salinity. LWT 2022, 158, 113118. [Google Scholar] [CrossRef]
- He, B.; Li, H.; Hu, Z.; Zhang, Y.; Sun, M.; Qiu, S.; Zeng, B. Difference in microbial community and taste compounds between Mucor-type and Aspergillus-type Douchi during koji-making. Food Res. Int. 2019, 121, 136–143. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, C.; Wang, X.; Li, X.; Zheng, Y.; Song, J.; Xia, M.; Zhang, R.; Wang, M. Bioaugmentation by Pediococcus acidilactici AAF1-5 Improves the bacterial activity and diversity of cereal vinegar under solid-state fermentation. Front. Microbiol. 2021, 11, 603721. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Peng, Y.; Ao, X.; Pan, W.; Chen, S.; He, L.; Yang, Y.; Chen, F.; Du, D.; Liu, S. Effects of Aspergillus niger biofortification on the microbial community and quality of Baoning vinegar. LWT 2020, 131, 109728. [Google Scholar] [CrossRef]
- Peng, M.; Zhang, X.; Huang, T.; Zhong, X.; Chai, L.; Lu, Z.; Shi, J.; Xu, Z. Komagataeibacter europaeus improves community stability and function in solid-state cereal vinegar fermentation ecosystem: Non-abundant species plays important role. Food Res. Int. 2021, 150, 110815. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.; Hu, Y.; Wang, C.; Gao, B.; Xu, N. Effect of a halophilic aromatic yeast together with Aspergillus oryzae in koji making on the volatile compounds and quality of soy sauce moromi. Int. J. Food Sci. Technol. 2015, 50, 1352–1358. [Google Scholar] [CrossRef]
- Gupte, A.; Madamwar, D. Solid state fermentation of lignocellulosic waste for cellulase and β-glucosidase production by cocultivation of Aspergillus ellipticus and Aspergillus fumigatus. Biotechnol. Prog. 1997, 13, 166–169. [Google Scholar] [CrossRef]
- Verma, P.; Madamwar, D. Production of ligninolytic enzymes for dye decolorization by cocultivation of white-rot fungi Pleurotus ostreatus and phanerochaete chrysosporium under solid-state fermentation. Appl. Biochem. Biotechnol. 2002, 102, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Niu, C.T.; Lu, Z.M.; Zhang, X.J.; Chai, L.J.; Shi, J.S.; Xu, Z.H.; Li, Q. A bottom-up approach to develop a synthetic microbial community model: Application for efficient reduced-salt broad bean paste fermentation. Appl. Environ. Microbiol. 2020, 86, e00306-20. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, M.; Zhai, S.; Chen, H.; Li, A.; Lv, X.; Deng, H. Application of autochthonous mixed starter for controlled Kedong Sufu fermentation in pilot plant tests. J. Food Sci. 2015, 80, M129–M136. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, H.; Lv, X.T.; Deng, H.L.; Chen, X.; Li, J.J.; Guo, L. Accelerated ripening of Kedong sufu with autochthonous starter cultures Kocuria rosea KDF3 and its protease KP3 as adjuncts. J. Appl. Microbiol. 2014, 116, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Chen, K. Mechanisms and clinical application of tetramethylpyrazine (an interesting natural compound isolated from Ligusticum Wallichii): Current status and perspective. Oxidative Med. Cell. Longev. 2016, 2016, 2124638. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Liu, J.; Huang, Y.; Zhou, M.; Hu, Y.; Fu, C.; Dai, J.; Wang, C.; Li, D.; Gao, B.; et al. Effects of a mixed koji culture of Aspergillus oryzae HG-26 and Aspergillus niger HG-35 on the levels of enzymes, antioxidants and phenolic compounds in soy sauce during the fermentation process. Int. J. Food Sci. Technol. 2017, 52, 1585–1593. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, C.; Song, D.; Li, P.; Zhu, X. Enhancing vitamin B12 content in soy-yogurt by Lactobacillus reuteri. Int. J. Food Microbiol. 2015, 206, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Xiang, S.; Chen, J.; Shi, Y.; Chen, Y.; Wang, H.; Zhu, X. Effect of Lactobacillus reuteri on vitamin B12 content and microbiota composition of furu fermentation. LWT 2019, 100, 138–143. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villegas, J.M.; Savoy De Giori, G.; Saavedra, L.; Hebert, E.M. Enhancement of γ-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Sharma, N.; Bhardwaj, A.; Phimolsiripol, Y. Lactic acid bacteria: A bio-green preservative against mycotoxins for food safety and shelf-life extension. Qual. Assur. Saf. Crop. Foods 2022, 14, 13–31. [Google Scholar] [CrossRef]
- Feng, L.; Gu, J.; Guo, L.; Mu, G.; Tuo, Y. Safety evaluation and application of lactic acid bacteria and yeast strains isolated from Sichuan broad bean paste. Food Sci. Nutr. 2023, 11, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, L.; Gu, J.; Mu, G.; Tuo, Y. Screening lactic acid bacteria and yeast strains for soybean paste fermentation in northeast of China. Food Sci. Nutr. 2023, 11, 4502–4515. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.; Ho, C. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Feng, Z.; Huang, S.; Ai, Z.W.; Zhang, M.; Zhai, S.; Chen, X. Evaluation of autochthonous micrococcus strains as starter cultures for the production of Kedong sufu. J. Appl. Microbiol. 2016, 120, 671–683. [Google Scholar] [CrossRef]
- Guo, J.; Luo, W.; Fan, J.; Suyama, T.; Zhang, W. Co-inoculation of Staphylococcus piscifermentans and salt-tolerant yeasts inhibited biogenic amines formation during soy sauce fermentation. Food Res. Int. 2020, 137, 109436. [Google Scholar] [CrossRef]
- Qi, Q.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Characterising the mechanism of abating biogenic amines accumulation by cocultures of Zygosaccharomyces rouxii and Tetragenococcus halophilus. LWT 2022, 164, 113672. [Google Scholar] [CrossRef]
- Qi, Q.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Abating biogenic amines and improving the flavor profile of Cantonese soy sauce via co-culturing Tetragenococcus halophilus and Zygosaccharomyces rouxii. Food Microbiol. 2022, 106, 104056. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, D.; Liao, H.; Xia, X. Patterns of biogenic amine during broad bean paste fermentation: Microbial diversity and functionality via Bacillus bioaugmentation. J. Sci. Food Agric. 2023, 103, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, X.; Feng, L.; Mu, G.; Tuo, Y. The microbial community, biogenic amines content of soybean paste, and the degradation of biogenic amines by Lactobacillus plantarum HM24. Food Sci. Nutr. 2021, 9, 6458–6470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Niu, C.; Du, S.; Liu, C.; Zheng, F.; Wang, J.; Li, Q. Reduction of biogenic amines formation during soybean paste fermentation by using Staphylococcus carnosus M43 and Pediococcus acidilactici M28 as starter culture. LWT 2020, 133, 109917. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Fu, M.; Yu, Y.; Wu, J.; Xu, Y.; Li, L. Effects of selected Bacillus strains on the biogenic amines, bioactive ingredients and antioxidant capacity of shuidouchi. Int. J. Food Microbiol. 2023, 388, 110084. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, R.; Chen, G.; Wang, S.; Li, C.; Xu, Y.; Kan, J. Effect of different starter cultures on the control of biogenic amines and quality change of douchi by rapid fermentation. LWT 2019, 109, 395–405. [Google Scholar] [CrossRef]
- Mccarty, N.S.; Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019, 37, 181–197. [Google Scholar] [CrossRef]
- Liu, J.; Solem, C.; Lu, T.; Jensen, P.R. Harnessing lactic acid bacteria in synthetic microbial consortia. Trends Biotechnol. 2022, 40, 8–11. [Google Scholar] [CrossRef]
| Seasoning | Fungi | Bacterium | Reference |
|---|---|---|---|
| Vinegar | Aspergillus, Saccharomycopsis, Pichia, Alternaria, Candida, Issatchenkia, Monascus | Lactobacilli, Acetobacter, Gluconacetobacter, Komagataeibacter, Weissella, Bacillus, Staphylococcus, Enterobacter, Pseudomonas, Clostridium | [21,27,28,29,30,31,32,33] |
| Soy sauce | Candida, Pichia, Zygosaccharomyces | Weissella, Tetragenococcus, Staphylococcus, Bacillus, Lactobacilli | [34,35] |
| Sufu | Simplicillium, Verticillium, Actinomucor, Candida, Debaryomyces, Trichosporon, Rhizopus, Monascus, Debaryomyces, Rhodotorula | Weissella, Lactococcus, Enterococcus, Kurthia, Tetragenococcus, Lactobacillales, Enterococcus, Enterobacter, Leuconostoc, Pseudomonas | [36,37,38,39] |
| Doubanjiang | Aspergillus, Trichosporon, Zygosaccharomyces, Fusicolla, Candida, Pichia, Millerozyma | Staphylococcus, Weissella, Bacillus, Lactobacilli, Lysinibacillus, Enterococcus, Escherichia-Shigella, Sphingomonas, Leuconostoc | [40,41,42,43] |
| Dajiang | Penicillium, Aspergillus | Tetragenococcus, Weissella, Lactobacilli, Leuconostoc, Tetragenococcus, Pediococcus | [44,45] |
| Douchi | Debaryomyces, Fusarium, Pichia, Aspergillus, Saccharomyces, Petromyces, Rhizopus, Penicillium | Staphylococcus, Pediococcus, Bacillus, Weissella, Lactobacilli | [46,47,48,49] |
| Seasoning | Microorganism | Bioaugmentation Strategy | Efficacy on Flavor | Reference |
|---|---|---|---|---|
| Shanxi aged vinegar | Pichia manshurica Y14 | P. manshurica Y14 was inoculated (7%, v/v) in the Daqu-based fermentation. | The contents of ester compounds increased from 15.3 to 21.5 g/L. | [25] |
| Sichuan bran vinegar | Bacillus amyloliquefaciens | A new Daqu prepared by combining B. amyloliquefaciens-bioaugmented Daqu and traditional Daqu without Chinese herbs at a 1:1 (v/v) ratio was used as a starter. | The contents of ethyl acetate and tetramethyl pyrazine increased by 191.84% and 123.17%, respectively. | [23] |
| Zhenjiang aromatic vinegar | Lacticaseibacillus casei (formerly Lactobacillus casei) M1-6, Acetobacter pasteurianus G3-2 | Same number of Ls. casei M1-6 and Acetobacter pasteurianus G3-2 were inoculated. | The contents of acetoin, ethyl acetate, ethyl lactate, and Chuanqiongqin increased by 102.4%, 146.6%, 91.7%, and 52.1%, respectively. | [78] |
| Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) M10-1, Ls. casei (formerly Lactobacillus casei) 21M3-1 | One liter of each strain supernatant (1012 CFU/mL) was inoculated into 164 kg vinegar Pei. | The presence of Ls. casei 21M3-1 led to a four-fold increase in L-lactic acid production, whereas Lp. plantarum M10-1 enhanced the contents of both L-lactic acid and D-lactic acid by one-fold. | [22] | |
| Soy sauce | T. halophilus, Zygosaccharomyces rouxii, Torulopsis versatilis | After 15 d of moromi fermentation, T. halophilus (2 × 105 CFU/mL) was inoculated. Z. rouxii (106 CFU/mL) was then inoculated on day 30, followed by the inoculation of T. versatilis (106 CFU/mL) on the 45th day. | The fruity, saucy, alcoholic, and caramel-like flavors increased by 64.3%, 22.7%, 43.1%, and 36.2%, respectively, while the saline taste increased by 64.3%. | [79] |
| T. halophilus, Z. rouxii | During moromi fermentation, co-inoculation with T. halophilus and Z. rouxii, or inoculation firstly with T. halophilus, followed by the sequential inoculation of Z. rouxii. | The promotion of alcohol formation obtained through bioaugmentation led to the development of more intricate aroma characteristics. | [80] | |
| High-salt liquid-state fermentation soy sauce | Millerozyma farinosa CS2.23, Z. rouxii CS2.42, Candida parapsilosis CS2.53 | Each strain (107 cell/mL) was inoculated in high-salt liquid-state moromi fermented for 45 d. | The volatile esters content inoculated with M. farinosa CS2.23, Z. rouxii CS2.42, and C. parapsilosis CS2.53 increased by 108.85%, 166.71%, and 113.61%, respectively. | [81] |
| Wickerhamomyces anomalus ZMS55, W. anomalus ZMS102 | Following the fermentation of high-salt liquid-state moromi to a pH of 5, it was inoculated with each strain (2 × 106 cells/g). | The production of esters showed increased diversity, accompanied by significantly higher yields of ethanol, acids, and aldehydes. | [82] | |
| Z. rouxii QH-25, C. versatilis | On the first day of the high-salt liquid-state moromi fermentation, Z. rouxii QH-25 was inoculated, followed by the inoculation of C. versatilis on the fifth day. | The concentrations of volatile substances, including ketones, esters, phenols, and alcohols, increased by 3.07-, 1.91-, 1.36-, and 1.22-fold, respectively. Characteristic components, such as ethyl octanoate, 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone, 4-ethyl-2-methoxy-phenol, and 3-methyl-1-butanol, exhibited increases by 3.99-, 3.29-, 1.63-, and 0.70-fold, respectively. | [83] | |
| Gray sufu | Leuconostoc mesenteroides F24 | L. mesenteroides F24 was inoculated in the mixture of brine and yellow tofu serofluids at approximately 106 CFU/mL. | The contents of esters, alcohols, aldehydes, acids, and aromatic compounds increased. | [84] |
| Weissella confusa M1 | W. confusa M1 was added to the mixture of brine and yellow tofu serofluids at approximately 106 CFU/mL. | The contents of 13 free amino acids increased, particularly aspartic acid and glutamic acid. | [85] | |
| Doubanjiang | T. halophilus, W. confuse, Z. rouxii | Lactobacillales (T. halophilus and W. confuse) and Z. rouxii were inoculated into the mixed Pei at 106 CFU/g and 105 CFU/g, respectively. | The contents of amino acids, like glutamic acid and aspartic acid, along with volatile flavor compounds, such as esters, carbonyls, and phenols, increased. | [41] |
| Z. rouxii Y-8 | Z. rouxii Y-8 was inoculated at the beginning of Pei fermentation at 106 CFU/g Pei. | The total concentrations of volatile flavor compounds increased from 4767.22 to 72,813.09 μg/100 g dry Pei, with the presence of 33 new volatile flavor compounds, including alcohols, esters, acids, and carbonyl compounds. | [86] | |
| Aspergillus-type douchi | Meyerozyma Caribbica, Meyerozyma guilliermondii, Candida etchellsii, C. versatilis | Following a 3-day culture period, the purified strains were co-inoculated with A. oryzae to produce Aspergillus-type douchi. | The contents of amino acids, unsaturated fatty acids, and organic acids increased. | [87] |
| Seasoning | Microorganisms | Bioaugmentation Strategy | Efficacy on Safety | Reference |
|---|---|---|---|---|
| Soy sauce | Staphylococcus piscifermentans QR19 | S. piscifermentans QR19 was inoculated into the fermentation mash at the beginning of fermentation. | The biogenic amine content decreased by 63.25% compared to soy sauce without S. piscifermentans. Additionally, they were 81.19% and 71.87% lower, respectively, than two commercial soy sauces. | [110] |
| Z. rouxii, T. halophilus | During brine fermentation, T. halophilus (2.5 × 106 CFU/g) and Z. rouxii (2 × 106 CFU/g) were inoculated into moromi. | The biogenic amine content was reduced by 52.36~55.05%. | [111] | |
| Cantonese soy sauce | T. halophilus CGMCC3792, Z. rouxii CGMCC21865 | At the beginning of the brine fermentation, T. halophilius (2.1 × 106 CFU/g) and Z. rouxii (1.6 × 106 CFU/g) were inoculated into moromi. | The biogenic amine content was reduced by 67.68%. | [112] |
| Sufu | Lv. brevis (formerly Lactobacillus brevis) | Lv. brevis (3.8 × 106 CFU/mL) was added to the mixture of brine and yellow tofu serofluids. | The biogenic amine content was reduced significantly. | [59] |
| Doubanjiang | Lp. plantarum (formerly Lactobacillus plantarum) DPUL-J5 | Lp. plantarum DPUL-J5 (2%) was inoculated into brine containing 2% B. subtilis DPUL-J2. | The biogenic amine content was reduced significantly. | [105] |
| B. amyloliquefaciens 1-G6, Bacillus licheniformis 2-B3 | Each strain (106 CFU/g) was inoculated on the third day of fermentation. | Inoculation with B. amyloliquefaciens 1-G6 led to a 29% reduction in the biogenic amine content, while inoculation with B. licheniformis 2-B3 resulted in a 16% decrease in the biogenic amine content. | [113] | |
| Dajiang | Lp. plantarum (formerly Lactobacillus plantarum) HM24 | The Lp. plantarum HM24 supernatant (4%) was inoculated into a mixture of koji and brine. | The degradation rates of tryptamine, phenethylamine, putrescine, cadaverine, histamine, and tyramine were 35.31%, 43.14%, 30.18%, 33.44%, 32.74%, and 39.91%, respectively. | [114] |
| S. carnosus M43, Pediococcus acidilactici M28 | A mixed bacteria solution of each strain at 107 CFU/g was prepared at a ratio of 1:1 and inoculated for fermentation. | The biogenic amine content decreased by 39.69%. | [115] | |
| Lp. plantarum DPUL-J8, P. kudriavzevii DPUY-J8 | Lp. plantarum DPUL-J8 and P. kudriavzevii DPUY-J8 were co-inoculated. | The biogenic amine content decreased by 67.15%. | [106] | |
| Douchi | Bacillus tropicus A11, Bacillus siamensis D11, B. subtilis T2, B. subtilis U2 | Each strain was inoculated into soybeans at 3% (v/m). | Through the mono-fermentation of B. tropicus A11, B. siamensis D11, B. subtilis T2, and B. subtilis U2, the contents of biogenic amines decreased by 74.38%, 61.85%, 82.13%, and 65.43%, respectively. | [116] |
| Mucor racemosus (M1), Mucor wutungqiao (M2), Actinomucor elegans (M3), A. oryzae 2339 (A1), A. oryzae 41380 (A2), A. oryzae 40188 (A3) | Each strain was cultivated in potato-dextro agar at 28 °C for 3 d. Subsequently, 1 mL of sterilized water was added to the agar to obtain the spore suspension, which was then incorporated into the bran medium and incubated at 28 °C for 3 d. Following this, a mixture of 0.3~0.5% (w/w) of the bran medium containing the strains was mixed with steamed soybeans. | The biogenic amine content decreased by 38.76%, 32.11%, 36.27%, 21.44%, 25.06%, and 21.27% for douchi inoculated with A1, A2, A3, M1, M2, and M3, respectively. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Wu, J.; Zhou, W.; Li, J.; Hu, K.; Li, Q.; Zhao, N.; Yang, Y.; Liu, S. Research Progress on Bioaugmentation Technology for Improving Traditional Chinese Fermented Seasonings. Fermentation 2024, 10, 123. https://doi.org/10.3390/fermentation10030123
Liu A, Wu J, Zhou W, Li J, Hu K, Li Q, Zhao N, Yang Y, Liu S. Research Progress on Bioaugmentation Technology for Improving Traditional Chinese Fermented Seasonings. Fermentation. 2024; 10(3):123. https://doi.org/10.3390/fermentation10030123
Chicago/Turabian StyleLiu, Aiping, Jie Wu, Weixin Zhou, Jianlong Li, Kaidi Hu, Qin Li, Ning Zhao, Yong Yang, and Shuliang Liu. 2024. "Research Progress on Bioaugmentation Technology for Improving Traditional Chinese Fermented Seasonings" Fermentation 10, no. 3: 123. https://doi.org/10.3390/fermentation10030123
APA StyleLiu, A., Wu, J., Zhou, W., Li, J., Hu, K., Li, Q., Zhao, N., Yang, Y., & Liu, S. (2024). Research Progress on Bioaugmentation Technology for Improving Traditional Chinese Fermented Seasonings. Fermentation, 10(3), 123. https://doi.org/10.3390/fermentation10030123





