Recent Advances and Challenges in the Production of Hydroxylated Natural Products Using Microorganisms
Abstract
1. Introduction
2. Types of Hydroxylation of NPs
2.1. Cytochrome P450 Monooxygenase-Mediated Hydroxylation
2.2. Hydroxylation Mediated by Non-Heme Iron-Dependent Hydroxylases
2.3. Other Enzyme-Mediated Hydroxylation Reactions
3. Hydroxylation of Different Types of NPs and Their Derivatives
3.1. Hydroxylation of Amino Acids and Their Derivatives
3.2. Hydroxylation of Steroids and Their Derivatives
3.2.1. Hydroxylation of Steroid Nucleus
3.2.2. Hydroxylation of Steroid Side Chains
3.3. Hydroxylation of Terpenoids and Their Derivatives
3.3.1. Hydroxylation of Cyclic Terpenoids
3.3.2. Hydroxylation of Acyclic Terpenoids
3.4. Hydroxylation of Lipids and Their Derivatives
3.5. Hydroxylation of Phenylpropanoids and Their Derivatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Cheminform. 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, B.; Pei, L.; Wang, J.; Gan, Y.; Jiang, L.; Liu, B.; Cheng, J.; Li, W. Advances and Challenges in Biomanufacturing of Glycosylation of Natural Products. Fermentation 2024, 10, 349. [Google Scholar] [CrossRef]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Springob, K.; Kutchan, T.M. Introduction to the Different Classes of Natural Products. In Plant-Derived Natural Products; Springer: New York, NY, USA, 2009; pp. 3–50. [Google Scholar]
- Sun, D.; Liu, X.; Zhu, M.; Chen, Y.; Li, C.; Cheng, X.; Zhu, Z.; Lu, F.; Qin, H.M. Efficient Biosynthesis of High-Value Succinic Acid and 5-Hydroxyleucine Using a Multienzyme Cascade and Whole-Cell Catalysis. J. Agric. Food Chem. 2019, 67, 12502–12510. [Google Scholar] [CrossRef]
- Schmitz, D.; Zapp, J.; Bernhardt, R. Steroid conversion with CYP106A2—Production of pharmaceutically interesting DHEA metabolites. Microb. Cell Fact. 2014, 13, 81. [Google Scholar] [CrossRef][Green Version]
- Riyadi, S.A.; Naini, A.A.; Supratman, U. Sesquiterpenoids from Meliaceae Family and Their Biological Activities. Molecules 2023, 28, 4874. [Google Scholar] [CrossRef]
- Sordon, S.; Madej, A.; Poplonski, J.; Bartmanska, A.; Tronina, T.; Brzezowska, E.; Juszczyk, P.; Huszcza, E. Regioselective ortho-Hydroxylations of Flavonoids by Yeast. J. Agric. Food Chem. 2016, 64, 5525–5530. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
- Cheong, D.H.J.; Tan, D.W.S.; Wong, F.W.S.; Tran, T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020, 158, 104901. [Google Scholar] [CrossRef]
- Hu, X.; Guo, F. Amino Acid Sensing in Metabolic Homeostasis and Health. Endocr. Rev. 2021, 42, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.L.; Gaspar, A.B.; Contini, L.R.F.; Silva, M.F.; Chagas, E.G.L.; Bahu, J.O.; Concha, V.O.C.; Carvalho, R.A.; Severino, P.; Souto, E.B.; et al. Lemongrass (Cymbopogon citratus)-incorporated chitosan bioactive films for potential skincare applications. Int. J. Pharm. 2022, 628, 122301. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Domb, A.J. Antimicrobial Activities of Natural Bioactive Polyphenols. Pharmaceutics 2024, 16, 718. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, Y.; Hu, J. Tea-polyphenol treated skin collagen owns coalesced adaptive-hydration, tensile strength and shape-memory property. Int. J. Biol. Macromol. 2020, 158, 1–8. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Ma, T.; Cheng, H.; Pitchakuntla, M.; Ma, W.; Jia, Y. Total Synthesis of (-)-Principinol C. J. Am. Chem. Soc. 2022, 144, 20196–20200. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, T.; Wang, H.; Yang, C.; Cheng, L.; Yin, S.F.; Kambe, N.; Qiu, R. Recent Progress on Photocatalytic Synthesis of Ester Derivatives and Reaction Mechanisms. Top. Curr. Chem. 2021, 379, 42. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Z.; Zhuang, W.; Wang, T.; Xie, Y. Family characteristics, phylogenetic reconstruction, and potential applications of the plant BAHD acyltransferase family. Front. Plant Sci. 2023, 14, 1218914. [Google Scholar] [CrossRef] [PubMed]
- Shatskiy, A.; Stepanova, E.V.; Karkas, M.D. Exploiting photoredox catalysis for carbohydrate modification through C-H and C-C bond activation. Nat. Rev. Chem. 2022, 6, 782–805. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Gao, S. Trends in applying C-H oxidation to the total synthesis of natural products. Nat. Prod. Rep. 2016, 33, 562–581. [Google Scholar] [CrossRef]
- Yi, D.; Bayer, T.; Badenhorst, C.P.S.; Wu, S.; Doerr, M.; Höhne, M.; Bornscheuer, U.T. Recent trends in biocatalysis. Chem. Soc. Rev. 2021, 50, 8003–8049. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Zhuang, Y.; Quan, W.; Wang, X.; Cheng, Y.; Jiao, Y. Comprehensive Review of EGCG Modification: Esterification Methods and Their Impacts on Biological Activities. Foods 2024, 13, 1232. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Xu, W.; Zhang, C.; Zhu, Q.; Qin, X.; Zhang, H.; Liu, G. Effects of esterification and enzymatic modification on the properties of wheat starch and dough. Food Hydrocoll. 2025, 158, 110509. [Google Scholar] [CrossRef]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Syntheses of mono-acylated luteolin derivatives, evaluation of their antiproliferative and radical scavenging activities and implications on their oral bioavailability. Sci. Rep. 2021, 11, 12595. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Zhao, J.; Li, Q.; Yu, X.; Acevedo-Rocha, C.G.; Li, A. Bacterial cytochrome P450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresour. Bioprocess. 2020, 7, 2. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, B.; Wang, Q.; Zhang, H.; Fushinobu, S.; Yang, J.; Lin, S.; Sun, K.; Han, B.N.; Xu, L.H. Improved 2alpha-Hydroxylation Efficiency of Steroids by CYP154C2 Using Structure-Guided Rational Design. Appl. Environ. Microbiol. 2023, 89, e0218622. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Zou, H.; Long, T.; Osire, T.; Wang, L.; Wei, X.; Long, M.; Rao, Z.; Liao, G. Novel cytochrome P450s for various hydroxylation of steroids from filamentous fungi. Bioresour. Technol. 2024, 394, 130244. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zheng, P.; Chen, P.; Wu, D.; Xu, S. Engineering of 4-hydroxyphenylacetate 3-hydroxylase derived from Pseudomonas aeruginosa for the ortho-hydroxylation of ferulic acid. Int. J. Biol. Macromol. 2024, 264, 130545. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Pandey, R.P.; Jung, N.; Jung, H.J.; Kim, E.H.; Sohng, J.K. Hydroxylation of diverse flavonoids by CYP450 BM3 variants: Biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microb. Cell Fact. 2016, 15, 135. [Google Scholar] [CrossRef]
- Lee, U.-J.; Sohng, J.K.; Kim, B.-G.; Choi, K.-Y. Recent trends in the modification of polyphenolic compounds using hydroxylation and glycosylation. Curr. Opin. Biotechnol. 2023, 80, 102914. [Google Scholar] [CrossRef]
- Amor, I.L.-B.; Hehn, A.; Guedon, E.; Ghedira, K.; Engasser, J.-M.; Chekir-Ghedrira, L.; Ghoul, M. Biotransformation of Naringenin to Eriodictyol by Saccharomyces cerevisiea Functionally Expressing Flavonoid 3′ Hydroxylase. Nat. Prod. Commun. 2010, 5, 1893–1898. [Google Scholar] [CrossRef]
- Huo, T.; Zhao, X.; Cheng, Z.; Wei, J.; Zhu, M.; Dou, X.; Jiao, N. Late-stage modification of bioactive compounds: Improving druggability through efficient molecular editing. Acta Pharm. Sin. B 2024, 14, 1030–1076. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, X.; Fu, X.; Li, Q.; Dou, J.; Zhai, G. Current development in nanoformulations of docetaxel. Expert Opin. Drug Deliv. 2012, 9, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Kawakishi, S.; Morimitsu, Y.; Osawa, T. New potent antioxidative o-dihydroxyisoflavones in fermented Japanese soybean products. Biosci. Biotechnol. Biochem. 1999, 63, 1637–1639. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, K.W.; Byun, S.; Jung, S.K.; Song, N.; Lim, S.H.; Heo, Y.S.; Kim, J.E.; Kang, N.J.; Kim, B.Y.; et al. 7,3’,4’-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4. J. Biol. Chem. 2011, 286, 14246–14256. [Google Scholar] [CrossRef]
- Sun, C.; Hu, B.; Li, Y.; Wu, Z.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Zhao, X. Efficient stereoselective hydroxylation of deoxycholic acid by the robust whole-cell cytochrome P450 CYP107D1 biocatalyst. Synth. Syst. Biotechnol. 2023, 8, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.A.; Cao, N.T.; Nguyen, T.H.H.; Ji, J.H.; Cha, G.S.; Kang, H.S.; Yun, C.H. Enzymatic Production of 3-OH Phlorizin, a Possible Bioactive Polyphenol from Apples, by Bacillus megaterium CYP102A1 via Regioselective Hydroxylation. Antioxidants 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Kim, J.W.; Cho, I.J.; Lee, S.Y. Metabolic Engineering of Escherichia coli for the Production of 3-Hydroxypropionic Acid and Malonic Acid through beta-Alanine Route. ACS Synth. Biol. 2016, 5, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xiao, S.; Luo, Q.; Wang, B.; Zeng, R.; Zhao, L.; Zhang, J. Engineering an Artificial Pathway to Improve the Bioconversion of Lysine into Chiral Amino Alcohol 2-Hydroxycadaverine Using a Semi-Rational Design. Fermentation 2024, 10, 56. [Google Scholar] [CrossRef]
- Lu, Z.H.; Yang, L.R.; Wu, J.P. Efficient heterologous expression of nicotinate dehydrogenase in Comamonas testosteroni CNB-2 with transcriptional, folding enhancement strategy. Enzyme Microb. Technol. 2020, 134, 109478. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Wang, J.; Yu, Y.; Li, L.; Sun, P.; Fan, X.; Xu, Q. Metabolic engineering of Escherichia coli for efficient production of L-5-hydroxytryptophan from glucose. Microb. Cell Fact. 2022, 21, 198. [Google Scholar] [CrossRef]
- Li, S.; Chang, Y.; Liu, Y.; Tian, W.; Chang, Z. A novel steroid hydroxylase from Nigrospora sphaerica with various hydroxylation capabilities to different steroid substrates. J. Steroid Biochem. Mol. Biol. 2023, 227, 106236. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, C.; Shen, X.; Sun, X.; Wang, J.; Yuan, Q. Unlocking the potential of enzyme engineering via rational computa-tional design strategies. Biotechnol. Adv. 2024, 73, 108376. [Google Scholar] [CrossRef]
- Zheng, R.C.; Tang, X.L.; Suo, H.; Feng, L.L.; Liu, X.; Yang, J.; Zheng, Y.G. Biochemical characterization of a novel tyrosine phenol-lyase from Fusobacterium nucleatum for highly efficient biosynthesis of l-DOPA. Enzyme Microb. Technol. 2018, 112, 88–93. [Google Scholar] [CrossRef]
- Kurpejovic, E.; Wendisch, V.F.; Sariyar Akbulut, B. Tyrosinase-based production of L-DOPA by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2021, 105, 9103–9111. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, B.; Du, G.; Chen, J.; Zhou, J. Integrating enzyme evolution and high-throughput screening for efficient biosynthesis of L-DOPA. J. Ind. Microbiol. Biotechnol. 2019, 46, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.A.; Deive, F.J.; Alvarez, M.S.; Longo, M.A.; Rodriguez, A.; Bernal, C.; Martinez, R. Effect of process parameters and surfactant additives on the obtained activity of recombinant tryptophan hydroxylase (TPH1) for enzymatic synthesis of 5-hydroxytryptophan (5-HTP). Enzyme Microb. Technol. 2022, 154, 109975. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, W.; Shi, F.; Huang, L.; Lian, J.; Qu, L.; Cai, J.; Xu, Z. Metabolic pathway engineering for high-level production of 5-hydroxytryptophan in Escherichia coli. Metab. Eng. 2018, 48, 279–287. [Google Scholar] [CrossRef]
- Song, F.; Gu, T.; Zhang, L.; Zhang, J.; You, S.; Qi, W.; Su, R. Rational design of tryptophan hydroxylation 1 for improving 5-Hydroxytryptophan production. Enzyme Microb. Technol. 2023, 165, 110198. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, G.; Xie, H.; Yang, W.; Xu, H.; Han, S.; Wang, J.; Meng, Y.; Xu, Q.; Li, Y.; et al. Sustainable production of 4-hydroxyisoleucine with minimised carbon loss by simultaneously utilising glucose and xylose in engineered Escherichia coli. Bioresour. Technol. 2022, 354, 127196. [Google Scholar] [CrossRef]
- Du, P.; Yan, S.; Qian, X.L.; Pan, J.; Zhang, Z.J.; Yu, H.L.; Xu, J.H. Engineering Bacillus subtilis Isoleucine Dioxygenase for Efficient Synthesis of (2S,3R,4S)-4-Hydroxyisoleucine. J. Agric. Food Chem. 2020, 68, 14555–14563. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, R.; Ma, L.; Wang, S.; Li, C.; Xu, Q. Optimization of trans-4-hydroxyproline synthesis pathway by rearrangement center carbon metabolism in Escherichia coli. Microb. Cell Fact. 2023, 22, 240. [Google Scholar] [CrossRef]
- Shibasaki, T.; Mori, H.; Chiba, S.; Ozaki, A. Microbial proline 4-hydroxylase screening and gene cloning. Appl. Environ. Microbiol. 1999, 65, 4028–4031. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Ma, J.; Liu, Y.; He, J.; Li, Y.; Zhu, F.; Meng, J.; Zhan, J.; Li, Z.; et al. High production of 4-hydroxyisoleucine in Corynebacterium glutamicum by multistep metabolic engineering. Metab. Eng. 2018, 49, 287–298. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, X.; Rao, Z.; Xu, M.; Yang, T.; Li, H.; Xu, Z.; Yang, S. Efficient testosterone production by engineered Pichia pastoris co-expressing human 17β-hydroxysteroid dehydrogenase type 3 and Saccharomyces cerevisiae glucose 6-phosphate dehydrogenase with NADPH regeneration. Green Chem. 2016, 18, 1774–1784. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.; Xi, Y.; Dai, Z.; Bi, C.; Chen, X.; Fan, F.; Zhang, X. Production of 14alpha-hydroxysteroids by a recombinant Saccharomyces cerevisiae biocatalyst expressing of a fungal steroid 14alpha-hydroxylation system. Appl. Microbiol. Biotechnol. 2019, 103, 8363–8374. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, L.; Liu, Y.; Tu, L.; Wu, X.; Wang, J.; Li, D.; Zhang, X.; Gao, W.; Zhang, Y.; et al. CYP72D19 from Tripterygium wilfordii catalyzes C-2 hydroxylation of abietane-type diterpenoids. Plant Cell Rep. 2023, 42, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Görner, C.; Schrepfer, P.; Redai, V.; Wallrapp, F.; Loll, B.; Eisenreich, W.; Haslbeck, M.; Brück, T. Identification, characterization and molecular adaptation of class I redox systems for the production of hydroxylated diterpenoids. Microb. Cell Factories 2016, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, J.A.; Belmonte Del Ama, A.; Jayachandran, S.; Dahlin, J.; Rago, D.; Andersen, A.J.C.; Borodina, I. Engineering of Yarrowia lipolytica for the production of plant triterpenoids: Asiatic, madecassic, and arjunolic acids. Metab. Eng. Commun. 2022, 14, e00197. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Shi, Q.-Y.; Ma, S.-G.; Yu, S.-S. Minor Hydroxylated Triterpenoids Produced in Engineered Yeast by the Enzymes OSC and CYP716s from the Plant Enkianthus chinensis and Their Anti-Inflammatory and Hepatoprotective Activities. J. Nat. Prod. 2024, 87, 1036–1043. [Google Scholar] [CrossRef]
- Kaprakkaden, A.; Srivastava, P.; Bisaria, V.S. In vitro synthesis of 9,10-dihydroxyhexadecanoic acid using recombinant Escherichia coli. Microb. Cell Fact. 2017, 16, 85. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Lu, W. Biosynthesis of Long-Chain omega-Hydroxy Fatty Acids by Engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2019, 67, 4545–4552. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.; Jung, E.; Choi, K.Y.; Bae, J.H.; Kim, M.; Kim, J.; Kim, E.J.; Kim, P.I.; Kim, B.G. The production of omega-hydroxy palmitic acid using fatty acid metabolism and cofactor optimization in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 6667–6676. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, Y. Biotechnological production of plant-specific hydroxylated phenylpropanoids. Biotechnol. Bioeng. 2014, 111, 1895–1899. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zhang, W.; Yao, M.; Li, B.; Liu, D.; Yuan, Y. Engineering the Biosynthesis of Caffeic Acid in Saccharomyces cerevisiae with Heterologous Enzyme Combinations. Engineering 2019, 5, 287–295. [Google Scholar] [CrossRef]
- Jones, J.A.; Collins, S.M.; Vernacchio, V.R.; Lachance, D.M.; Koffas, M.A. Optimization of naringenin and p-coumaric acid hydroxylation using the native E. coli hydroxylase complex, HpaBC. Biotechnol. Prog. 2016, 32, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, Y.; Yang, Y.; Min, Q.; Li, H.; Chen, L. Cytochrome P450 Monooxygenases Catalyse Steroid Nucleus Hydroxylation with Regio- and Stereo-Selectivity. Adv. Synth. Catal. 2022, 364, 2701–2719. [Google Scholar] [CrossRef]
- Renata, H. Synthetic utility of oxygenases in site-selective terpenoid functionalization. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab002. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Su, Z.; Xiong, L.; Wang, P.; Lei, W.; Yan, X.; Ma, D.; Zhao, G.; Zhou, Z. Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol. Nat. Commun. 2024, 15, 2339. [Google Scholar] [CrossRef]
- Zhang, M.-F.; Xie, W.-L.; Chen, C.; Li, C.-X.; Xu, J.-H. Computational redesign of taxane-10β-hydroxylase for de novo biosynthesis of a key paclitaxel intermediate. Appl. Microbiol. Biotechnol. 2023, 107, 7105–7117. [Google Scholar] [CrossRef]
- Song, F.; Zheng, M.; Wang, J.; Liu, H.; Lin, Z.; Liu, B.; Deng, Z.; Cong, H.; Zhou, Q.; Qu, X. Chemoenzymatic synthesis of C14-functionalized steroids. Nat. Synth. 2023, 2, 729–739. [Google Scholar] [CrossRef]
- Felpeto-Santero, C.; Galan, B.; Garcia, J.L. Engineering the Steroid Hydroxylating System from Cochliobolus lunatus in Mycolicibacterium smegmatis. Microorganisms 2021, 9, 1499. [Google Scholar] [CrossRef]
- Solomon, E.I.; Light, K.M.; Liu, L.V.; Srnec, M.; Wong, S.D. Geometric and electronic structure contributions to function in non-heme iron enzymes. Accounts Chem. Res. 2013, 46, 2725–2739. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; An, J.; Jing, X.; Chen, C.-C.; Dai, L.; Xu, Y.; Liu, W.; Guo, R.-T.; Nie, Y. Molecular Insights into the Regioselectivity of the Fe(II)/2-Ketoglutarate-Dependent Dioxygenase-Catalyzed C–H Hydroxylation of Amino Acids. ACS Catal. 2022, 12, 11586–11596. [Google Scholar] [CrossRef]
- Cheung-Lee, W.L.; Kolev, J.N.; McIntosh, J.A.; Gil, A.A.; Pan, W.; Xiao, L.; Velasquez, J.E.; Gangam, R.; Winston, M.S.; Li, S.; et al. Engineering Hydroxylase Activity, Selectivity, and Stability for a Scalable Concise Synthesis of a Key Intermediate to Belzutifan. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316133. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Abe, I. Nonheme Iron- and 2-Oxoglutarate-Dependent Dioxygenases in Fungal Meroterpenoid Biosynthesis. Chem. Pharm. Bull. 2020, 68, 823–831. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Du, D.; Su, Y.; Shang, Q.; Chen, C.; Tang, W.; Zhang, L.; Ren, H.; Liu, W. Biomimetic synthesis of L-DOPA inspired by tyrosine hydroxylase. J. Inorg. Biochem. 2022, 234, 111878. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, W.; Bao, Y.; Huang, Q.; Ye, K.; Liu, P.; Chu, X. Modular reconstruction and optimization of the trans-4-hydroxy-L-proline synthesis pathway in Escherichia coli. Microb. Cell Fact. 2022, 21, 159. [Google Scholar] [CrossRef]
- Li, X.; Awakawa, T.; Mori, T.; Ling, M.; Hu, D.; Wu, B.; Abe, I. Heterodimeric Non-heme Iron Enzymes in Fungal Meroterpenoid Biosynthesis. J. Am. Chem. Soc. 2021, 143, 21425–21432. [Google Scholar] [CrossRef]
- Janzen, D.J.; Wang, H.; Li, S.-M. A Flavin-Dependent Oxygenase Catalyzes Hydroxylation and Simultaneous Pyrrolidine Ring Formation in Protubonine Biosynthesis in Aspergillus ustus. J. Nat. Prod. 2023, 86, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Kunishita, A.; Ertem, M.Z.; Okubo, Y.; Tano, T.; Sugimoto, H.; Ohkubo, K.; Fujieda, N.; Fukuzumi, S.; Cramer, C.J.; Itoh, S. Active site models for the Cu(A) site of peptidylglycine alpha-hydroxylating monooxygenase and dopamine beta-monooxygenase. Inorg. Chem. 2012, 51, 9465–9480. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Janzen, D.J.; Guan, Z.; Ye, Y.; Zhang, Y.; Li, S.M. The Promiscuous Flavin-Dependent Monooxygenase PboD from Aspergillus ustus Increases the Structural Diversity of Hydroxylated Pyrroloindoline Diketopiperazines. J. Nat. Prod. 2024, 87, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Dippe, M.; Pecher, P.; Funke, E.; Pietzsch, M.; Wessjohann, L.A. Engineered Bacterial Flavin-Dependent Monooxygenases for the Regiospecific Hydroxylation of Polycyclic Phenols. ChemBioChem 2022, 23, e202100480. [Google Scholar] [CrossRef] [PubMed]
- Klinman, J.P. The Copper-Enzyme Family of Dopamine β-Monooxygenase and Peptidylglycine α-Hydroxylating Monooxygenase: Resolving the Chemical Pathway for Substrate Hydroxylation. J. Biol. Chem. 2006, 281, 3013–3016. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, S.; Zhao, X.; Zhao, L.; Zhang, Y.; Cheng, J.; Zhang, J. Recent Advances in the Hydroxylation of Amino Acids and Its Derivatives. Fermentation 2023, 9, 285. [Google Scholar] [CrossRef]
- Sun, D.Y.; Cheng, X.T.; Gao, D.K.; Xu, P.P.; Guo, Q.Q.; Zhu, Z.L.; Zhu, M.L.; Wang, X.Y.; Qin, H.M.; Lu, F.P. Properties, biosynthesis, and catalytic mechanisms of hydroxy-amino-acids. IOP Conf. Ser. Earth Environ. Sci. 2018, 188, 012084. [Google Scholar] [CrossRef]
- Choroba, O.W.; Williams, D.H.; Spencer, J.B.J.J.o.t.A.C.S. Biosynthesis of the Vancomycin Group of Antibiotics: Involvement of an Unusual Dioxygenase in the Pathway to (S)-4-Hydroxyphenylglycine. J. Am. Chem. Soc. 2000, 122, 256–258. [Google Scholar] [CrossRef]
- Sutanto, C.N.; Xia, X.; Heng, C.W.; Tan, Y.S.; Lee, D.P.S.; Fam, J.; Kim, J.E. The impact of 5-hydroxytryptophan supplementation on sleep quality and gut microbiota composition in older adults: A randomized controlled trial. Clin. Nutr. 2024, 43, 593–602. [Google Scholar] [CrossRef]
- Zafar, M.I.; Gao, F. 4-Hydroxyisoleucine: A Potential New Treatment for Type 2 Diabetes Mellitus. BioDrugs 2016, 30, 255–262. [Google Scholar] [CrossRef]
- Hao, M.; He, Y.; Song, T.; Guo, H.; Rayman, M.P.; Zhang, J. Dopamine and its precursor levodopa inactivate SARS-CoV-2 main protease by forming a quinoprotein. Free Radic. Biol. Med. 2024, 220, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Taga, Y.; Zientek, K.; Mizuno, N.; Salo, A.M.; Semenova, O.; Tufa, S.F.; Keene, D.R.; Holden, P.; Mizuno, K.; et al. Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER. J. Biol. Chem. 2021, 296, 100453. [Google Scholar] [CrossRef] [PubMed]
- Orieshyna, A.; Puetzer, J.L.; Amdursky, N. Proton Transport Across Collagen Fibrils and Scaffolds: The Role of Hydroxyproline. Biomacromolecules 2023, 24, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.J.; Yan, F.; Liu, P.; Liu, H.W.; Drennan, C.L.J.N. Structural insight into antibiotic fosfomycin biosynthesis by a mononuclear iron enzyme. Nature 2005, 437, 838. [Google Scholar] [CrossRef] [PubMed]
- Bodner, M.J.; Phelan, R.M.; Freeman, M.F.; Li, R.; Townsend, C.A. Non-Heme Iron Oxygenases Generate Natural Structural Diversity in Carbapenem Antibiotics. J. Am. Chem. Soc. 2010, 132, 12–13. [Google Scholar] [CrossRef][Green Version]
- Blaskovich, M.A.; Evindar, G.; Rose, N.G.W.; Wilkinson, S.; Luo, Y.; Lajoie, G.A. Stereoselective Synthesis of Threo and Erythro β-Hydroxy and β-Disubstituted-β-Hydroxy α-Amino Acids. J. Org. Chem. 1998, 63, 3631–3646. [Google Scholar] [CrossRef]
- Palomo, C.; Arrieta, A.; Cossío, F.P.; Aizpurua, J.M.; Mielgo, A.; Aurrekoetxea, N. Highly stereoselective synthesis of α-hydroxy β-amino acids through β-lactams: Application to the synthesis of the taxol and bestatin side chains and related systems. Tetrahedron Lett. 1990, 31, 6429–6432. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Raines, R.T. Insights on the conformational stability of collagen. Nat. Prod. Rep. 2002, 19, 49–59. [Google Scholar] [CrossRef]
- Fordjour, E.; Adipah, F.K.; Zhou, S.; Du, G.; Zhou, J. Metabolic engineering of Escherichia coli BL21 (DE3) for de novo production of L-DOPA from D-glucose. Microb. Cell Fact. 2019, 18, 74. [Google Scholar] [CrossRef]
- Rolf, J.; Nerke, P.; Britner, A.; Krick, S.; Lütz, S.; Rosenthal, K. From Cell-Free Protein Synthesis to Whole-Cell Biotransformation: Screening and Identification of Novel α-Ketoglutarate-Dependent Dioxygenases for Preparative-Scale Synthesis of Hydroxy-l-Lysine. Catalysts 2021, 11, 1038. [Google Scholar] [CrossRef]
- Prell, C.; Vonderbank, S.-A.; Meyer, F.; Pérez-García, F.; Wendisch, V.F. Metabolic engineering of Corynebacterium glutamicum for de novo production of 3-hydroxycadaverine. Curr. Res. Biotechnol. 2022, 4, 32–46. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Peng, S.; Chen, Y.; Chen, F.; Zhang, A.; Chen, K. Enhancing thermostability of tryptophan hydroxylase via protein engineering and its application in 5-hydroxytryptophan production. Int. J. Biol. Macromol. 2024, 264, 130609. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Chen, Q.; Zhou, N.; Wang, X.; Zhang, A.; Chen, K.; Ouyang, P. Construction of cell factory capable of efficiently converting L-tryptophan into 5-hydroxytryptamine. Microb. Cell Fact. 2022, 21, 47. [Google Scholar] [CrossRef]
- Nagy, P.I. Competing intramolecular vs. intermolecular hydrogen bonds in solution. Int. J. Mol. Sci. 2014, 15, 19562–19633. [Google Scholar] [CrossRef]
- Caron, G.; Kihlberg, J.; Ermondi, G. Intramolecular hydrogen bonding: An opportunity for improved design in medicinal chemistry. Med. Res. Rev. 2019, 39, 1707–1729. [Google Scholar] [CrossRef] [PubMed]
- Krane, S.M. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 2008, 35, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, H.; Zhang, T.; Pijning, T.; Yu, L.; Zhang, W.; Liu, W.; Meng, X. Biochemical and genetic characterization of fungal proline hydroxylase in echinocandin biosynthesis. Appl. Microbiol. Biotechnol. 2018, 102, 7877–7890. [Google Scholar] [CrossRef]
- Prier, C.K.; Lo, M.M.C.; Li, H.; Yasuda, N. Stereodivergent Synthesis of 3-Hydroxyprolines and 3-Hydroxypipecolic Acids via Ketoreductase-Catalyzed Dynamic Kinetic Reduction. Adv. Synth. Catal. 2019, 361, 5140–5143. [Google Scholar] [CrossRef]
- Klein, C.; Hüttel, W. A Simple Procedure for Selective Hydroxylation of L-Proline and L-Pipecolic Acid with Recombinantly Expressed Proline Hydroxylases. Adv. Synth. Catal. 2011, 353, 1375–1383. [Google Scholar] [CrossRef]
- Abas, H.; Blencowe, P.; Brookfield, J.L.; Harwood, L.A. Selective Hydroxylation of C(sp(3) )-H Bonds in Steroids. Chemistry 2023, 29, e202301066. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pramanik, J.; Mahata, B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes Immun. 2021, 22, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, K.B. An intracrine view of sex steroids, immunity, and metabolic regulation. Mol. Metab. 2018, 15, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.S.; Gametchu, B. Membrane estrogen and glucocorticoid receptors—Implications for hormonal control of immune function and autoimmunity. Int. Immunopharmacol. 2001, 1, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Feng, J.; Chen, X.; Bao, Y.J.; Wang, Y.; Wu, Q.; Ma, Y.; Zhu, D. Distinct Regioselectivity of Fungal P450 Enzymes for Steroidal Hydroxylation. Appl. Environ. Microbiol. 2019, 85, e01182-19. [Google Scholar] [CrossRef]
- He, P.; Li, H.; Sun, J.; Zhang, X.; Gong, J.; Shi, J.; Xu, Z. Identification of a fungal cytochrome P450 with steroid two-step ordered selective hydroxylation characteristics in Colletotrichum lini. J. Steroid Biochem. Mol. Biol. 2022, 220, 106096. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, P.; Zhao, J.; Chen, Y.; Li, X.; Huang, J.-W.; Zhang, L.; Li, Q.; Gao, C.; Xing, Q.; et al. Rationally Controlling Selective Steroid Hydroxylation via Scaffold Sampling of a P450 Family. ACS Catal. 2023, 13, 1280–1289. [Google Scholar] [CrossRef]
- Jeyaprakash, N.; Maeder, S.; Janka, H.; Stute, P. A systematic review of the impact of 7-keto-DHEA on body weight. Arch. Gynecol. Obstet. 2023, 308, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Templeton, J.F.; Kumar, V.P.; Bose, D.; Smyth, D.D.; Kim, R.S.; LaBella, F.S. Digitalis-like pregnanes. Cardiac and renal effects of a glycoside of 14 beta-hydroxyprogesterone. Can. J. Physiol. Pharmacol. 1988, 66, 1420–1424. [Google Scholar] [CrossRef]
- Lathe, R. Steroid and sterol 7-hydroxylation: Ancient pathways. Steroids 2002, 67, 967–977. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kumar, A.; Rohela, A.; Arya, V.; Gautam, A.K.; Sharma, H.; Rai, P.; Kumari, A.; Amarowicz, R. Traditional uses, hepatoprotective potential, and phytopharmacology of Tinospora cordifolia: A narrative review. J. Pharm. Pharmacol. 2024, 76, 183–200. [Google Scholar] [CrossRef]
- Reese, P.B. Remote functionalization reactions in steroids, discovery and application. Steroids 2024, 204, 109362. [Google Scholar] [CrossRef] [PubMed]
- Kollerov, V.; Shutov, A.; Kazantsev, A.; Donova, M. Hydroxylation of pregnenolone and dehydroepiandrosterone by zygomycete Backusella lamprospora VKM F-944: Selective production of 7alpha-OH-DHEA. Appl. Microbiol. Biotechnol. 2022, 106, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Liu, Y.J.; Ji, W.T.; Liu, K.; Gao, B.; Tao, X.Y.; Zhao, M.; Wang, F.Q.; Wei, D.Z. One-pot biosynthesis of 7beta-hydroxyandrost-4-ene-3,17-dione from phytosterols by cofactor regeneration system in engineered mycolicibacterium neoaurum. Microb. Cell Fact. 2022, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.; Borah, K.; Amin, B.; Griffiths, H.R.; Sassi, K.; Lizard, G.; Iriondo, A.; Martinez-Lage, P. Localisation of oxysterols at the sub-cellular level and in biological fluids. J. Steroid Biochem. Mol. Biol. 2019, 193, 105426. [Google Scholar] [CrossRef]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef]
- Hayashi, K.; Yasuda, K.; Sugimoto, H.; Ikushiro, S.; Kamakura, M.; Kittaka, A.; Horst, R.L.; Chen, T.C.; Ohta, M.; Shiro, Y.; et al. Three-step hydroxylation of vitamin D3 by a genetically engineered CYP105A1: Enzymes and catalysis. FEBS J. 2010, 277, 3999–4009. [Google Scholar] [CrossRef]
- Schmitz, L.M.; Kinner, A.; Althoff, K.; Rosenthal, K.; Lutz, S. Investigation of Vitamin D(2) and Vitamin D(3) Hydroxylation by Kutzneria albida. ChemBioChem 2021, 22, 2266–2274. [Google Scholar] [CrossRef]
- Putkaradze, N.; Litzenburger, M.; Hutter, M.C.; Bernhardt, R. CYP109E1 from Bacillus megaterium Acts as a 24- and 25-Hydroxylase for Cholesterol. ChemBioChem 2019, 20, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Rugor, A.; Tataruch, M.; Staron, J.; Dudzik, A.; Niedzialkowska, E.; Nowak, P.; Hogendorf, A.; Michalik-Zym, A.; Napruszewska, D.B.; Jarzebski, A.; et al. Regioselective hydroxylation of cholecalciferol, cholesterol and other sterol derivatives by steroid C25 dehydrogenase. Appl. Microbiol. Biotechnol. 2017, 101, 1163–1174. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Wang, Y.; Shao, M.; Xu, Z.; Rao, Z. Identification of a novel cytochrome P450 17A2 enzyme catalyzing the C17α hydroxylation of progesterone and its application in engineered Pichia pastoris. Biochem. Eng. J. 2022, 177, 108264. [Google Scholar] [CrossRef]
- Zielinska-Blajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives-Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 9078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Q.Y.; Ge, Y.; Huang, Z.Y.; Hong, R.; Li, A.; Xu, J.H.; Yu, H.L. Hydroxylases involved in terpenoid biosynthesis: A review. Bioresour. Bioprocess. 2023, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Tan, H.; Sultan, S.; Faridz, M.; Shah, M.; Nurfazilah, S.; Hussain, M. Microbial-Catalyzed Biotransformation of Multifunctional Triterpenoids Derived from Phytonutrients. Int. J. Mol. Sci. 2014, 15, 12027–12060. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Halitschke, R.; Li, D.; Paetz, C.; Su, H.; Heiling, S.; Xu, S.; Baldwin, I.T. Controlled hydroxylations of diterpenoids allow for plant chemical defense without autotoxicity. Science 2021, 371, 255–260. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, T.; Liu, Y.; Hou, G.; Wang, Q.; Zhao, F.; Li, F.; Meng, Q. Research Advances in Clinical Applications, Anticancer Mechanism, Total Chemical Synthesis, Semi-Synthesis and Biosynthesis of Paclitaxel. Molecules 2023, 28, 7517. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef]
- Cannazza, P.; Rabuffetti, M.; Donzella, S.; De Vitis, V.; Contente, M.L.; de Oliveira, M.d.C.F.; de Mattos, M.C.; Barbosa, F.G.; de Souza Oliveira, R.P.; Pinto, A.; et al. Whole cells of recombinant CYP153A6-E. coli as biocatalyst for regioselective hydroxylation of monoterpenes. AMB Express 2022, 12, 48. [Google Scholar] [CrossRef]
- Ly, T.T.; Khatri, Y.; Zapp, J.; Hutter, M.C.; Bernhardt, R. CYP264B1 from Sorangium cellulosum So ce56: A fascinating norisoprenoid and sesquiterpene hydroxylase. Appl. Microbiol. Biotechnol. 2012, 95, 123–133. [Google Scholar] [CrossRef]
- Cruz de Carvalho, T.; de Oliveira Silva, E.; Soares, G.A.; Parreira, R.L.T.; Ambrosio, S.R.; Jacometti Cardoso Furtado, N.A. Fungal biocatalysts for labdane diterpene hydroxylation. Bioprocess. Biosyst. Eng. 2020, 43, 1051–1059. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Ma, L.; Tu, L.; Hu, T.; Wu, X.; Su, P.; Zhao, Y.; Liu, Y.; Li, D.; et al. Tandemly duplicated CYP82Ds catalyze 14-hydroxylation in triptolide biosynthesis and precursor production in Saccharomyces cerevisiae. Nat. Commun. 2023, 14, 875. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Sun, Z.; Wang, D.; Qu, G.; Ma, X.; Fan, F.; Zhang, L.; Li, S.; Zhang, X. Identification of a novel cytochrome P450 enzyme that catalyzes the C-2α hydroxylation of pentacyclic triterpenoids and its application in yeast cell factories. Metab. Eng. 2019, 51, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Schewe, H.; Buchhaupt, M.; Holtmann, D.; Schrader, J. Efficient hydroxylation of 1,8-cineole with monoterpenoid-resistant recombinant Pseudomonas putida GS1. World J. Microbiol. Biotechnol. 2016, 32, 112. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Li, Y.; Meng, L.; Ouyang, J.; Jin, D.; Wu, L.; Zhang, X.; Jia, X.; You, S. “Mirror-Image” Manipulation of Curdione Stereoisomer Scaffolds by Chemical and Biological Approaches: Development of a Sesquiterpenoid Library. J. Nat. Prod. 2015, 78, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Q.; Cao, Y.; Liu, X.; Cheng, Z.-H. Regio- and stereo-selective hydroxylations of ingenane diterpenoids by Mortierella ramanniana and Gibberella fujikuroi. Chin. J. Nat. Med. 2016, 14, 939–945. [Google Scholar] [CrossRef]
- Bergman, M.E.; Franks, A.E.; Phillips, M.A. Biosynthesis, natural distribution, and biological activities of acyclic monoterpenes and their derivatives. Phytochem. Rev. 2022, 22, 361–384. [Google Scholar] [CrossRef]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in essential oils: Chemistry, classification, and potential impact on human health and industry. Phytomed. Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Nankai, H.; Miyazawa, M.; Kameoka, H. Hydroxylation of Two Saturated Acyclic Monoterpenoids, Tetrahydrogeraniol and Tetrahydrolavandulol, by the Plant Pathogenic Fungus Glomerella cingulata. J. Nat. Prod. 1997, 60, 287–289. [Google Scholar] [CrossRef]
- Davies, M.E.; Tsyplenkov, D.; Martin, V.J.J. Engineering Yeast for De Novo Synthesis of the Insect Repellent Nepetalactone. ACS Synth. Biol. 2021, 10, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Sintupachee, S.; Promden, W.; Ngamrojanavanich, N.; Sitthithaworn, W.; De-Eknamkul, W. Functional expression of a putative geraniol 8-hydroxylase by reconstitution of bacterially expressed plant CYP76F45 and NADPH-cytochrome P450 reductase CPR I from Croton stellatopilosus Ohba. Phytochemistry 2015, 118, 204–215. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Purriños, L.; Munekata, P.E.S.; Echegaray, N.; Lorenzo, J.M. Introduction and classification of lipids. In Food Lipids; Academic Press: New York, NY, USA, 2022; pp. 1–16. [Google Scholar]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Lim, S.A.; Su, W.; Chapman, N.M.; Chi, H. Lipid metabolism in T cell signaling and function. Nat. Chem. Biol. 2022, 18, 470–481. [Google Scholar] [CrossRef]
- Ibarguren, M.; Lopez, D.J.; Escriba, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Gimenez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Oh, D.K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yu, J.Q. Lactonization as a general route to beta-C(sp(3))-H functionalization. Nature 2020, 577, 656–659. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kobayashi, A.; Kohyama, A.; Sakai, H.; Matsuya, Y. Divinylcarbinol Desymmetrization Strategy: A Concise and Reliable Approach to Chiral Hydroxylated Fatty Acid Derivatives. J. Org. Chem. 2021, 86, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Löwe, J.; Gröger, H. Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts 2020, 10, 287. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, T.; Zhao, G.; Niu, W.; Guo, J.; Xian, M.; Liu, H. Metabolic engineering of Escherichia coli for the production of hydroxy fatty acids from glucose. BMC Biotechnol. 2016, 16, 26. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zheng, Y.; Zou, H.; Cao, Y.; Liu, H.; Liu, W.; Xian, M. Biosynthesis of long chain hydroxyfatty acids from glucose by engineered Escherichia coli. Bioresour. Technol. 2012, 114, 561–566. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, Y.; Shao, Z.; Wang, Y.; Guo, Z.; Gao, R.; Eser, B.E. Optimization and Engineering of a Self-Sufficient CYP102 Enzyme from Bacillus amyloliquefaciens towards Synthesis of In-Chain Hydroxy Fatty Acids. Catalysts 2021, 11, 665. [Google Scholar] [CrossRef]
- Gomez de Santos, P.; Gonzalez-Benjumea, A.; Fernandez-Garcia, A.; Aranda, C.; Wu, Y.; But, A.; Molina-Espeja, P.; Mate, D.M.; Gonzalez-Perez, D.; Zhang, W.; et al. Engineering a Highly Regioselective Fungal Peroxygenase for the Synthesis of Hydroxy Fatty Acids. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ko, Y.-J.; Park, J.-B.; Oh, D.-K. Biotransformation of C20- and C22-polyunsaturated fatty acids and fish oil hydrolyzates to R,R-dihydroxy fatty acids as lipid mediators using double-oxygenating 15R-lipoxygenase. Green Chem. 2024, 26, 4665–4676. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.-A.; Shin, K.C.; Park, J.-B.; Oh, D.-K. Efficient biotransformation of docosahexaenoic acid-rich oils into the lipid mediator resolvin D5 by cells expressing 15S-lipoxygenase using a bioreactor. Bioresour. Technol. 2023, 388, 129750. [Google Scholar] [CrossRef] [PubMed]
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharma-ceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sansinenea, E. Phenylpropanoid Derivatives and Their Role in Plants’ Health and as antimicrobials. Curr. Microbiol. 2023, 80, 380. [Google Scholar] [CrossRef]
- Koshak, A.E.; Elfaky, M.A.; Albadawi, D.A.I.; Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Alzain, A.A.; Khafagy, E.S.; Elsayed, E.M.; Hegazy, W.A.H. Piceatannol: A renaissance in antibacterial innovation unveiling synergistic potency and virulence disruption against serious pathogens. Int. Microbiol. 2024. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- de Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxid. Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef]
- Lv, Y.; Marsafari, M.; Koffas, M.; Zhou, J.; Xu, P. Optimizing Oleaginous Yeast Cell Factories for Flavonoids and Hydroxylated Flavonoids Biosynthesis. ACS Synth. Biol. 2019, 8, 2514–2523. [Google Scholar] [CrossRef]
- Wang, L.; Lui, A.C.W.; Lam, P.Y.; Liu, G.; Godwin, I.D.; Lo, C. Transgenic expression of flavanone 3-hydroxylase redirects flavonoid biosynthesis and alleviates anthracnose susceptibility in sorghum. Plant Biotechnol. J. 2020, 18, 2170–2172. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhao, X.; Zhou, J.; Li, J.; Chen, J.; Du, G. Efficient hydroxylation of flavonoids by using whole-cell P450 sca-2 biocatalyst in Escherichia coli. Front. Bioeng. Biotechnol. 2023, 11, 1138376. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, X.; Ruan, H.; Chen, Y.; Gao, L.; Lei, T.; Li, Y.; Gui, L.; Guo, L.; Xia, T.; et al. Optimization of the Biosynthesis of B-Ring Ortho-Hydroxy Lated Flavonoids Using the 4-Hydroxyphenylacetate 3-Hydroxylase Complex (HpaBC) of Escherichia coli. Molecules 2021, 26, 2919. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, H.; Lyu, Y.; Zeng, W.; Zhou, J. Enhanced Biosynthesis of Dihydromyricetin in Saccharomyces cerevisiae by Coexpression of Multiple Hydroxylases. J. Agric. Food Chem. 2020, 68, 14221–14229. [Google Scholar] [CrossRef]
- Jeong, H.C.; Cha, G.S.; Yun, C.-H.; Park, C.M. Production of eriodictyol and dihydrotricetin from naringenin by recombinant tyrosinase of Bacillus megaterium DY804 strain. Process. Biochem. 2024, 138, 111–119. [Google Scholar] [CrossRef]

| Types of Natural Products | Product | Chemical Structure | Host | Enzymes | Engineered Strategy | Titer | Reference |
|---|---|---|---|---|---|---|---|
| Hydroxylation of amino acids and their derivatives | L-DOPA |  | E. coli | Fn-TPL | Overexpression of a novel TPL | 110 g/L | [50] |
| L-DOPA |  | C. glutamicum | Ralstonia solanacearum tyrosinase | Heterologous expression of Ralstonia solanacearum tyrosinase in C. glutamicum | 0.26 g/L | [51] | |
| L-DOPA | 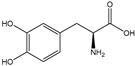 | E. coli | Eh-TPL | Enzyme evolution and high-throughput screening method | 69.1 g/L | [52] | |
| 5-HTP | 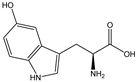 | E. coli | TPH1 | Heterologous expression of human tryptophan hydroxylase (TPH1) in E. coli | 0.02 g/L | [53] | |
| 5-HTP | 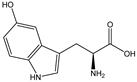 | E. coli | TPH1 | By protein engineering, manipulation of plasmid copy number, and fine-tuning of transcriptional regulation strategies | 5.1 g/L | [54] | |
| 5-HTP | 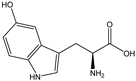 | E. coli | TPH1 | By rational design and molecular dynamics simulations | 0.91 g/L | [55] | |
| 4-HIL | 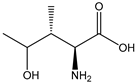 | E. coli | IDO | Dynamically regulate ODHC activity | 29.16 g/L | [56] | |
| (2S,3R,4S)-4-HIL |  | E. coli | IDO | A high-throughput screening method was developed | 80.8 g/L/d | [57] | |
| T-4-HYP |  | E. coli | P4H | Introduced a NOG pathway and redesigned key regulatory genes | 89.4 g/L | [58] | |
| T-4-HYP | 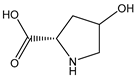 | E. coli | P4H | Heterologous expression of P4H from Dactylosporangium sp. strain RH1 in E. coli | 41.0 g/L | [59] | |
| 4-HIL |  | C. glutamicum | IDO | By employing L-isoleucine-responsive transcriptional regulation or attenuation strategies | 34.21 g/L | [60] | |
| Hydroxylation of steroids and their derivatives | Testosterone |  | P. pastoris | 17β-HSD3 | Optimization of the gene codons of human 17β-HSD3 | 11.6 g/L | [61] |
| 2α-Hydroxylated steroids | 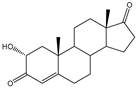 | E. coli | CYP154C2 | Rational engineering | 15–20 mg/L | [32] | |
| 11α-OH-4AD |  | S. cerevisiae or Aspergillus oryzae | CYP68N1 | Introducing a rapid identification strategy for filamentous fungi P450 enzymes | 0.845 g/L | [33] | |
| 14α-OH-AD, 14α-OH-RSS | 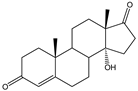 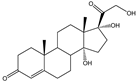 | S. cerevisiae | P-450lun | Increasing the copies of P-450lun and CPRlun | 150 mg/L, 64 mg/L | [62] | |
| Hydroxylation of terpenoids and their derivatives | 2-Hydroxy-dehydroabietic acid | 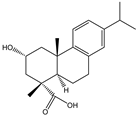 | S. cerevisiae | CYP72D19 | Molecular docking and site-directed mutagenesis were used | - | [63] |
| Trihydroxylated diterpene cyclooctatin | 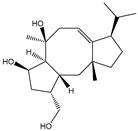 | E. coli | P450s CotB3/CotB4 | A new reductase/ferredoxin system was identified and characterized | 15 mg/L | [64] | |
| Ursolic acid, oleanolic acid, asiatic acid, madecassic acid, arjunolic acid | 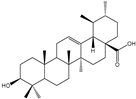   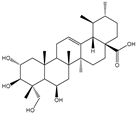 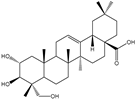 | Yarrowia lipolytica | CaCYP716C11p, CaCYP714E19p, and CaCYP716E41p | Reconstructing the metabolic pathways in Y. lipolytica | 11.6 mg/g, 10.2 mg/g, 0.12 mg/g, 8.9 mg/g, 4.4 mg/g | [65] | |
| 6β-Hydroxy-α-amyrin, 6β-hydroxy-β-amyrin, (2α,3β)-urs-12-ene-2,3-diol, (2α,3β)-olean-12-ene-2,3-diol, uvaol, erythrodiol |  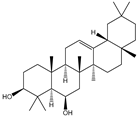 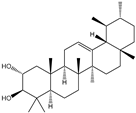 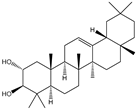 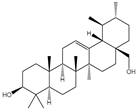 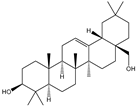 | S. cerevisiae | EcOSC, CYP716E60, CYP716C80, CYP716A86, and CYP716A862 | Identified a oxidosqualene cyclase (EcOSC) gene and four CYP716 genes | - | [66] | |
| Hydroxylation of lipids and their derivatives | 9,10-Dihydroxyhexadecanoic |  | E. coli | FAD, EH, EPOX | The heterologous expression of fatty acid hydroxylases | - | [67] |
| ω-HFAs | 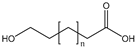 | S. cerevisiae | CYP52M1 | Constructing a self-sufficient cytochrome P450 enzyme system | 347 mg/L | [68] | |
| ω-HPA | 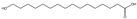 | E. coli | CYP153A, camA/camB | By enhancing fatty acid synthesis, blocking the β-oxidation pathway, and optimizing NADH supply | 610 mg/L | [69] | |
| Hydroxylation of phenylpropanoids and their derivatives | Esculetin, piceatannol |   | E. coli | HpaBC | Overexpression of HpaBC | 2.7 g/L, 1.2 g/L. | [70] |
| Caffeic acid |  | S. cerevisiae | 4HPA3H | A functional 4HPA3H was constructed to replace plant-derived cytochrome P450 enzymes | 289.4 mg/L | [71] | |
| Eriodictyol, catechin, caffeic acid | 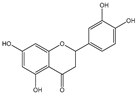  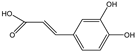 | E. coli | HpaBC | Optimization of media, induction temperature, induction point, and substrate delay time | 62.7 mg/L, 34.7 mg/L, 3.5 g/L | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Zeng, R.; Chen, T.; Yang, Y.; Song, Y.; Li, Q.; Cheng, J.; Liu, B. Recent Advances and Challenges in the Production of Hydroxylated Natural Products Using Microorganisms. Fermentation 2024, 10, 604. https://doi.org/10.3390/fermentation10120604
Sun C, Zeng R, Chen T, Yang Y, Song Y, Li Q, Cheng J, Liu B. Recent Advances and Challenges in the Production of Hydroxylated Natural Products Using Microorganisms. Fermentation. 2024; 10(12):604. https://doi.org/10.3390/fermentation10120604
Chicago/Turabian StyleSun, Chang, Rumei Zeng, Tianpeng Chen, Yibing Yang, Yi Song, Qiang Li, Jie Cheng, and Bingliang Liu. 2024. "Recent Advances and Challenges in the Production of Hydroxylated Natural Products Using Microorganisms" Fermentation 10, no. 12: 604. https://doi.org/10.3390/fermentation10120604
APA StyleSun, C., Zeng, R., Chen, T., Yang, Y., Song, Y., Li, Q., Cheng, J., & Liu, B. (2024). Recent Advances and Challenges in the Production of Hydroxylated Natural Products Using Microorganisms. Fermentation, 10(12), 604. https://doi.org/10.3390/fermentation10120604







