Characterization of Novel Exopolysaccharides from Weissella cibaria and Lactococcus lactis Strains and Their Potential Application as Bio-Hydrocolloid Agents in Emulsion Stability
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Sampling and Isolation of LAB
2.3. Screening of EPS-Producing LAB
2.4. Identification of the EPS-Producing LAB
2.5. EPS Characterization
2.5.1. EPS Quantification and Total Protein Assay
2.5.2. UV–Vis Analysis
2.5.3. FTIR Spectrometer
2.6. Techno-Functional Properties
2.6.1. Zeta Potential Measurement
2.6.2. Measurement of EPS Solubility
2.6.3. Interfacial Properties
2.7. Multilayer Emulsion Systems Prepared Using EPS Fractions
2.7.1. Solution Preparation
2.7.2. Emulsion Preparation
2.7.3. Droplet Size and Zeta Potential Measurements
2.8. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Preliminary Identification of LAB
3.2. Screening of EPS-Producing Strains
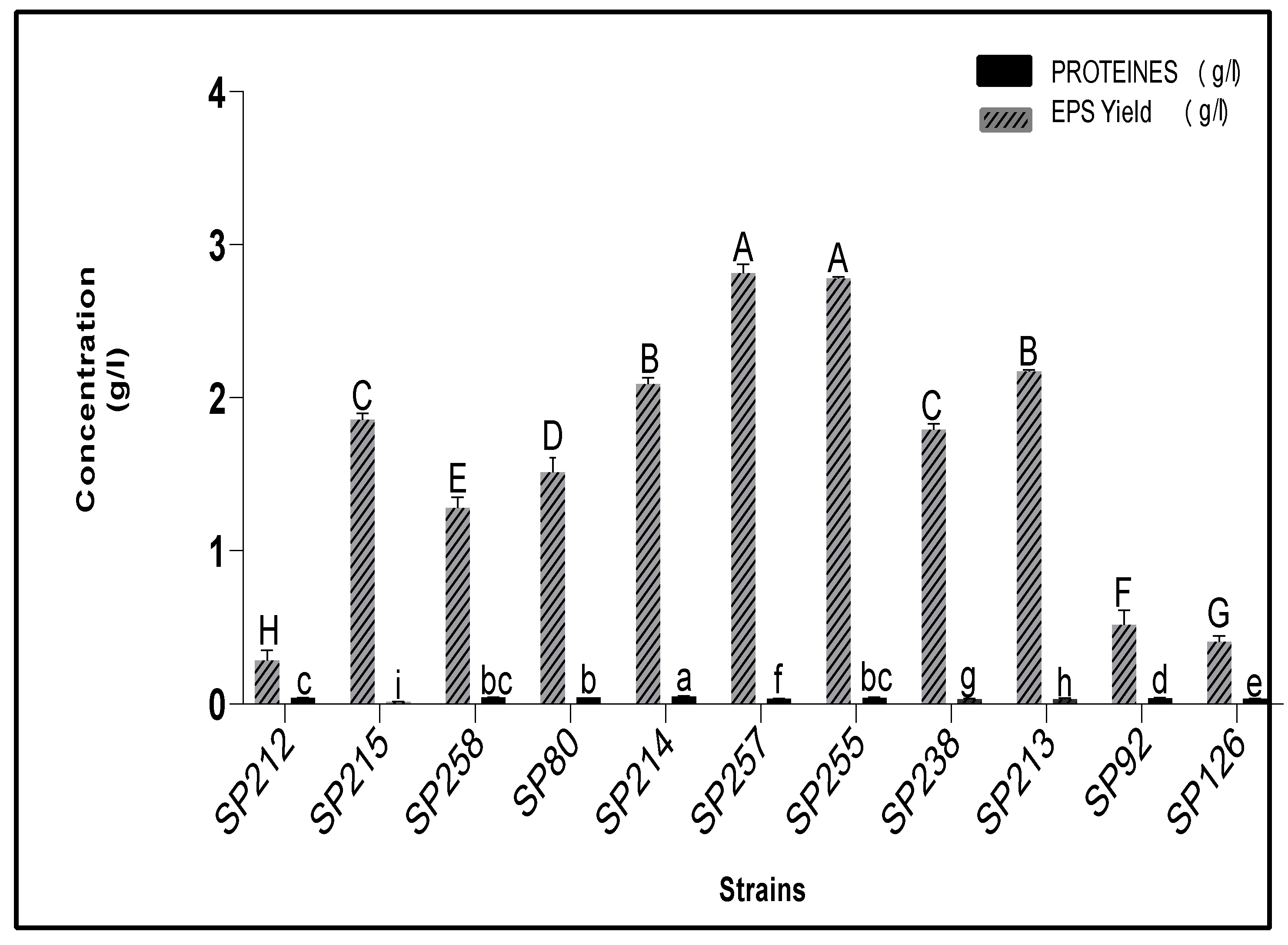
3.3. Estimation of Carbohydrates and Protein Contents of Crude EPS Extracts
3.4. Molecular Identification of SP213, SP255, and SP257 Strains
3.5. UV-Visible Analyses
3.6. Chemical Structure of EPS
3.7. Zeta Potential Measurement (ζ)
3.8. EPS Solubility
3.9. Interfacial Properties
3.10. Formation of Multilayer Emulsions
3.10.1. Zeta Potential Analysis
3.10.2. Particle Size Measurements
3.10.3. Influence of EPS Concentration on Emulsion Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPS | Exopolysaccharide |
| IEP | Isoelectric Point |
| LAB | Lactic Acid Bacteria |
| CAGR | Compound Annual Growth |
| HoPS | Homopolysaccharides |
| HeEPS | Heteropolysaccharides |
| MRS | de Man, Rogosa, and Sharpe |
| UV | Ultraviolet |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| CAS | Colony Absorption Spectroscopy |
| BSA | Bovine Serum Albumin |
References
- Singh, S. Natural Food Additives Market Size, Share, Trends. Industry Reports. Available online: https://www.marketresearchfuture.com/reports/natural-food-additives (accessed on 24 August 2024).
- Ramos, I.M.; Seseña, S.; Poveda, J.M.; Palop, M.L. Screening of Lactic Acid Bacteria Strains to Improve the Properties of Non-fat Set Yogurt by in situ EPS Production. Food Bioprocess Technol. 2023, 16, 2541–2558. [Google Scholar] [CrossRef]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and health-promoting properties of probiotics’ exopolysaccharides; isolation, characterization, and applications in the food industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 8194–8225. [Google Scholar] [CrossRef]
- Fuso, A.; Bancalari, E.; Castellone, V.; Caligiani, A.; Gatti, M.; Bottari, B. Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods 2023, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, O.N.; Sasmal, S.; Kataria, A.K.; Devi, I. Application of microbial extracellular carbohydrate polymeric substances in food and allied industries. 3 Biotech 2020, 10, 221. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, F.; Zhu, X.; Zhang, C.; Jiang, M.; Lu, Z. An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Int. J. Biol. Macromol. 2020, 143, 775–784. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from lactic acid bacteria, as an alternative to antibiotics, on regulation of intestinal health and the immune system. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef]
- Abid, Y.; Casillo, A.; Gharsallah, H.; Joulak, I.; Lanzetta, R.; Corsaro, M.M.; Attia, H.; Azabou, S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int. J. Biol. Macromol. 2018, 108, 719–728. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Derdak, R.; Sakoui, S.; Pop, O.L.; Vodnar, D.C.; Elmakssoudi, A.; Errachidi, F.; Suharoschi, R.; Soukri, A.; El Khalfi, B.; AddoumElmakssoudi, B. Screening, optimization and characterization of exopolysaccharides produced by novel strains isolated from Moroccan raw donkey milk. Food Chem. X 2022, 14, 100305. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, G.; van Berkum, P.; Amarger, N.; Prévost, D. Genetic diversity of rhizobial symbionts isolated from legume species within the genera Astragalus, Oxytropis, and Onobrychis. Appl. Environ. Microbiol. 1997, 63, 4748–4758. [Google Scholar] [CrossRef] [PubMed]
- Oussaief, O.; Jrad, Z.; Sbissi, I.; Nasri, W.; Khorchani, T.; El-Hatmi, H. Technological and probiotic potential of autochthonous lactic acid bacteria from spontaneously fermented dromedary milk. J. Food Process. Preserv. 2020, 44, e14685. [Google Scholar] [CrossRef]
- Ziadi, M.; Bouzaiene, T.; M’hir, S.; Zaafouri, K.; Mokhtar, F.; Hamdi, M.; Boisset-Helbert, C. Evaluation of the Efficiency of Ethanol Precipitation and Ultrafiltration on the Purification and Characteristics of Exopolysaccharides Produced by Three Lactic Acid Bacteria. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hamidi, M.; Okoro, O.V.; Ianiri, G.; Jafari, H.; Rashidi, K.; Ghasemi, S.; Castoria, R.; Palmieri, D.; Delattre, C.; Pierre, G.; et al. Exopolysaccharide from the yeast Papiliotrema terrestris PT22AV for skin wound healing. J. Adv. Res. 2023, 46, 61–74. [Google Scholar] [CrossRef]
- Liao, W.; Gharsallaoui, A.; Dumas, E.; Elaissari, A. Understanding of the key factors influencing the properties of emulsions stabilized by sodium caseinate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5291–5317. [Google Scholar] [CrossRef]
- Karakas-Sen, A.; Karakas, E. Isolation, identification and technological properties of lactic acid bacteria from raw cow milk. Biosci. J. 2018, 34, 985–999. [Google Scholar] [CrossRef]
- Zantar, S.; El Galiou, O.; Zerrouk, H.M.; Laglaoui, A. Elaboration d’un fromage de chèvre semi-affiné à partir d’une sélection de souches lactiques autochtones isolées du lait de chèvres du Nord du FAO. Options Méditerranéennes Série A 2014, 108, 191–197. [Google Scholar]
- Shokryazdan, P.; Liang, J.B.; Abdullah, N.; Jahromi, M.F. Probiotic Potential of Lactic Acid Bacteria Isolated From Mulberry Silage. J. Pure Appl. Microbiol. 2015, 9, 443–452. [Google Scholar]
- Stephen, J.M.; Saleh, A.M. Homofermentative Lactobacilli isolated from organic sources exhibit potential ability of lactic acid production. Front. Microbiol. 2023, 14, 1297036. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Wadehra, A.; Munjal, K.; Behare, P. Isolation of exopolysaccharides producing lactic acid bacteria from dairy products. Asian J. Dairy Food Res. 2015, 34, 280–284. [Google Scholar] [CrossRef]
- Taj, R.; Masud, T.; Sohail, A.; Sammi, S.; Naz, R.; Khanal, B.K.S.; Nawaz, M.A. In vitro screening of EPS-producing Streptococcus thermophilus strains for their probiotic potential from Dahi. Food Sci. Nutr. 2022, 10, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Benhadria, M.K.; Touil, M.A.T.; Meddah, B. Optimization of production of Microbial Exopolysaccharides (EPS) with essential oils from two medicinal plants. J. Appl. Biosci. 2017, 111, 10925. [Google Scholar] [CrossRef]
- Almalki, M.A. Exopolysaccharide production by a new Lactobacillus lactis isolated from the fermented milk and its antioxidant properties. J. King Saud Univ. Sci. 2020, 32, 1272–1277. [Google Scholar] [CrossRef]
- Şentürk, D.Z.; Dertli, E.; Erten, H.; Şimşek, Ö. Structural and technological characterization of ropy exopolysaccharides produced by Lactobacillus plantarum strains isolated from Tarhana. Food Sci. Biotechnol. 2020, 29, 121–129. [Google Scholar] [CrossRef]
- Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703. [Google Scholar] [CrossRef]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the exemplary knowledge of microbial exopolysaccharides in a nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Shetty, P.H. Overview of exopolysaccharides produced by Weissella genus—A review. Int. J. Biol. Macromol. 2020, 164, 2964–2973. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Fguiri, I.; Ziadi, M.; Atigui, M.; Ayeb, N.; Arroum, S.; Assadi, M.; Khorchani, T. Isolation and characterisation of lactic acid bacteria strains from raw camel milk for potential use in the production of fermented Tunisian dairy products. Int. J. Dairy Technol. 2015, 69, 103–113. [Google Scholar] [CrossRef]
- Saleena, L.A.K.; Teo, M.Y.M.; How, Y.H.; In, L.L.A.; Pui, L.P. Immunomodulatory action of Lactococcus lactis. J. Biosci. Bioeng. 2023, 135, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Li, J.; Ju, X.; Wang, L. Impact of exopolysaccharides-producing lactic acid bacteria on the chemical, rheological properties of buckwheat sourdough and the quality of buckwheat bread. Food Chem. 2023, 425, 136369. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, D.; Özcan, A.; Yibar, A.; Dertli, E. Genetic diversity and phylogenetic relationships of Streptococcus thermophilus isolates from traditional Turkish yogurt: Multilocus sequence typing (MLST). Arch. Microbiol. 2024, 206, 121. [Google Scholar] [CrossRef]
- Power, A.C.; Chapman, J.; Chandra, S.; Cozzolino, D. Ultraviolet-visible spectroscopy for food quality analysis. In Evaluation Technologies for Food Quality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–104. [Google Scholar] [CrossRef]
- Giglio, C.; Yang, Y.; Kilmartin, P. Analysis of phenolics in New Zealand Pinot noir wines using UV-visible spectroscopy and chemometrics. J. Food Compos. Anal. 2023, 117, 105106. [Google Scholar] [CrossRef]
- Dash, H.R.; Shrivastava, P.; Das, S. Quantification of DNA by Using UV–Visible Spectrophotometer. In Principles and Practices of DNA Analysis: A Laboratory Manual for Forensic DNA Typing, in Springer Protocols Handbooks; Springer: New York, NY, USA, 2020; pp. 127–132. [Google Scholar] [CrossRef]
- Passos, M.L.; Saraiva, M.L.M. Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Bhat, V.T.; James, N.R.; Jayakrishnan, A. A photochemical method for immobilization of azidated dextran onto aminated poly(ethylene terephthalate) surfaces. Polym. Int. 2008, 57, 124–132. [Google Scholar] [CrossRef]
- Spangenberg, M.; Bryant, J.I.; Gibson, S.J.; Mousley, P.J.; Ramachers, Y.; Bell, G.R. Ultraviolet absorption of contaminants in water. Sci. Rep. 2021, 11, 3682. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Joulak, I.; Casillo, A.; Lanzetta, R.; Corsaro, M.M.; Gharsallaoui, A.; Attia, H. Potential biotechnological properties of an exopolysaccharide produced by newly isolated Bacillus tequilensis-GM from spontaneously fermented goat milk. LWT 2019, 105, 135–141. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Mishra, A.; Clark, J.; Farmer, T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. Comptes Rendus Chim. 2018, 21, 112–130. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Han, Y.; Zhou, Z. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int. J. Biol. Macromol. 2019, 141, 21–28. [Google Scholar] [CrossRef]
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Pichyangkura, R.; Nepogodiev, S.A.; Wonganan, P.; Mahalapbutr, P.; Field, R.A. Characterization of a nanoparticulate exopolysaccharide from Leuconostoc holzapfelii KM01 and its potential application in drug encapsulation. Int. J. Biol. Macromol. 2021, 187, 690–698. [Google Scholar] [CrossRef]
- Maajid, H.S.; Nurliyani, N.; Widodo, W. Exopolysaccharide production in fermented milk using Lactobacillus casei strains AP and AG. AIMS Microbiol. 2022, 8, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, I.C.F.; Ferreira, J.d.A.S.; Crugeira, P.J.L.; Oliveira, I.M.d.S.; dos Santos, J.N.; Matos, J.B.T.L.; Pinheiro, A.L.B.; de Almeida, P.F. Oilfield Carbonated Produced Water Recycling Coupled to Exopolysaccharide Transformation by Lelliottia amnigena. Waste Biomass Valorization 2024, 15, 1309–1322. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, T.; Wei, H.; Zhang, M.; Zou, Y.; Mao, G.; Wu, X. Carboxymethylation of polysaccharides from Auricularia auricula and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2011, 49, 1124–1130. [Google Scholar] [CrossRef]
- Hu, S.-M.; Zhou, J.-M.; Zhou, Q.-Q.; Li, P.; Xie, Y.-Y.; Zhou, T.; Gu, Q. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus rhamnosus ZFM231 isolated from milk. LWT 2021, 147, 111561. [Google Scholar] [CrossRef]
- Amiri, S.; Reza, M.; Sowti, K.M.; Alizadeh, M. Optimization of food-grade medium for co-production of bioactive substances by Lactobacillus acidophilus LA-5 for explaining pharmabiotic mechanisms of probiotic. J. Food Sci. Technol. 2021, 58, 1–12. [Google Scholar] [CrossRef]
- Kansandee, W.; Moonmangmee, D.; Moonmangmee, S.; Itsaranuwat, P. Characterization and Bifidobacterium sp. growth stimulation of exopolysaccharide produced by Enterococcus faecalis EJRM152 isolated from human breast milk. Carbohydr. Polym. 2019, 206, 102–109. [Google Scholar] [CrossRef]
- Naibaho, J.; Butula, N.; Jonuzi, E.; Korzeniowska, M.; Chodaczek, G.; Yang, B. The roles of brewers’ spent grain derivatives in coconut-based yogurt-alternatives: Microstructural characteristic and the evaluation of physico-chemical properties during the storage. Curr. Res. Food Sci. 2022, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Kim, T.J.; Kim, M.J.; Yoo, J.Y.; Kim, J.H. Isolation of Exopolysaccharide-Producing Lactic Acid Bacteria from Pa-Kimchi and Characterization of Exopolysaccharides. Microbiol. Biotechnol. Lett. 2023, 51, 157–166. [Google Scholar] [CrossRef]
- Jiang, G.; Gan, L.; Li, X.; He, J.; Zhang, S.; Chen, J.; Zhang, R.; Xu, Z.; Tian, Y. Characterization of Structural and Physicochemical Properties of an Exopolysaccharide Produced by Enterococcus sp. F2 From Fermented Soya Beans. Front. Microbiol. 2021, 12, 744007. [Google Scholar] [CrossRef]
- Delvart, A.; Moreau, C.; Cathala, B. Dextrans and dextran derivatives as polyelectrolytes in layer-by-layer processing materials —A review. Carbohydr. Polym. 2022, 293, 119700. [Google Scholar] [CrossRef]
- Boukhelata, N.; Taguett, F.; Kaci, Y. Characterization of an extracellular polysaccharide produced by a Saharan bacterium Paenibacillus tarimensis REG 0201M. Ann. Microbiol. 2018, 69, 93–106. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Guo, Z.; Zheng, Y.; Rea, M.C.; Su, H.; Zheng, X.; Zheng, B.; Miao, S. Using polysaccharides for the enhancement of functionality of foods: A review. Trends Food Sci. Technol. 2019, 86, 311–327. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal extracellular polymeric substances and their interactions with metal(loid)s: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Joulak, I.; Azabou, S.; Finore, I.; Poli, A.; Nicolaus, B.; Donato, P.D.; Bkhairia, I.; Dumas, E.; Gharsallaoui, A.; Immirzi, B.; et al. Structural characterization and functional properties of novel exopolysaccharide from the extremely halotolerant Halomonas elongata S6. Int. J. Biol. Macromol. 2020, 164, 95–104. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, H.; Liu, W.; Zheng, H.; Wu, H.; Guan, Y.; Lu, Q.; Lv, Z. Effects of EPS-producing Leuconostoc mesenteroides XR1 on texture, rheological properties, microstructure and volatile flavor of fermented milk. Food Biosci. 2023, 56, 103371. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.M.; Pluta, A.S.; Garbowska, M.; Stasiak-Różańska, L. Exopolysaccharide-Producing Lactic Acid Bacteria—Health-Promoting Properties and Application in the Dairy Industry. Postępy Mikrobiol. Adv. Microbiol. 2019, 58, 191–204. [Google Scholar] [CrossRef]
- Sabbah, M.; Esposito, M. Insight into Zeta Potential Measurements in Biopolymer Film Preparation. J. Biotechnol. Biomater. 2016, 6, 2–4. [Google Scholar] [CrossRef]
- Kamarajan, B.P.; Ananthasubramanian, M. Nanoemulsion-based antimicrobial systems. In Antimicrobial Nanosystems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 61–78. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Abdelsadig, M.S.; Conway, B.R.; Merchant, H.A. Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int. J. Pharm. X 2019, 1, 100024. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Characterization of pharmaceutical nanocarriers: In vitro and in vivo studies. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–58. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers 2022, 14, 3986. [Google Scholar] [CrossRef] [PubMed]
- Mende, S.; Rohm, H.; Jaros, D. Influence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int. Dairy J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Schmidt, W.; Brouwers, H.J.H.; Kühne, H.-C.; Meng, B. Interactions of polysaccharide stabilising agents with early cement hydration without and in the presence of superplasticizers. Constr. Build. Mater. 2017, 139, 584–593. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Li, L. The relationship between charge intensity and bioactivities/processing characteristics of exopolysaccharides from lactic acid bacteria. LWT 2022, 153, 112345. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and Solubility. In Solubility of Polysaccharides; Xu, Z., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Castellane, T.C.L.; Persona, M.R.; Campanharo, J.C.; Lemos, E.G.d.M. Production of exopolysaccharide from rhizobia with potential biotechnological and bioremediation applications. Int. J. Biol. Macromol. 2015, 74, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; El Enshasy, H.A.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides Producing Bacteria for the Amelioration of Drought Stress in Wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Lobo, R.E.; Gómez, M.I.; de Valdez, G.F.; Torino, M.I. Physicochemical and antioxidant properties of a gastroprotective exopolysaccharide produced by Streptococcus thermophilus CRL1190. Food Hydrocoll. 2019, 96, 625–633. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Y.; Cai, Z.; Guo, Y.; Wu, M.; Wang, P.; Li, R.; Zhang, H. Interfacial and emulsion characterisation of chemically modified polysaccharides through a multiscale approach. J. Colloid Interface Sci. 2020, 580, 480–492. [Google Scholar] [CrossRef]
- Liu, J.; Tan, J.; Hua, X.; Jiang, Z.; Wang, M.; Yang, R.; Cao, Y. Interfacial properties of ultrahigh methoxylated pectin. Int. J. Biol. Macromol. 2020, 152, 403–410. [Google Scholar] [CrossRef]
- Elmanan, M.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia exudate gums: Part VI. Interfacial rheology of Acacia senegal and Acacia seyal. Food Hydrocoll. 2008, 22, 682–689. [Google Scholar] [CrossRef]
- Schroën, K.; de Ruiter, J.; Berton-Carabin, C. The Importance of Interfacial Tension in Emulsification: Connecting Scaling Relations Used in Large Scale Preparation with Microfluidic Measurement Methods. ChemEngineering 2020, 4, 63. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Yang, L.; Liu, H. Preparations, application of polysaccharide–protein nanoparticles and their assembly at the oil–water interface. Food Sci. Biotechnol. 2024, 33, 13–22. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Cases, E.; Voilley, A.; Cayot, P. Utilisation of pectin coating to enhance spray-dry stability of pea protein-stabilised oil-in-water emulsions. Food Chem. 2010, 122, 447–454. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Rubilar, M.; Morales, E.; Medina, C.; Acevedo, F.; Marqués, A.M.; Shene, C. Naturally occurring protein–polysaccharide complexes from linseed (Linum usitatissimum) as bioemulsifiers. Eur. J. Lipid Sci. Technol. 2016, 118, 165–174. [Google Scholar] [CrossRef]
- Liao, W.; Dumas, E.; Elaissari, A.; Gharsallaoui, A. The formation mechanism of multilayer emulsions studied by isothermal titration calorimetry and dynamic light scattering. Food Hydrocoll. 2023, 136, 108275. [Google Scholar] [CrossRef]
- Wang, J.; Souihi, S.; Ben Amara, C.; Dumas, E.; Gharsallaoui, A. Influence of low methoxyl pectin on the physicochemical properties of sodium caseinate-stabilized emulsions. J. Food Process Eng. 2018, 41, e12906. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.-L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Interfacial deposition of an anionic polysaccharide (fucoidan) on protein-coated lipid droplets: Impact on the stability of fish oil-in-water emulsions. Food Hydrocoll. 2015, 51, 252–260. [Google Scholar] [CrossRef]
- Shi, F.; Chang, Y.; Shen, J.; Chen, G.; Xue, C. A comparative investigation of anionic polysaccharides (sulfated fucan, ι-carrageenan, κ-carrageenan, and alginate) on the fabrication, stability, rheology, and digestion of multilayer emulsion. Food Hydrocoll. 2023, 134, 108081. [Google Scholar] [CrossRef]
- McClements, D.J. Theoretical Analysis of Factors Affecting the Formation and Stability of Multilayered Colloidal Dispersions. Langmuir 2005, 21, 9777–9785. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, M.; McClements, D.J. Improving the stability of wheat protein-stabilized emulsions: Effect of pectin and xanthan gum addition. Food Hydrocoll. 2015, 43, 377–387. [Google Scholar] [CrossRef]
- Sheng, B.; Li, L.; Zhang, X.; Jiao, W.; Zhao, D.; Wang, X.; Wan, L.; Li, B.; Rong, H. Physicochemical Properties and Chemical Stability of β-Carotene Bilayer Emulsion Coated with Bovine Serum Albumin and Arabic Gum Compared to Monolayer Emulsions. Molecules 2018, 23, 495. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Dickinson, E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2019, 96, 209–223. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Decker, E.A.; McClements, D.J. Competitive Adsorption of Mixed Anionic Polysaccharides at the Surfaces of Protein-Coated Lipid Droplets. Langmuir 2009, 25, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Surber, G.; Spiegel, T.; Dang, B.P.; Pombo, A.W.; Rohm, H.; Jaros, D. Cream cheese made with exopolysaccharide-producing Lactococcus lactis: Impact of strain and curd homogenization pressure on texture and syneresis. J. Food Eng. 2021, 308, 110664. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Rad, A.H.; Ghanbarzadeh, B.; Torbati, M.; Falcone, P.M. The emulsifying and foaming properties of Amuniacum gum (Dorema ammoniacum) in comparison with gum Arabic. Food Sci. Nutr. 2020, 8, 3716–3730. [Google Scholar] [CrossRef] [PubMed]






| Strain | Origin | EPS Production | Identification | Accession Number |
|---|---|---|---|---|
| SP80 | raw camel milk | + | Weissella cibaria | PP911463 |
| SP92 | raw camel milk | + | Leuconostoc mesenteroides subsp. | - |
| SP126 | fermented camel milk | + | Weissella cibaria | - |
| SP212 | fermented goat milk | + | Leuconostoc mesenteroides subsp. | PP911461 |
| SP213 | fermented goat milk | + | Weissella cibaria | PP911457 |
| SP214 | fermented goat milk | + | Leuconostoc mesenteroides subsp. | PP911462 |
| SP215 | fermented goat milk | + | Weissella cibaria | - |
| SP238 | raw sheep milk | + | Weissella cibaria | PP911460 |
| SP255 | raw camel milk | + | Lactococcus lactis subsp. Lactis | PP911458 |
| SP257 | raw camel milk | + | Lactococcus lactis subsp. Lactis | PP911459 |
| SP258 | raw camel milk | + | Leuconostoc mesenteroides subsp. | - |
| Emulsion | D (4.3) (μm) |
|---|---|
| Primary emulsion | |
| 2 wt % CAS, 10 wt % oil | 22.70 ± 0.95 |
| Secondary emulsion | |
| 0.75 wt % CAS/EPS 257 | 55.90 ± 2.72 |
| 1.25 wt % CAS/EPS 257 | 55.20 ± 2.24 |
| 1.7 wt % CAS/EPS 257 | 31.24 ± 2.27 |
| 0.75 wt % CAS/EPS 255 | 68.16 ± 4.27 |
| 1.5 wt % CAS/EPS 255 | 36.30 ± 1.37 |
| 1.25 wt % CAS/EPS213 | 47.13 ± 3.25 |
| 1.7 wt % CAS/EPS213 | 56.55 ± 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammouri, A.; Ziadi, M.; Gharsallaoui, A.; Fguiri, I.; Sbissi, I.; Hammadi, M.; Khorchani, T. Characterization of Novel Exopolysaccharides from Weissella cibaria and Lactococcus lactis Strains and Their Potential Application as Bio-Hydrocolloid Agents in Emulsion Stability. Fermentation 2024, 10, 532. https://doi.org/10.3390/fermentation10100532
Zammouri A, Ziadi M, Gharsallaoui A, Fguiri I, Sbissi I, Hammadi M, Khorchani T. Characterization of Novel Exopolysaccharides from Weissella cibaria and Lactococcus lactis Strains and Their Potential Application as Bio-Hydrocolloid Agents in Emulsion Stability. Fermentation. 2024; 10(10):532. https://doi.org/10.3390/fermentation10100532
Chicago/Turabian StyleZammouri, Amal, Manel Ziadi, Adem Gharsallaoui, Imen Fguiri, Imed Sbissi, Mohamed Hammadi, and Touhami Khorchani. 2024. "Characterization of Novel Exopolysaccharides from Weissella cibaria and Lactococcus lactis Strains and Their Potential Application as Bio-Hydrocolloid Agents in Emulsion Stability" Fermentation 10, no. 10: 532. https://doi.org/10.3390/fermentation10100532
APA StyleZammouri, A., Ziadi, M., Gharsallaoui, A., Fguiri, I., Sbissi, I., Hammadi, M., & Khorchani, T. (2024). Characterization of Novel Exopolysaccharides from Weissella cibaria and Lactococcus lactis Strains and Their Potential Application as Bio-Hydrocolloid Agents in Emulsion Stability. Fermentation, 10(10), 532. https://doi.org/10.3390/fermentation10100532







