Clavulanic Acid Overproduction: A Review of Environmental Conditions, Metabolic Fluxes, and Strain Engineering in Streptomyces clavuligerus
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Operational Conditions during Submerging of Cultures of S. clavuligerus
3.2. Carbon Sources Used for High Clavulanic Acid Titers in Submerged Cultures
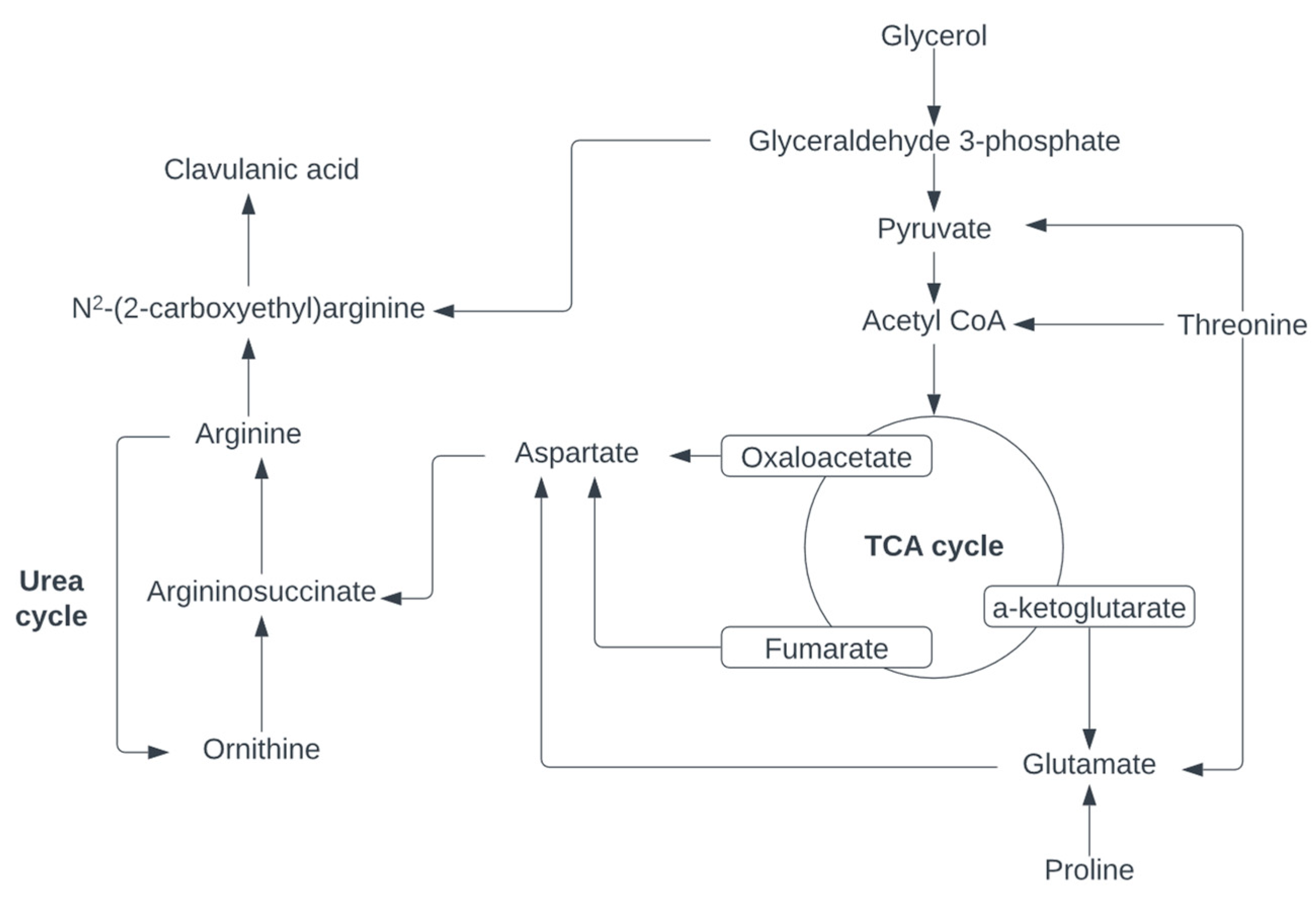
3.3. Amino Acids and Complex Nitrogen Sources for Enhancing Clavulanic Acid Biosynthesis
3.4. Mutant and Engineered S. clavuligerus Strains for Clavulanic Acid Overproduction
4. Discussion
5. Concluding Remarks
6. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paradkar, A. Clavulanic Acid Production by Streptomyces clavuligerus: Biogenesis, Regulation and Strain Improvement. J. Antibiot. 2013, 66, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Liras, P.; Martín, J.F. Streptomyces clavuligerus: The Omics Era. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab072. [Google Scholar] [CrossRef] [PubMed]
- López-Agudelo, V.A.; Gómez-Ríos, D.; Ramirez-Malule, H. Clavulanic Acid Production by Streptomyces clavuligerus: Insights from Systems Biology, Strain Engineering, and Downstream Processing. Antibiotics 2021, 10, 84. [Google Scholar] [CrossRef]
- Ser, H.-L.; Law, J.W.-F.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Fermentation Conditions That Affect Clavulanic Acid Production in Streptomyces clavuligerus: A Systematic Review. Front. Microbiol. 2016, 7, 522. [Google Scholar] [CrossRef]
- Agudelo-Arenas, Y.; Ríos-Estepa, R.; Gómez-Ríos, D. Evaluation of Clavulanic Acid Recovery Techniques from Streptomyces clavuligerus Cultivation Broths. J. Appl. Pharm. Sci. 2024, 14, 52–60. [Google Scholar] [CrossRef]
- Song, J.Y.; Jensen, S.E.; Lee, K.J. Clavulanic Acid Biosynthesis and Genetic Manipulation for Its Overproduction. Appl. Microbiol. Biotechnol. 2010, 88, 659–669. [Google Scholar] [CrossRef]
- Hamed, R.B.; Gomez-Castellanos, J.R.; Henry, L.; Ducho, C.; McDonough, M.A.; Schofield, C.J. The Enzymes of β-Lactam Biosynthesis. Nat. Prod. Rep. 2013, 30, 21–107. [Google Scholar] [CrossRef]
- Jensen, S.E. Biosynthesis of Clavam Metabolites. J. Ind. Microbiol. Biotechnol. 2012, 39, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Townsend, C.A. Kinetic Mechanism of the β-Lactam Synthetase of Streptomyces clavuligerus. Biochemistry 2000, 39, 11187–11193. [Google Scholar] [CrossRef]
- Tahlan, K.; Park, H.U.; Wong, A.; Beatty, P.H.; Jensen, S.E. Two Sets of Paralogous Genes Encode the Enzymes Involved in the Early Stages of Clavulanic Acid and Clavam Metabolite Biosynthesis in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 2004, 48, 930–939. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, J.-S.; Harlos, K.; McKinnon, C.H.; Clifton, I.J.; Schofield, C.J. Crystal Structure of a Clavaminate Synthase-Fe(II)-2-Oxoglutarate-Substrate-NO Complex: Evidence for Metal Centred Rearrangements. FEBS Lett. 2002, 517, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Caines, M.E.C.; Elkins, J.M.; Hewitson, K.S.; Schofield, C.J. Crystal Structure and Mechanistic Implications of N 2-(2-Carboxyethyl)Arginine Synthase, the First Enzyme in the Clavulanic Acid Biosynthesis Pathway. J. Biol. Chem. 2004, 279, 5685–5692. [Google Scholar] [CrossRef]
- Shrestha, B.; Dhakal, D.; Darsandhari, S.; Pandey, R.P.; Pokhrel, A.R.; Jnawali, H.N.; Sohng, J.K. Heterologous Production of Clavulanic Acid Intermediates in Streptomyces venezuelae. Biotechnol. Bioprocess Eng. 2017, 22, 359–365. [Google Scholar] [CrossRef]
- Ramirez-Malule, H.; Restrepo, A.; Cardona, W.; Junne, S.; Neubauer, P.; Rios-Estepa, R. Inversion of the Stereochemical Configuration (3S, 5S)-Clavaminic Acid into (3R, 5R)-Clavulanic Acid: A Computationally-Assisted Approach Based on Experimental Evidence. J. Theor. Biol. 2016, 395, 40–50. [Google Scholar] [CrossRef]
- Gómez, S.; Ramírez-Malule, H.; Cardona-G, W.; Osorio, E.; Restrepo, A. Double-Ring Epimerization in the Biosynthesis of Clavulanic Acid. J. Phys. Chem. A 2020, 124, 9413–9426. [Google Scholar] [CrossRef]
- Arulanantham, H.; Kershaw, N.J.; Hewitson, K.S.; Hughes, C.E.; Thirkettle, J.E.; Schofield, C.J. ORF17 from the Clavulanic Acid Biosynthesis Gene Cluster Catalyzes the ATP-Dependent Formation of N-Glycyl-Clavaminic Acid. J. Biol. Chem. 2006, 281, 279–287. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, A.K.; Kershaw, N.J.; Hernandez, H.; Robinson, C.V.; Schofield, C.J.; Andersson, I. Clavulanic Acid Dehydrogenase: Structural and Biochemical Analysis of the Final Step in the Biosynthesis of the β-Lactamase Inhibitor Clavulanic Acid. Biochemistry 2007, 46, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, K.; Anders, C.; Wong, A.; Mosher, R.H.; Beatty, P.H.; Brumlik, M.J.; Griffin, A.; Hughes, C.; Griffin, J.; Barton, B.; et al. 5S Clavam Biosynthetic Genes Are Located in Both the Clavam and Paralog Gene Clusters in Streptomyces clavuligerus. Chem. Biol. 2007, 14, 131–142. [Google Scholar] [CrossRef]
- Goomeshi Nobary, S.; Jensen, S.E. A Comparison of the Clavam Biosynthetic Gene Clusters in Streptomyces antibioticus Tü1718 and Streptomyces clavuligerus. Can. J. Microbiol. 2012, 58, 413–425. [Google Scholar] [CrossRef]
- Kwong, T.; Zelyas, N.J.; Cai, H.; Tahlan, K.; Wong, A.; Jensen, S.E. 5S Clavam Biosynthesis Is Controlled by an Atypical Two-Component Regulatory System in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 2012, 56, 4845–4855. [Google Scholar] [CrossRef]
- Zelyas, N.J.; Cai, H.; Kwong, T.; Jensen, S.E. Alanylclavam Biosynthetic Genes Are Clustered Together with One Group of Clavulanic Acid Biosynthetic Genes in Streptomyces clavuligerus. J. Bacteriol. 2008, 190, 7957–7965. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, E.R.; Baptista-Neto, A.; Azevedo, A.G.; Badino, A.C., Jr.; Hokka, C.O. Improvement of Clavulanic Acid Production by Streptomyces clavuligerus in Medium Containing Soybean Derivatives. World J. Microbiol. Biotechnol. 1999, 15, 623–627. [Google Scholar] [CrossRef]
- Gouveia, E.R.; Baptista-Neto, A.; Badino, A.C.; Hokka, C.O. Optimisation of Medium Composition for Clavulanic Acid Production by Streptomyces clavuligerus. Biotechnol. Lett. 2001, 23, 157–161. [Google Scholar] [CrossRef]
- Maranesi, G.L.; Baptista-Neto, A.; Hokka, C.O.; Badino, A.C. Utilization of Vegetable Oil in the Production of Clavulanic Acid by Streptomyces clavuligerus ATCC 27064. World J. Microbiol. Biotechnol. 2005, 21, 509–514. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, B.; Ren, J.; Dong, M.L.; Liang, D.; Xu, A.L. Optimization of Medium Composition for the Production of Clavulanic Acid by Streptomyces clavuligerus. Process Biochem. 2005, 40, 1161–1166. [Google Scholar] [CrossRef]
- Belmar-Beiny, M.T.; Thomas, C.R. Morphology and Clavulanic Acid Production of Streptomyces clavuligerus: Effect of Stirrer Speed in Batch Fermentations. Biotechnol. Bioeng. 1991, 37, 456–462. [Google Scholar] [CrossRef]
- Rosa, J.C.; Neto, A.B.; Hokka, C.O.; Badino, A.C. Influence of Dissolved Oxygen and Shear Conditions on Clavulanic Acid Production by Streptomyces clavuligerus. Bioprocess Biosyst. Eng. 2005, 27, 99–104. [Google Scholar] [CrossRef]
- Teodoro, J.C.; Baptista-Neto, A.; Cruz-Hernández, I.L.; Hokka, C.O.; Badino, A.C. Influence of Feeding Conditions on Clavulanic Acid Production in Fed-Batch Cultivation with Medium Containing Glycerol. Appl. Microbiol. Biotechnol. 2006, 72, 450–455. [Google Scholar] [CrossRef]
- Li, R.; Townsend, C.A. Rational Strain Improvement for Enhanced Clavulanic Acid Production by Genetic Engineering of the Glycolytic Pathway in Streptomyces clavuligerus. Metab. Eng. 2006, 8, 240–252. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, Y. Application of Lat Gene Disruption to Increase the Clavulanic Acid Production of Streptomyces clavuligerus. J. Mol. Catal. B Enzym. 2006, 43, 102–107. [Google Scholar] [CrossRef]
- Salem-Bekhit, M.M.; Alanazi, F.K.; Alsarra, I.A. Improvement and Enhancement of Clavulanic Acid Production in Streptomyces clavuligerus Using Vegetable Oils. Afr. J. Biotechnol. 2010, 9, 6806–6812. [Google Scholar]
- Korbekandi, H.; Darkhal, P.; Hojati, Z.; Abedi, D.; Hamedi, J.; Pourhosein, M. Overproduction of Clavulanic Acid by UV Mutagenesis of Streptomyces clavuligerus. Iran. J. Pharm. Res. 2010, 9, 177–181. [Google Scholar] [CrossRef]
- Saudagar, P.S.; Shaligram, N.S.; Singhal, R.S. Immobilization of Streptomyces clavuligerus on Loofah Sponge for the Production of Clavulanic Acid. Bioresour. Technol. 2008, 99, 2250–2253. [Google Scholar] [CrossRef]
- Costa, C.L.L.; Badino, A.C. Production of Clavulanic Acid by Streptomyces clavuligerus in Batch Cultures without and with Glycerol Pulses under Different Temperature Conditions. Biochem. Eng. J. 2012, 69, 1–7. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, Y.; Zhang, Y.; Wang, C.; Heng, C. Mutation of the Streptomyces clavuligerus by Traditional and Molecular Breeding to Increase the Biosynthesis of Clavulanic Acid. Afr. J. Microbiol. Res. 2012, 6, 3144–3153. [Google Scholar]
- da Silva Vasconcelos, E.; de Lima, V.A.; Goto, L.S.; Cruz-Hernández, I.L.; Hokka, C.O. Clavulanic Acid Production by the MMS 150 Mutant Obtained from Wild Type Streptomyces clavuligerus ATCC 27064. Braz. J. Microbiol. 2013, 44, 1049–1057. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Y.; Yang, K. Coordination of Glycerol Utilization and Clavulanic Acid Biosynthesis to Improve Clavulanic Acid Production in Streptomyces clavuligerus. Sci. China Life Sci. 2013, 56, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Said, I.G.; Abdelwahed, N.A.M.; Awad, H.M.; Shallan, M.A.; El-Shahed, K.Y.I.; Abdel-Rahim, E.A. Enhancement of Clavulanic Acid Production by Streptomyces Sp MU-NRC77 via Mutation and Medium Optimization. Trop. J. Pharm. Res. 2017, 16, 31. [Google Scholar] [CrossRef]
- Qin, R.; Zhong, C.; Zong, G.; Fu, J.; Pang, X.; Cao, G. Improvement of Clavulanic Acid Production in Streptomyces clavuligerus F613-1 by Using a ClaR-Neo Reporter Strategy. Electron. J. Biotechnol. 2017, 28, 41–46. [Google Scholar] [CrossRef]
- da Silva Rodrigues, K.C.; de Souza, A.T.; Badino, A.C.; Pedrolli, D.B.; Cerri, M.O. Screening of Medium Constituents for Clavulanic Acid Production by Streptomyces clavuligerus. Braz. J. Microbiol. 2018, 49, 832–839. [Google Scholar] [CrossRef]
- Fu, J.; Xie, X.; Zhang, S.; Kang, N.; Zong, G.; Zhang, P.; Cao, G. Rich Organic Nitrogen Impacts Clavulanic Acid Biosynthesis through the Arginine Metabolic Pathway in Streptomyces clavuligerus F613-1. Microbiol. Spectr. 2022, 11, e02017-22. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.C.; Kim, C.H.; Hong, S.I.; Kim, S.W. Fed-Batch Cultivation for the Production of Clavulanic Acid by an Immobilized Streptomyces clavuligerus Mutant. World J. Microbiol. Biotechnol. 2001, 17, 869–872. [Google Scholar] [CrossRef]
- Ortiz, S.C.A.; Hokka, C.O.; Badino, A.C. Utilization of Soybean Derivatives on Clavulanic Acid Production by Streptomyces clavuligerus. Enzym. Microb. Technol. 2007, 40, 1071–1077. [Google Scholar] [CrossRef]
- Saudagar, P.S.; Singhal, R.S. A Statistical Approach Using L25 Orthogonal Array Method to Study Fermentative Production of Clavulanic Acid by Streptomyces clavuligerus MTCC 1142. Appl. Biochem. Biotechnol. 2007, 136, 345–359. [Google Scholar] [CrossRef]
- Saudagar, P.S.; Singhal, R.S. Optimization of Nutritional Requirements and Feeding Strategies for Clavulanic Acid Production by Streptomyces clavuligerus. Bioresour. Technol. 2007, 98, 2010–2017. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, J.O.; Shin, C.H.; Park, H.W.; Kim, C.W. An Approach to Strain Improvement and Enhanced Production of Clavulanic Acid in Streptomyces clavuligerus. Biosci. Biotechnol. Biochem. 2009, 73, 160–164. [Google Scholar] [CrossRef]
- Teodoro, J.C.; Baptista-Neto, A.; Araujo, M.L.G.C.; Hokka, C.O.; Badino, A.C. Influence of Glycerol and Ornithine Feeding on Clavulanic Acid Production by Streptomyces clavuligerus. Braz. J. Chem. Eng. 2010, 27, 499–506. [Google Scholar] [CrossRef]
- Cerri, M.O.; Badino, A.C. Shear Conditions in Clavulanic Acid Production by Streptomyces clavuligerus in Stirred Tank and Airlift Bioreactors. Bioprocess Biosyst. Eng. 2012, 35, 977–984. [Google Scholar] [CrossRef]
- Bellão, C.; Antonio, T.; Araujo, M.L.G.C.; Badino, A.C. Production of Clavulanic Acid and Cephamycin c by Streptomyces clavuligerus under Different Fed-Batch Conditions. Braz. J. Chem. Eng. 2013, 30, 257–266. [Google Scholar] [CrossRef]
- Kurt-Kizildoğan, A.; Vanli-Jaccard, G.; Mutlu, A.; Sertdemir, I.; Özcengiz, G. Genetic Engineering of an Industrial Strain of Streptomyces clavuligerus for Further Enhancement of Clavulanic Acid Production. Turk. J. Biol. 2017, 41, 342–353. [Google Scholar] [CrossRef]
- Cruz-Hernández, I.L.; Vasconcelos, E.D.S.; Teodoro, J.C.; De Baptista-Neto, A.; Da Costa Araujo, M.L.G.; Badino, A.C. Exploring the Optimization of UV Mutants of Streptomyces clavuligerus for Clavulanic Acid Production. Microbiol. Res. J. Int. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Cho, H.S.; Jo, J.C.; Shin, C.-H.; Lee, N.; Choi, J.-S.; Cho, B.-K.; Roe, J.-H.; Kim, C.-W.; Kwon, H.J.; Yoon, Y.J. Improved Production of Clavulanic Acid by Reverse Engineering and Overexpression of the Regulatory Genes in an Industrial Streptomyces clavuligerus Strain. J. Ind. Microbiol. Biotechnol. 2019, 46, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Li, Y.; Efthimiou, G. Olive Pomace Oil Can Be Used as an Alternative Carbon Source for Clavulanic Acid Production by Streptomyces clavuligerus. Waste Biomass Valoriz. 2020, 11, 3965–3970. [Google Scholar] [CrossRef]
- Ribeiro, R.M.M.G.P.; Esperança, M.N.; Sousa, A.P.A.; Neto, Á.B.; Cerri, M.O. Individual Effect of Shear Rate and Oxygen Transfer on Clavulanic Acid Production by Streptomyces clavuligerus. Bioprocess Biosyst. Eng. 2021, 44, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhao, J.; Chu, J.; Wang, Y.-H.; Zhuang, Y.-P. Statistical Optimizing of Medium for Clavulanic Acid Production by Streptomyces clavuligerus Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2021, 193, 3936–3948. [Google Scholar] [CrossRef]
- Shin, C.-H.; Cho, H.S.; Won, H.-J.; Kwon, H.J.; Kim, C.-W.; Yoon, Y.J. Enhanced Production of Clavulanic Acid by Improving Glycerol Utilization Using Reporter-Guided Mutagenesis of an Industrial Streptomyces clavuligerus Strain. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab004. [Google Scholar] [CrossRef]
- Domingues, L.C.G.; Teodoro, J.C.; Hokka, C.O.; Badino, A.C.; Araujo, M.L.G.C. Optimisation of the Glycerol-to-Ornithine Molar Ratio in the Feed Medium for the Continuous Production of Clavulanic Acid by Streptomyces clavuligerus. Biochem. Eng. J. 2010, 53, 7–11. [Google Scholar] [CrossRef]
- Chen, K.-C.; Lin, Y.-H.; Tsai, C.-M.; Hsieh, C.-H.; Houng, J.-Y. Optimization of Glycerol Feeding for Clavulanic Acid Production by Streptomyces clavuligerus with Glycerol Feeding. Biotechnol. Lett. 2002, 24, 455–458. [Google Scholar] [CrossRef]
- Neto, A.B.; Hirata, D.B.; Cassiano Filho, L.C.M.; Bellão, C.; Badino Júnior, A.C.; Hokka, C.O. A Study on Clavulanic Acid Production by Streptomyces clavuligerus in Batch, Fed-Batch and Continuous Processes. Braz. J. Chem. Eng. 2005, 22, 557–563. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Junne, S.; Neubauer, P.; Ochoa, S.; Ríos-Estepa, R.; Ramírez-Malule, H. Characterization of the Metabolic Response of Streptomyces clavuligerus to Shear Stress in Stirred Tanks and Single-Use 2D Rocking Motion Bioreactors for Clavulanic Acid Production. Antibiotics 2019, 8, 168. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ríos-Estepa, R.; Ochoa, S. Tuning of Fed-Batch Cultivation of Streptomyces clavuligerus for Enhanced Clavulanic Acid Production Based on Genome-Scale Dynamic Modeling. Biochem. Eng. J. 2022, 185, 108534. [Google Scholar] [CrossRef]
- Gómez-Gaona, L.M.; Ramírez-Malule, H.; Gómez-Ríos, D. Flux Dynamics of C-5 Amino Acid Precursors in Fed-Batch Cultures of Streptomyces clavuligerus during Clavulanic Acid Biosynthesis. J. Appl. Pharm. Sci. 2024, 14, 77–87. [Google Scholar] [CrossRef]
- Carvalho, V.; Brandão, J.F.; Brandão, R.; Rangel-yagui, C.O.; Couto, J.A.; Converti, A.; Pessoa, A. Stability of Clavulanic Acid under Variable PH, Ionic Strength and Temperature Conditions. A New Kinetic Approach. Biochem. Eng. J. 2009, 45, 89–93. [Google Scholar] [CrossRef]
- Brethauer, S.; Held, M.; Panke, S. Clavulanic Acid Decomposition Is Catalyzed by the Compound Itself and by Its Decomposition Products. J. Pharm. Sci. 2008, 97, 3451–3455. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ríos-Estepa, R. Data of Clavulanic Acid and Clavulanate-Imidazole Stability at Low Temperatures. Data Brief 2019, 23, 103775. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ríos-Estepa, R. Degradation Kinetics of Clavulanic Acid in Fermentation Broths at Low Temperatures. Antibiotics 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.C.S.; Costa, C.L.L.; Badino, A.C.; Pedrolli, D.B.; Pereira, J.F.B.; Cerri, M.O. Application of Acid and Cold Stresses to Enhance the Production of Clavulanic Acid by Streptomyces clavuligerus. Appl. Biochem. Biotechnol. 2019, 188, 706–719. [Google Scholar] [CrossRef]
- Bersanetti, P.A.; Almeida, R.M.R.G.; Barboza, M.; Araújo, M.L.G.C.; Hokka, C.O. Kinetic Studies on Clavulanic Acid Degradation. Biochem. Eng. J. 2005, 23, 31–36. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; López-Agudelo, V.A.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ochoa, S.; Ríos-Estepa, R. A Genome-Scale Insight into the Effect of Shear Stress during the Fed-Batch Production of Clavulanic Acid by Streptomyces clavuligerus. Microorganisms 2020, 8, 1255. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, N.; Olmos, E.; Marchal, P.; Goergen, J.L.; Delaunay, S. Relation between Pristinamycins Production by Streptomyces pristinaespiralis, Power Dissipation and Volumetric Gas-Liquid Mass Transfer Coefficient, KLa. Process Biochem. 2010, 45, 1779–1786. [Google Scholar] [CrossRef]
- Yagüe, P.; López-García, M.T.; Rioseras, B.; Sánchez, J.; Manteca, Á. Pre-Sporulation Stages of Streptomyces Differentiation: State-of-the-Art and Future Perspectives. FEMS Microbiol. Lett. 2013, 342, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Gomez, N.; Quintero, J.C. Producción de Ácido Clavulánico Por Fermentación de Streptomyces clavuligerus: Evaluación de Diferentes Medios de Cultivo y Modelado Matemático. Dyna 2012, 79, 158–165. [Google Scholar]
- Bussari, B.; Saudagar, P.S.; Shaligram, N.S.; Survase, S.A.; Singhal, R.S. Production of Cephamycin C by Streptomyces clavuligerus NT4 Using Solid-State Fermentation. J. Ind. Microbiol. Biotechnol. 2008, 35, 49–58. [Google Scholar] [CrossRef]
- Antonio, T.; Bellão, C.; Corrêa, T.; Cavallieri, A.P.; Badino, A.C.; Araujo, M.L.G. da C. Evaluation of Different Media for the Production of Cephalosporins by Streptomyces clavuligerus ATCC 27064. Braz. Arch. Biol. Technol. 2012, 55, 819–825. [Google Scholar] [CrossRef]
- Gómez-Rios, D.; Ramírez-Malule, H.; Ochoa, S.; Ríos-Estepa, R. Rational Selection of Culture Medium for Clavulanic Acid Production by Streptomyces clavuligerus Based on a Metabolic Modeling Approach. Agric. Nat. Resour. 2022, 56, 267–276. [Google Scholar] [CrossRef]
- Ramirez-Malule, H.; López-Agudelo, V.A.; Gómez-Ríos, D.; Ochoa, S.; Ríos-Estepa, R.; Junne, S.; Neubauer, P. TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus. Bioengineering 2021, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Cavallieri, A.P.; Baptista, A.S.; Leite, C.A.; da Costa Araujo, M.L.G. A Case Study in Flux Balance Analysis: Lysine, a Cephamycin C Precursor, Can Also Increase Clavulanic Acid Production. Biochem. Eng. J. 2016, 112, 42–53. [Google Scholar] [CrossRef]
- Medema, M.H.; Alam, M.T.; Heijne, W.H.M.; Van Den Berg, M.A.; Müller, U.; Trefzer, A.; Bovenberg, R.A.L.; Breitling, R.; Takano, E. Genome-Wide Gene Expression Changes in an Industrial Clavulanic Acid Overproduction Strain of Streptomyces clavuligerus. Microb. Biotechnol. 2011, 4, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, D.; Ramírez-Malule, H.; López-Agudelo, V.A. Dynamic in Silico Assessment of Potential Gene Targets for Enhancing Clavulanic Acid Production in Streptomyces clavuligerus Submerged Cultures. J. Appl. Pharm. Sci. 2022, 13, 61–68. [Google Scholar] [CrossRef]
- Kirk, S.; Avignone-rossa, C.A.; Bushell, M.E. Growth Limiting Substrate Affects Antibiotic Production and Associated Metabolic Fluxes in Streptomyces clavuligerus. Biotechnol. Lett. 2000, 22, 1803–1809. [Google Scholar] [CrossRef]
- Esnault, C.; Dulermo, T.; Smirnov, A.; Askora, A.; David, M.; Deniset-Besseau, A.; Holland, I.B.; Virolle, M.J. Strong Antibiotic Production Is Correlated with Highly Active Oxidative Metabolism in Streptomyces Coelicolor M145. Sci. Rep. 2017, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Millan-Oropeza, A.; Henry, C.; Lejeune, C.; David, M.; Virolle, M.-J. Expression of Genes of the Pho Regulon Is Altered in Streptomyces Coelicolor. Sci. Rep. 2020, 10, 8492. [Google Scholar] [CrossRef] [PubMed]
- Virolle, M.-J. A Challenging View: Antibiotics Play a Role in the Regulation of the Energetic Metabolism of the Producing Bacteria. Antibiotics 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]



| Ref. | Type | Strain | Scale | Medium (g/L) | Volume (mL) | Time (h) | Aeration (vvm) | pH | Temperature (°C) | Agitation (rpm) | Supplement (g/L) | CA (mg/L) | Biomass (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [22] | Wild | NRRL 3585 | Bench | Glycerol (15); bacteriological peptone (10); K2HPO4 (0.8) | 500 | 96 | - | 6.5 | 28 | 250 | Samprosoy 90NB (27) | 920 | 4.8 |
| Soybean meal (80) | 472 | 4.8 | |||||||||||
| [23] | Wild | NRRL 3585 | Bench | Glycerol (15); K2HPO4 (0.8) | 500 | 72 | - | 6.5 | 28 | 250 | Corn steep liquor (36.5); protein extract of soybean (27) | 400 | - |
| Yeast extract (12.8); protein extract of soybean (27) | 725 | - | |||||||||||
| Bacteriological peptone (10); protein extract of soybean (27) | 900 | - | |||||||||||
| Protein extract of soybean (37) | 1140 | - | |||||||||||
| [24] | Wild | ATCC 27064 | Bench | Soluble starch (10); soybean flour (20); soybean oil (23); K2HPO4 (1.2) | 500 | 100 | - | 7.0 | 28 | 250 | - | 458 | - |
| Soybean flour (20); soybean oil (28); K2HPO4 (1.2) | 500 | 120 | - | 7.0 | 28 | 250 | - | 478 | - | ||||
| Glycerol (10); soybean flour (20); soybean oil (23); K2HPO4 (1.2) | 500 | 120 | - | 7.0 | 28 | 250 | Soybean oil (16) | 420 | - | ||||
| Soybean oil (23) | 722 | - | |||||||||||
| Soybean oil (30) | 753 | - | |||||||||||
| Corn oil (23) | 680 | - | |||||||||||
| Sunflower oil (23) | 660 | - | |||||||||||
| [25] | Wild | ATCC 27064 | Bench | Glycerol (18.75); soybean meal powder (42); KH2PO4 (0.125); MgSO4·7H2O (0.25); FeSO4·7H2O (0.3); ornithine (1.32) | 300 | - | - | - | 28 | 250 | - | 526 | - |
| [26] | Wild | ATCC 20764 | Bioreactor | Glycerol (20); malt extract (10); bacteriological peptone (10) | 5000 | 120 | 0.5 | 7.0 | 26 | 1300 | - | 250 | 5.4 |
| [27] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); Samprosoy 90NB (10); malt extract (10); yeast extract (1); K2HPO4 (2.5) | 4000 | 80 | 0.5 | 6.8 | 28 | 600 | - | 254 | - |
| 800 | - | 475 | - | ||||||||||
| 1000 | - | 614 | - | ||||||||||
| 800 | - | 482 | - | ||||||||||
| [28] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); malt extract (10); yeast extract (1); K2HPO4 (2.5); MgSO4·7H2O (0.75); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | 4000 | 64 | 0.5 | 6.8 | 28 | 800 | Samprosoy 90NB (20) | 376 | |
| [29] | Mutant | Gap-15-7-30 | - | Glycerol (20); soybean protein extract (5.5); K2HPO4 (0.8) | - | 216 | - | 7.0 | 28 | 300 | - | 282 | 3.0 1 |
| [30] | Mutant | pKC1139-lat gene disrupted | Bench | Soybean flour (15); soluble starch (4.7); K2HPO4 (0.1); FeSO4·7H2O (0.2) | 250 | 96 | - | 6.8 | 28 | 220 | - | 269.8 | 6.0 |
| [31] | Wild | ATCC 27064 | Bench | Starch (10); soybean flour (20); oil (23); phosphate (1.2); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | 500 | 140 | - | 7.0 | 28 | 250 | Olive oil | 1120 | - |
| [32] | Wild | DSM 738 | Bench | Malt extract (0.3); glycerol (0.05); soy flour (0.05); FeSO4 (0.00001); and MnSO4 (0.00001) | 250 | 72 | - | - | 28 | 220 | - | 343.87 | - |
| [33] | Wild | MTCC 1142 | Bench | Glycerol (15); sucrose (20); L-arginine (1.7); L-glutamic acid (16.8); CaCl2 (0.4); FeCl3·6H2O (0.1); K2HPO4 (2); NaCl (5); MnCl2·4H2O (0.1); ZnCl2 (0.05); MgSO4·7H2O (1) | 250 | 120 | - | 7.0 | 25 | 200 | - | 1129 | - |
| [34] | Wild | ATCC 27064 | Bench | Glycerol (15); soybean protein isolate (25); K2HPO4 (0.8); MgSO4·7H2O (0.75) | 500 | 250 | - | 6.8 | 20 | 250 | - | 1266.2 | - |
| - | 631.6 2 | - | |||||||||||
| Glycerol (100) | 1534.3 | - | |||||||||||
| Glycerol (200) | 1495.1 | - | |||||||||||
| Glycerol (300) | 1465.4 | - | |||||||||||
| Glycerol (400) | 1460.0 | - | |||||||||||
| [35] | Mutant | lat::scar | Bench | - | - | 120 | - | - | 28 | - | - | 790.68 | 6 |
| [36] | Wild | ATCC 27064 | Bench | Soybean flour (20); glycerol (10); K2HPO4 (1.2); soy oil (23); MnCl2.4H2O (0.001); FeSO47H2O (0.001); ZnSO47H2O (0.001) | 500 | 120 | - | 7.2 | 25 | 250 | - | 815.8 | 8.8 |
| [37] | Mutant | pSGR | Bench | Soybean flour (20); dextrin (10); glycerol (5); KH2PO4 (0.6); MOPS (8) | 500 | 72 | - | 7.1 | 28 | 250 | - | 838.7 | - |
| [38] | Mutant | 120A3 | Bench | Soluble starch (25); glycerol (10 mL); soybean meal (30); KH2PO4 (0.1); FeSO47H2O (0.1) | 250 | 144 | - | 7.0 | 28 | 200 | - | 275 | 5.6 |
| [39] | Mutant | M3-19 | Bench | Soybean powder (1.7); soybean protein extract (2.2); maize starch (3); KCl (0.15); MgCl2·6H2O (0.1); MgSO4·7H2O (0.2); CaCl2·2H2O (0.04) 3 | 100 | 120 | - | 7.1 | 25 | 250 | - | 4330 | - |
| F613-1 | Bench | 3260 | - | ||||||||||
| [40] | Wild | ATCC 27064 | Bench | Glycerol (15); yeast extract (1); soybean protein isolate (20); K2HPO4 (0.5); MgSO4·7H2O (0.4); FeSO4·7H2O (0.4); MnCl2·4H2O (0.001); ZnSO4·7H2O (0.001) | 250 | 72–84 | - | 6.8 | 27 | 250 | - | 437 | - |
| [41] | Mutant | F613-1 | Bench | Peptone from soybean (0.08); dextrin (0.01); glycerol trioleate (0.008); KCl (0.015); MgCl2·6H2O (0.01); K2HPO4 (0.02); CaCl2·2H2O (0.004); FeCl2·6H2O (0.0008); ZnCl2 (0.0001); NaCl (0.0018); MOPS (0.08) | 250 | 144 | - | 7.1 | 25 | 200 | - | 390 | 18.7 (wet weight) |
| [42] | Mutant | KK | Bench | Soybean flour (10); dextrin (20); soybean oil (2 4) | 250 | 192 | 1 | 7.0 | 28 | 400–500 | - | 1750 | - |
| Bioreactor | Soybean flour (15); glycerol (12.6); KH2PO4 (1); MgSO4 (4); soybean oil (2 5) | 2500 | 192 | 1 | 7.0 | 28 | 400–500 | - | 3000 | - | |||
| [43] | Wild | ATCC 27064 | Bench | Glycerol (10); soybean flour (20); soybean oil (23); K2HPO4 (1.2); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | 500 | 120–160 | - | 6.8 | 28 | 250 | - | 698 | - |
| Bioreactor | 4000 | 100 | 0.5 | 6.8 | 28 | 800 | - | 796 | - | ||||
| [44] | Wild | MTCC 1142 | Bioreactor | Soybean oil (4); soybean flour (88); yeast extract (15); dextrin (10); K2HPO4 (2) | - | 96 | - | 7.0 | 25 | 200 | L-arginine (17.42) | 1400 | - |
| 7.5 | 25 | 200 | - | 495 | - | ||||||||
| [45] | Wild | MTCC 1142 | Bioreactor | Glycerol (15); L-proline (22.4); L-glutamic acid (16.6); sucrose (20); K2HPO4 (2); CaCl2 (0.4); FeCl3·6H2O (0.1); NaCl (5); MnCl2·4H2O (0.1); ZnCl2 (0.05); MgSO4·7H2O (1) | - | 96 | - | 7.0 | - | - | L-threonine (11.9) | 1700 | - |
| K2HPO4 (1.742) | 878 | 24.0 | |||||||||||
| [46] | Wild | NRRL 3585 | Bioreactor | Starch (10); soybean flour (20); oil (23); phosphate (1.2); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | 7000 | 140 | - | 7.0 | 28 | 220 | Triolein (22) | 989 | - |
| Mutant | OL13 | Bioreactor | N-Z amine type A (2); yeast extract (1); beef extract (1); oleic acid (0.5–3) | 7000 | 140 | - | 8.0 | 28 | 250 | Triolein | 1950 | 2.0 | |
| Olive oil | 500 | - | |||||||||||
| [47] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); soybean protein isolate (20); K2HPO4 (0.8); MgSO4·7H2O (0.75); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001); salt solution (1); soybean oil (1) | 5000 | 66 | 0.5 | 6.8 | 28 | 800 | Ornithine (0.66) | 560 | - |
| [48] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); soybean protein isolated (10); malt extract (10); yeast extract (1); K2HPO4 (2.5); MgSO4·7H2O (0.75); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | 6000 | 60 | 3.0 | 6.8 | 30 | - | - | 454 | - |
| 4000 | 60 | 0.5 | 6.8 | 30 | 800 | - | 402 | - | |||||
| [49] | Wild | DSM 41826 | Bioreactor | Glycerol (10); soybean meal (11); L-lysine (18.3); yeast extract (0.5); K2HPO4 (1.75); MgSO4·7H2O (0.75); CaCl2·2H2O (0.2); NaCl (2.0); FeSO4·7H2O (0.005); MnCl2·4H2O (0.005); ZnSO4·7H2O (0.005); sodium thiosulfate (1) | 5000 | 80 | 0.5 | 6.8 | 28 | 800 | Glycerol (15) | 348.5 | - |
| [50] | Mutant | IDG3 | Bioreactor | Soybean flour (20); dextrin (10); KH2PO4 (0.6); GTO (5); MOPS (10.5); oligo element solution (CaCl2) (10); MgCl2.6H2O (10); FeCl3 (3); ZnCl2 (0.5); MnSO4 H2O (0.5); NaCl (10) (10 mL) | - | - | - | - | 23.5 | - | Glycerol trioleate (0.8 mL/40 mL) | 6647 | - |
| [51] | Mutant | Mutant 70 | Bioreactor | Glycerol (15); malt extract (10); yeast extract (1); soytone (15); arginine (2.62); KH2PO4 (0.63); MgSO47H2O (0.75); salt solution (1 mL); MnCl2.4H2O (0.001); FeSO47H2O (0.001); ZnSO47H2O (0.001); MOPS buffer (21) | 5000 | 48 | 0.5 | 6.8 | 28 | 800 | - | 500 | - |
| [52] | Mutant | OR/pCCAR-CLAR | Bioreactor | Starch (10); soybean meal (20); triolein (23); phosphate (1.2); trace elements; antifoam (1 mL/L) | 7000 | - | Over 0.3 | 7.0 | - | 300–700 | - | 6010 | - |
| [53] | Wild | DM738 | Bioreactor | Yeast extract (4); malt extract (10); dextrose (4) | 1800 | 48 | 0.25 | 7.0 | 30 | 1000 | Olive pomace oil (0.6% v/v) | 325 | 36.0 |
| [54] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); soybean protein isolate (20); MOPS (21); MgSO47H2O (0.75); K2HPO4 (0.80); and salt solutions (1) | 5100 | 72 | 0.5 | 6.8 | 28 | 1000 | - | 917.5 | 38.4 |
| [55] | Wild | ATCC 27064 | Bioreactor | Glycerol (20); dextrin (12.37); soybean meal (39.75); KH2PO4 (1.2); triolein (26.98 mL); trace element solution (2 mL) | 50,000 | 62 | 0.28 | 7.0 | 28 | 300 | - | 1322 | - |
| [56] | Mutant | ORUN | Bioreactor | Glycerol (0.3); soy flour (0.47); MOPS (0.105); NaH2PO4 (0.016); NaCl (0.001); MgSO4·7H2O (0.001); FeCl3·6H2O (0.0004); MnSO4·H2O (0.00005); CuCl2·2H2O (0.00005); ZnCl2 (0.00005) | 7000 | 136 | - | - | 28 | 220 | Glycerol (10) | 893 | - |
| Ref. | Type | Strain | Scale | Medium (g/L) | Feed (g/L) | Volume (mL) | Feed Time (h) | Flow (mL/h) | Time (h) | Aeration (vvm) | pH | Temperature (°C) | Agitation (rpm) | CA (mg/L) | Biomass (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [29] | Mutant | Gap 15-7-30 | Bench | Glycerol (20); protein extract from soybean (5.5); K2HPO4 (0.8); MOPS (21) | Arginine (2.35) | - | 60 | - | 216 | - | 7.0 | 28 | 300 | 417 | 25.0 1 |
| [57] | Wild | ATCC 27064 | Bench | Glycerol (15); soybean protein isolate (15.5); yeast extract (1); K2HPO4 (0.8); MgSO4·7H2O (0.75); MnCl2·4H2O (1); FeSO4·7H2O (1); ZnSO4·7H2O (1) | Glycerol:ornithine (40:1) 2 | 500 | 48 | - | 144 | - | 6.8 | 28 | 250 | 390 | 8.0 |
| Bioreactor | Glycerol:ornithine (40:1) 2 | 5000 | 48 | - | 144 | 1 | 6.8 | 28 | - | 650 | 7.2 | ||||
| [42] | Mutant | KK | Bioreactor | Soybean flour (15); glycerol (12.6); KH2PO4 (1); MgSO4 (4); soybean oil (2 3) | Glycerol (66 4) | 2500 | 48 | - | 192 | 1 | 7.0 | 28 | 400–500 | 3250 | - |
| [58] | Wild | ATCC 27064 | Bioreactor | Glycerol (20); soy meal extract (1000) 5; peptone (10); KH2PO4 (1); MgSO4·7H2O (1) | Glycerol (250) | 5000 | 60 | 10 | 120 | 1 | 7.0 | 28 | 500 | 270 | 10.6 |
| [59] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); Samprosoy 90NB (10); malt extract (10); yeast extract (1); K2HPO4 (2.5); MgSO4·7H2O (0.75); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | Glycerol (10); Samprosoy 90NB (10); malt extract (10); yeast extract (1); K2HPO4 (2.5); MgSO4·7H2O (0.75); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001) | 3000 | 24 | 80 | 96 | 0.5 | 6.8 | 28 | 800 | 404 | 9.9 |
| [45] | Wild | MTCC 1142 | Bioreactor | Glycerol (15); L-proline (22.4); L-glutamic acid (16.6); sucrose (20); K2HPO4 (2); CaCl2 (0.4); FeCl3·6H2O (0.1); NaCl (5); MnCl2·4H2O (0.1); ZnCl2 (0.05); MgSO4·7H2O (1) | Threonine (595.6) | - | 60 | - | 130 | - | 7.0 | - | - | 1863 | 13.0 |
| [47] | Wild | ATCC 27064 | Bioreactor | Glycerol (15); soybean protein isolate (20); K2HPO4 (0.8); MgSO4·7H2O (0.75); MnCl2·4H2O (0.001); FeSO4·7H2O (0.001); ZnSO4·7H2O (0.001); salt solution (1); soybean oil (1) | Glycerol (180); ornithine (3.7) | 5000 | 24 | 10 | 120 | 0.5 | 6.8 | 28 | 800 | 1506 | - |
| 10,000 | 24 | 20 | 120 | 0.5 | 6.8 | 28 | 800 | 1560 | - | ||||||
| [49] | Wild | DSM 41826 | Bioreactor | Glycerol (10); soybean meal (11); L-lysine (18.3); yeast extract (0.5); K2HPO4 (1.75); MgSO4·7H2O (0.75); CaCl2·2H2O (0.2); NaCl (2.0); FeSO4·7H2O (0.005); MnCl2·4H2O (0.005); ZnSO4·7H2O (0.005); sodium thiosulfate (1) | Glycerol (201) | 5000 | 24 | 5 | - | 0.5 | 6.8 | 28 | 800 | 982.1 | - |
| [60] | Wild | DSM 41826 | Bioreactor | Glycerol (9.3); K2HPO4 (0.8); (NH4)2SO4 (1.26); monosodium glutamate (9.8); FeSO4·7H2O (0.18); MgSO4·7H2O (0.72) | Glycerol (120); K2HPO4 (2); (NH4)2SO4 (8) | 15,000 | 37 | 35 | 157 | 0.6 | 6.8 | 28 | 300–500 | 422.7 | 12.3 |
| [61] | Wild | DSM 41826 | Bioreactor | Glycerol (0.8); (NH4)2SO4 (1.26); monosodium glutamate (9.8); FeSO47H2O (0.18); MgSO47H2O (0.72); MOPS (10.5); trace element solution (1.44 mL) | Glycerol (120); K2HPO4 (2); (NH4)2SO4 (8) | 15,000 | 37 | 35 | 110 | 0.5–0.7 | 6.8 | 28 | 300–500 | 467.2 | 13.8 |
| [62] | Wild | DSM 41826 | Bioreactor | Glycerol (15); L-proline (22.4); L-glutamic acid (16.6); sucrose (20); K2HPO4 (2); CaCl2 (0.4); FeCl3·6H2O (0.1); NaCl (5); MnCl2·4H2O (0.1); ZnCl2 (0.05); MgSO4·7H2O (1) | Arginine (21.07); threonine (119.12); K2HPO4 (2); (NH4)2SO4 (8) | 5000 | 18 | 0.02 L−1 | 120 | 0.6 | 6.8 | 28 | 300–600 | 3293.3 | 21.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Ríos, D.; Gómez-Gaona, L.M.; Ramírez-Malule, H. Clavulanic Acid Overproduction: A Review of Environmental Conditions, Metabolic Fluxes, and Strain Engineering in Streptomyces clavuligerus. Fermentation 2024, 10, 526. https://doi.org/10.3390/fermentation10100526
Gómez-Ríos D, Gómez-Gaona LM, Ramírez-Malule H. Clavulanic Acid Overproduction: A Review of Environmental Conditions, Metabolic Fluxes, and Strain Engineering in Streptomyces clavuligerus. Fermentation. 2024; 10(10):526. https://doi.org/10.3390/fermentation10100526
Chicago/Turabian StyleGómez-Ríos, David, Luisa María Gómez-Gaona, and Howard Ramírez-Malule. 2024. "Clavulanic Acid Overproduction: A Review of Environmental Conditions, Metabolic Fluxes, and Strain Engineering in Streptomyces clavuligerus" Fermentation 10, no. 10: 526. https://doi.org/10.3390/fermentation10100526
APA StyleGómez-Ríos, D., Gómez-Gaona, L. M., & Ramírez-Malule, H. (2024). Clavulanic Acid Overproduction: A Review of Environmental Conditions, Metabolic Fluxes, and Strain Engineering in Streptomyces clavuligerus. Fermentation, 10(10), 526. https://doi.org/10.3390/fermentation10100526






