Abstract
Basic fibroblast growth factor (bFGF) is a crucial protein with diverse applications in biotechnology and medicine. This study aims to investigate the use of EL222-based optogenetic control systems to fine-tune the expression of porcine (Sus scrofa) bFGF in Escherichia coli. The bioactivity and the productivity of blue light-induced bFGF were demonstrated to be comparable to those achieved using a conventional T7-expression system. Secondly, through systematic optimization of regulatory elements, optimal expression of bFGF was achieved using a medium-strength promoter for EL222 expression, a strong RBS upstream of the bFGF gene, and an optimized C120 configuration within the blue light-inducible promoter. Moreover, various parameters of blue light illumination during fermentation were investigated, including initial cell density, light intensity, illumination duration, and pulsed illumination patterns. The results identified optimal conditions for maximizing bFGF yield in E. coli, specifically an initial OD600 of 0.6, 800 lux blue light intensity, and 8 h total illumination in a 2 h on/off pattern. Overall, this successful implementation of optogenetically controlled bFGF expression in E. coli serves as a proof-of-concept for light-responsive systems in industrial biotechnology, highlighting the potential of optogenetic control for biologically active protein production.
1. Introduction
In cell biology, serum-free cell culture media are essential for studying cellular processes and enabling commercial-scale cell culture applications [1,2,3]. However, their high production costs, primarily due to required supplements such as growth factors and hormones, limit widespread adoption [4,5]. Recent advances in microbial genetic engineering and synthetic biology have created opportunities for efficient microbial synthesis of these essential media components [6,7,8]. This approach shows promise for both reducing production costs and advancing cell culture technology for commercial applications [9,10,11]. Basic fibroblast growth factor (bFGF/FGF2) is a key member of the fibroblast growth factor family and an essential component in serum-free cell culture media [12,13]. bFGF functions as both a growth factor that stimulates cellular proliferation and a neurotrophic factor promoting cellular differentiation [14,15,16]. Its ability to restore cellular activity has led to its incorporation in anti-aging cosmetics [17]. However, bFGF occurs naturally in only trace amounts in animal tissues, limiting both research and industrial applications. To address this constraint, microbial cell factories have emerged as promising platforms for bFGF biosynthesis [18,19,20]. Research into green, safe, and efficient microbial expression systems for bFGF is essential for advancing both fundamental research and meeting increasing market demands.
Significant advancements have been made in the cloning and expression of bFGF genes derived from various species, particularly humans and cattle. Multiple research teams have successfully constructed expression vectors to produce biologically active bFGF in E. coli [21,22,23]. Notably, Akitsu Masuda and colleagues utilized insect cell expression systems to promote the expression of active human bFGF [24]. In eukaryotic expression systems, Xu et al. introduced a synthetic bFGF gene into Pichia pastoris, expressing biologically active bFGF [20]. Further, Tsutomu Kurokawa et al. transformed COS7 cells with a modified bFGF gene, and the cell culture medium was able to stimulate the growth of NIH3T3 cells, indicating that the bFGF gene was expressed in COS7 cells and partially secreted into the culture medium [25]. Expanding the understanding of bFGF regulation, Chen et al. studied the effect of prostaglandin E2 on the expression of bFGF in mouse mullerian duct cells, finding that prostaglandin E2 significantly stimulated the expression of bFGF, with a concentration of 10 μM increasing bFGF expression in cells by 3.5 times that of the control group [26]. Despite these advancements, there remains a paucity of studies regarding the cloning and expression of bFGF derived specifically from porcine sources. Therefore, conducting research directly on porcine bFGF has the potential to promote the development of serum-free culture media suitable for culturing porcine stem cells.
Traditional methods for recombinant protein production often rely on chemical inducers, which can be costly and may introduce unwanted contaminants into the final product [9,27,28]. With the rapid development of synthetic biology, more novel expression systems, such as those incorporating optogenetic control systems, offer an attractive alternative, allowing for precise, reversible, and contamination-free regulation of gene expression using light [29,30]. In contrast to chemical inducers, light offers several advantages as a stimulus for gene expression control, including its eco-friendly nature and cost-effectiveness [31,32]. Contemporary advancements in illumination technology have facilitated the provision of multiple wavelengths of light through both customized and commercially available devices, significantly reducing associated costs [33]. Light stimuli can be rapidly and easily manipulated or terminated without introducing exogenous chemicals or perturbing cellular growth conditions [34]. Moreover, optogenetic systems exhibit high controllability and precision, enabling researchers to modulate gene expression with unprecedented accuracy and flexibility [35]. Previous studies have demonstrated that various parameters, including light wavelength, intensity, pulse duration, and timing, can be finely tuned to achieve spatiotemporal regulation of gene expression [36]. This level of control allows for the implementation of sophisticated expression strategies, such as dynamic pathway regulation and adaptive responses to metabolic states [37]. The non-invasive nature of light induction also facilitates real-time monitoring and adjustment of protein expression levels, potentially improving product yield and quality [38].
In this study, we engineered and implemented a novel optogenetic system for the precise regulation of porcine bFGF expression in E. coli. The optogenetic system was predicated on the blue light-responsive transcription factor EL222, derived from the marine bacterium Erythrobacter litoralis, which was selected for its rapid and reversible photoswitching capabilities in response to blue light stimulation [39,40,41,42]. By integrating this photosensitive regulatory element into the genetic architecture of E. coli, we aimed to achieve dynamic, non-invasive control over bFGF production. The comparative analysis of bFGF expression under optogenetic regulation in E. coli allowed us to elucidate the relative strengths, limitations, and unique characteristics of this approach. We systematically evaluated various parameters, including light intensity, exposure duration, and pulsing strategies, to optimize the system’s performance. Furthermore, this study sought to demonstrate the broad applicability and potential advantages of optogenetic regulation in the context of recombinant protein production, particularly for the synthesis of this therapeutically and biotechnologically significant growth factor. Our approach might not only address the current challenges in bFGF production but also provide a framework for the application of optogenetic control in diverse bioprocessing scenarios.
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
The bacterial strains, as well as the plasmids employed in this study, were comprehensively detailed in Supplementary Table S1. E. coli DH5α and BL21(DE3) were used as the primary host organisms for molecular cloning and protein expression, respectively. Custom-synthesized oligonucleotide primers were obtained from Sangon Biotech (Shanghai, China), and their complete sequences and specifications are provided in Supplementary Table S2. Genomic DNA was extracted using the Bacterial Genomic DNA Extraction Kit (Vazyme Biotech Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplifications were performed using Phanta Super-Fidelity DNA Polymerase (Vazyme Biotech, Nanjing, China) in a DNA Thermal Cycler 9700 (Applied Biosystems, Foster, CA, USA) under the following conditions: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation (95 °C, 15 s), annealing (55–65 °C depending on the primer melting temperature, 15 s), and extension (72 °C, 1 min/kb), with a final extension at 72 °C for 5 min. All constructed plasmids were verified by DNA sequencing before use in subsequent experiments.
2.2. Media and Culture Conditions
Bacterial strains were cultivated in two distinct growth media, depending on the experimental requirements. For routine culture maintenance and general growth studies, Luria–Bertani (LB) medium (5 g/L yeast extract, 10 g/L tryptone, 10 g/L NaCl) was employed. For plasmid extraction, Terrific Broth (TB) medium (24 g/L yeast extract, 12 g/L tryptone, 4 mL glycerol, 2.3 g/L KH2PO4, 12.5 g/L K2HPO4) was utilized. To maintain selective pressure for plasmid retention and to prevent contamination, antibiotics were supplemented to the growth media when appropriate. The antibiotics were used at the following final concentrations: ampicillin at 100 μg/mL, kanamycin at 30 μg/mL, and chloramphenicol at 25 μg/mL. Cultures were incubated at 37 °C with orbital shaking at 200 rpm unless otherwise specified for particular experimental conditions.
2.3. Construction of Expression Strains
To enhance the expression of porcine bFGF in E. coli, a comprehensive molecular cloning strategy was implemented. The initial phase involved the design and synthesis of a codon-optimized bFGF gene sequence, incorporating a C-terminal histidine tag for subsequent protein purification. This synthetic gene construct was commissioned and produced by Sangon Biotech (Shanghai, China). The optimized sequence was strategically inserted into the pET-28a (+) expression vector, generating the pET28-bFGF construct, which was engineered to maximize protein expression efficiency in the bacterial host system. The development of a blue light-inducible expression system necessitated the construction of the pEL222-bFGF vector through a series of sophisticated molecular cloning procedures. Initially, the bFGF gene was amplified from the pET28-bFGF template via high-fidelity PCR. The thermal cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 15 s, annealing at 58 °C for 15 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 5 min. Concurrently, a DNA fragment encompassing the EL222 transcriptional activator expression cassette and the blue light-inducible promoter (PBLI) was amplified from the pKEIR plasmid using similar PCR conditions [43]. The amplified bFGF gene and the EL222-PBLI fragment were subsequently assembled into a single construct employing the ClonExpress MultiS One Step Cloning Kit (Vazyme, Nanjing, China), following the manufacturer’s recommended protocol. To ensure the fidelity of the cloned sequences, the resulting plasmid constructs underwent DNA sequencing analysis (Genewiz, Suzhou, China), confirming the absence of unintended mutations or sequence alterations. Following sequence validation, the fully validated pEL222-bFGF expression plasmids were introduced into chemically competent E. coli DH5α cells through heat shock transformation.
2.4. Blue Light-Induced Expression System
The blue light-induced expression system was implemented using a modified protocol based on previous reports [41,43]. Overnight cultures were inoculated into 100 mL of M9 minimal medium contained in 250 mL Erlenmeyer flasks. The cultures were incubated at 37 °C with orbital shaking at 200 rpm under controlled blue light illumination conditions. The illumination system consisted of a customized 4 × 4 LED panel (dimensions: 70 mm × 110 mm; power: 5 W; wavelength: 450 nm) positioned 20 cm above the culture vessels to ensure uniform light distribution across all samples. The light intensity incident on the cell cultures was measured using a calibrated luminometer (TASI TA632B, Suzhou, China) and adjusted to 500 lux. To establish appropriate controls, dark cultures were prepared by completely shielding the flasks from light exposure using aluminum foil. These control cultures were placed alongside the illuminated samples within the same incubator to ensure identical environmental conditions, with the exception of light exposure.
2.5. Protein Expression, Purification and Analysis
The expression of bFGF was conducted using strain DHELF harboring the pEL222-bFGF plasmid. A single colony was inoculated into 20 mL of LB broth and cultivated at 30 °C with orbital shaking at 200 rpm for 16–18 h. Subsequently, 1 mL of this overnight culture was used to inoculate 100 mL of fresh LB broth, which was then incubated under identical conditions until the optical density at 600 nm (OD600) was approximately 0.5. At this point, bFGF expression was induced by activating the blue light illumination system, and cultivation was continued for an additional 10 h under constant illumination. Post-induction, bacterial cells were harvested by centrifugation at 8014 relative centrifugal force (rcf) for 10 min at 4 °C using an Eppendorf 5804 R centrifuge. The resultant cell pellet was resuspended in a lysis buffer comprising 20 mM Tris-HCl (pH 8.0), 200 mM NaCl, and 100 µg/mL lysozyme. To facilitate cell lysis, the suspension was incubated at 37 °C for 1 h, followed by sonication-mediated mechanical disruption conducted under controlled temperature conditions using an ice bath. The lysate was clarified by centrifugation, and the supernatant was subjected to affinity chromatography using a Ni-NTA column (Genscript, Nanjing, China) for the purification of His-tagged bFGF. The purification was performed using a 5 mL Ni-NTA column pre-equilibrated with binding buffer (50 mM sodium dihydrogen phosphate, 150 mM NaCl, pH 8.0). The clarified lysate was loaded onto the column at a flow rate of 1 mL/min. Non-specifically bound proteins were removed through sequential washing steps using wash buffer (50 mM sodium dihydrogen phosphate, 150 mM NaCl, 5 mM imidazole, pH 8.0). The His-tagged bFGF was eluted using a stepwise gradient of imidazole concentrations: 5 mM, 20 mM, 50 mM, and 100 mM. To assess the purity and molecular weight of the expressed protein, samples from various stages of the purification process were analyzed by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) using a 12% polyacrylamide gel. Protein bands were visualized by staining with Coomassie Brilliant Blue R-250. Further characterization of the purified bFGF was performed using Western blot analysis with anti-His tag antibodies to confirm the identity of the recombinant protein. Additionally, protein concentration was determined using the Bradford assay kit (Beyotime, Beijing, China), and the biological activity of the purified bFGF was assessed through a cell proliferation assay using fibroblast cells [44].
2.6. RNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated from the induced transformants using the Bacteria Total RNA Isolation Kit (Sangon Biotech, Shanghai, China), following the manufacturer’s recommended protocol. The quantity and quality of the extracted RNA were assessed using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA), with concentration measurements recorded at 260 nm. To ensure the removal of genomic DNA contamination and to generate cDNA for subsequent analysis, 1 µg of total RNA was reverse transcribed using the HiScript III RT SuperMix for qPCR Kit (Vazyme, Nanjing, China) in accordance with the supplier’s instructions. Quantitative real-time PCR (qPCR) was performed to evaluate the relative transcription levels of the target gene across different strains. The qPCR reactions were carried out using the HiScript II Q RT SuperMix kit (Vazyme, Nanjing, China) on a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA). Each reaction was performed in technical triplicates, with appropriate negative controls included to ensure the absence of contamination or non-specific amplification.
2.7. Genetic Optimization of Engineered bFGF-Producing Strain
To optimize the blue light-inducible expression system, a series of genetic modifications were implemented. Initially, the promoter BBa_J23118 of plasmid pEL222-bFGF was systematically replaced with other synthetic promoters to evaluate the impact of promoter strength on system performance. The resulting plasmids were then transformed into E. coli DH5α. Next, a thorough examination of the PBLI promoter was conducted by modifying the −35 and −10 hexamer sequences, which were critical for RNA polymerase binding and transcription initiation. Site-directed mutagenesis was employed to generate a library of promoter variants with altered spacer lengths and consensus sequence similarities, allowing for fine-tuning of promoter activity. To optimize translation initiation efficiency, various ribosome binding sequences (RBS) were inserted upstream of the bFGF gene. These RBS variants, designed to modulate the strength of ribosome recruitment, were cloned into the plasmid using overlap extension PCR. Finally, the impact of the EL222-binding region (C120) length and copy number on system performance was investigated to elucidate the relationship between operator site availability and transcriptional output. Several new constructs were designed and synthesized, featuring varying lengths of the C120 sequence. Specifically, C120 sequences of 20, 25, 30, 35, and 45 base pairs were individually generated and subsequently inserted into the plasmid vector. Plasmids containing one, two, three, four, and five copies of the C120 sequence were constructed, allowing for the evaluation of binding site saturation effects. All genetic modifications were confirmed by Sanger sequencing before functional characterization.
2.8. Optimization of Blue Light-Induced bFGF Fermentation
To optimize the blue light-induced bFGF fermentation process, four key parameters were further investigated: initial cell density at light induction, light intensity, illumination duration, and pulsed illumination patterns. The engineered strain DHELF-Opto, harboring the optimized blue light-responsive bFGF expression system, was cultivated in 500 mL Erlenmeyer flasks containing 100 mL of LB medium. To determine the optimal cell density for initiating blue light induction, cultures were grown to various OD600 ranging from 0.2 to 0.8, with increments of 0.1. Subsequently, the effect of blue light intensity on bFGF expression was evaluated by exposing cultures to blue light at intensities of 200, 400, 600, 800, and 1000 lux. To optimize the illumination period during fermentation, the total duration of blue light exposure varied, with testing periods of 2, 4, 6, 8, 10, and 12 h. Lastly, the impact of pulsed illumination on bFGF production was investigated. Various pulsed illumination cycles were tested, including light:dark ratios of 1h:1h, 2h:2h, 4h:4h, and continuous OFF (Dark) or continuous ON (Full light). The optimal combination of initial OD600, light intensity, illumination duration, and pulse pattern was determined based on the highest bFGF yield and production rate.
3. Results and Discussion
3.1. Bioinformatics Analysis of Porcine bFGF
The amino acid sequence of Sus scrofa bFGF protein (NCBI Reference Sequence: NP_001392443.1) was subjected to a comprehensive BLAST search against both the NCBI and UniProt databases to identify homologous sequences. As illustrated in Figure 1A, multiple sequence alignment revealed a high degree of conservation among bFGF proteins across various species. Notably, only two amino acid substitutions were observed between the human and porcine bFGF sequences, highlighting the potential for cross-species applicability and functional conservation [45]. To further investigate the evolutionary relationships among bFGF proteins, a phylogenetic tree was constructed using the neighbor-joining method based on the aligned sequences (Figure 1B) [46]. The resulting dendrogram demonstrated close phylogenetic proximity among the analyzed bFGF proteins, corroborating the high sequence similarity observed in the multiple sequence alignment and suggesting a conserved evolutionary history among these bFGF proteins. Subsequent analytical efforts were conducted on the physicochemical properties and structural features of porcine bFGF. The hydropathicity profile was generated using the ProtScale tool (https://web.expasy.org/protscale/ (accessed on 5 September 2022)), revealing an overall hydrophilic character for the bFGF protein (Figure 1C). This hydrophilic nature suggested favorable solubility in aqueous environments, which might facilitate purification processes in recombinant bFGF production systems. Transmembrane topology prediction was performed using the DeepTMHMM-1.0 algorithm (https://services.healthtech.dtu.dk/services/DeepTMHMM-1.0/ (accessed on 6 September 2022)) [47]. As depicted in Figure S1A, no transmembrane domains were identified in the porcine bFGF protein sequence, indicating that all amino acid residues were likely to be located on the intracellular side of the cell membrane. This prediction aligned with the hydrophilicity analysis and suggested that the full-length bFGF protein could be expressed without the need for complex membrane integration processes, potentially simplifying heterologous expression strategies and facilitating the production of recombinant bFGF. Finally, signal peptide prediction was conducted using SignalP-6.0 (https://services.healthtech.dtu.dk/services/SignalP-6.0/ (accessed on 8 September 2022)) [48]. The analysis, presented in Figure S1B, revealed the absence of a canonical signal peptide sequence, indicating that bFGF was not naturally secreted and was likely to accumulate intracellularly when expressed in heterologous systems. These findings provided valuable insights into sequence conservation, evolutionary relationships, and structural characteristics, offering the groundwork for future studies on recombinant bFGF protein production and purification.
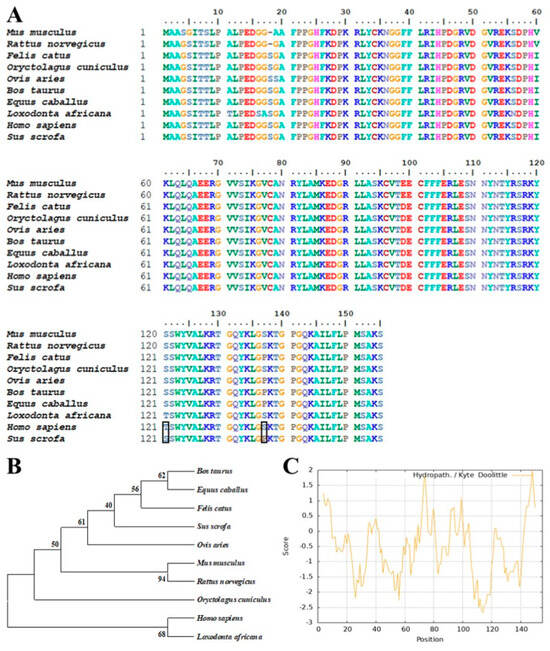
Figure 1.
Analysis of bFGF sequences and properties. (A) ClustalW alignment of bFGF amino acid sequences from Homo sapiens and Sus scrofa. Black boxes highlight the differences between the two species. (B) Neighbor-joining phylogenetic tree of 10 bFGF sequences constructed using MEGA 11.0 with 1000 bootstrap replicates. (C) Hydropathicity profile of bFGF (S. scrofa) predicted using the ProtScale tool.
3.2. Expression and Purification of Recombinant Porcine bFGF
To ensure the successful soluble expression of porcine bFGF protein, the bFGF gene was initially subjected to codon optimization for optimal expression in E. coli. The optimized gene was subsequently ligated into the pET-28a (+) expression vector, resulting in the construction of the pET28-bFGF plasmid. This expression vector was then transformed into E. coli BL21 cells. A single colony of the transformed E. coli BL21 cells, designated as BLETF, was cultivated under standard conditions at 37 °C. Protein expression was induced when the OD600 of the culture reached 0.5 by adding IPTG to a final concentration of 0.5 mM. After 10 h of cultivation, the expression of bFGF was analyzed using SDS-PAGE. Initial results revealed a significant formation of inclusion bodies at 37 °C, necessitating optimization of the expression conditions. To address this issue, the induction temperature was reduced to 25 °C. This modification resulted in successful soluble expression of bFGF in E. coli, as evidenced by SDS-PAGE analysis (Figure 2A). The recombinant porcine bFGF protein, which included a histidine tag for purification purposes, was successfully isolated using nickel-affinity chromatography. The majority of contaminating proteins were eliminated during the flow-through and washing steps, while the target protein, appearing as a distinct band at approximately 18.1 kDa, was eluted using 100 mM imidazole. The resulting product was confirmed to be the porcine bFGF protein through Western blot analysis (Figure 2B). The concentration of the purified recombinant bFGF protein was determined using the bicinchoninic acid (BCA) assay.
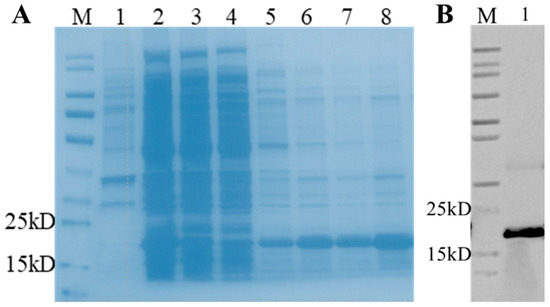
Figure 2.
Purification and analysis of recombinant His-tagged bFGF expressed in E. coli BLETF cells. (A) SDS-PAGE analysis of bFGF purification steps. Recombinant His-tagged bFGF was expressed and purified with high yield and purity. Lane M: Protein molecular weight marker; Lane 1: Precipitated fraction; Lane 2: Supernatant; Lane 3: Flow-through; Lane 4: Wash fraction; Lanes 5–8: Elution fractions with increasing imidazole concentrations (5 mM, 20 mM, 50 mM, and 100 mM, respectively). (B) Western blot analysis of the expressed bFGF product using anti-His tag antibody.
The construction of the recombinant plasmid pEL222-bFGF involved the fusion of the EL222 cassette with the bFGF gene. The EL222 gene was placed under the regulation of the constitutive synthetic promoter BBa_J23118 (medium-strength), which was known for its robust and reliable expression in various genetic backgrounds. Concurrently, the bFGF gene was controlled by the blue light-inducible promoter (PBLI), a regulatory element that allows for the precise temporal control of gene expression in response to blue light signals [41,43]. This particular configuration within the pEL222-bFGF plasmid ensured the independent and constitutive expression of EL222 alongside the inducible expression of bFGF under dark conditions, thereby facilitating the study of bFGF expression dynamics in response to blue light stimuli. To assess the efficacy of blue light-induced bFGF expression, the pEL222-bFGF plasmid was transformed into E. coli DH5α competent cells, resulting in the generation of a recombinant strain designated as DHELF. Post-transformation, a single colony was selected and cultured in fresh LB medium at 37 °C for a duration of 12 h under both dark and blue light illumination conditions. Subsequent analysis using SDS-PAGE revealed a faint band at approximately 18.1 kDa in the precipitate fraction obtained from cultures maintained under dark conditions (Figure 3A). This observation indicated a basal level of bFGF expression, which was insufficient for effective protein purification due to the minimal elution observed, even with increasing concentrations of imidazole during the purification process. In stark contrast, upon exposure to blue light, a prominent protein band was observed in the supernatant fraction of DHELF cells, unambiguously demonstrating the successful induction of bFGF expression by blue light illumination (Figure 3B). Notably, the quantity of bFGF protein induced under blue light conditions was substantially higher than that observed under dark conditions, suggesting that the blue light-induced expression strategy yielded a sufficient amount of protein for purification and subsequent experiments. To assess the biological activity of the recombinant bFGF protein induced by blue light and evaluate any potential impact of the histidine tag on its functionality, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed using NIH3T3 cells. The results, as illustrated in Figure 3C, demonstrated that the proliferation of NIH3T3 cells treated with the blue light-induced bFGF protein was comparable to that observed with commercial human bFGF protein treatment. This finding suggested that the presence of blue light did not significantly affect the biological activity of the recombinant porcine bFGF protein, indicating its potential utility for following research and applications.
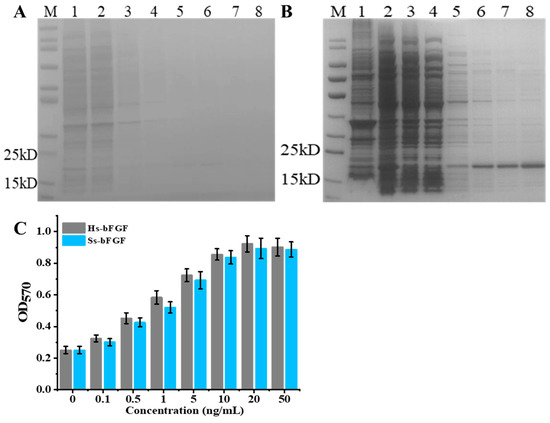
Figure 3.
SDS-PAGE analysis of His-tagged bFGF purification from E. coli DHELF cells under (A) dark and (B) blue light illumination conditions. M: Molecular weight marker; Lanes: 1, Precipitate; 2, Supernatant; 3, Flow-through; 4, Wash fraction; 5–8, Elution fractions with increasing imidazole concentrations (5, 20, 50, and 100 mM, respectively). (C) Dose-dependent effect of different bFGF sources on cell viability measured by MTT assay. Hs-bFGF represented commercial human bFGF, while Ss-bFGF indicated blue light-induced porcine bFGF. Cell viability was assessed by measuring the optical density (OD) at 570 nm. bFGF concentrations ranged from 0 to 50 ng/mL.
3.3. Optimization of EL222-Based Engineered Strain
To enhance the efficiency and precision of blue light-induced bFGF expression in the recombinant strain DHELF, a systematic optimization strategy was implemented, focusing on the pivotal regulatory components of the optogenetic system (Figure 4A) [43,49]. The primary objective was to investigate the interplay between the expression levels of the EL222 gene and the production of bFGF protein, thereby enabling the fine-tuning of the light-responsive mechanism. The optimization process commenced with an investigation into the impact of EL222 transcription rates. This was achieved by replacing the original promoter with a series of variant promoters exhibiting differing strengths, all of which were sourced from the Anderson promoter library. Specifically, the constitutive promoter BBa_J23118 was systematically replaced with a series of promoters of varying strengths: two weak (BBa_J23112, BBa_J23103), two medium-strength (BBa_J23106, BBa_J23108), and two strong (BBa_J23100, BBa_J23102) promoters. As shown in Figure 4B, the relationship between EL222 transcription levels and bFGF protein expression exhibited a non-linear response curve. When the transcription of the EL222 gene was incrementally increased, the expression of bFGF protein initially rose, reached a maximum, and subsequently declined. Among the tested groups, the highest expression of bFGF protein was achieved using the medium-strength promoter (BBa_J23106). This obtained phenomenon suggested that suboptimal EL222 levels were insufficient to efficiently induce the expression of bFGF protein, while excessive EL222 expression potentially led to competitive effects, possibly attributable to the sequestration of cellular resources or negative feedback mechanisms. Furthermore, at low concentrations, EL222 dimerization was efficient, leading to robust binding to the PBLI promoter and subsequent strong transcriptional activity. However, with increased EL222 concentrations, the number of EL222 dimers activated by blue light surpassed the limited availability of C120 binding sites within the PBLI promoter. Once these sites were saturated, the additional EL222 dimers could no longer bind to the promoter, culminating in a plateau in bFGF expression and, thus, a non-linear response [42,50]. These findings underscored the critical importance of achieving a balanced expression between the optogenetic regulator (EL222) and the target protein (bFGF) in optimizing the performance of the light-inducible system. Importantly, this balance was considered to be essential for maximizing the efficiency of the optogenetic system and ensuring the robustness of the light-induced bFGF expression.
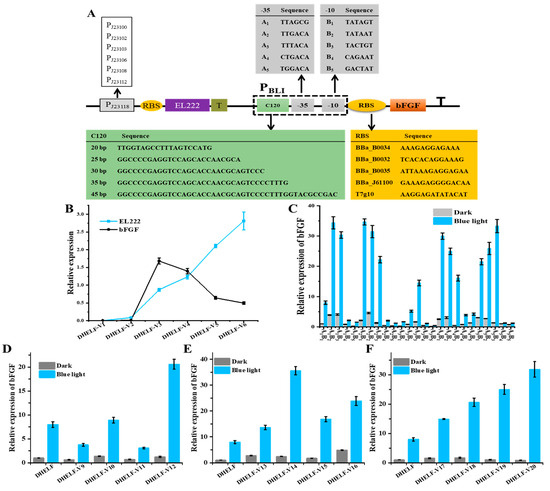
Figure 4.
Optimization strategies for enhancing bFGF expression in DHELF strain. (A) Schematic of the optimization strategy implemented in DHELF. This panel illustrates the key components involved in the optimization process. (B) Correlation between EL222 transcription levels and bFGF expression. (C) Comparative analysis of bFGF expression levels induced by various combinations of −35 and −10 hexamer sequences. (D) Effect of ribosome binding site (RBS) strength on bFGF expression. (E) Impact of C120 sequence length on bFGF expression. (F) Influence of C120 copy number on bFGF expression.
Subsequently, the correlation between the strength of the PBLI promoter and the transcriptional activity of the bFGF gene was investigated [41,49]. To this end, we conducted a comprehensive analysis of the PBLI promoter architecture by systematically modifying the −35 and −10 hexamer sequences, which were critical determinants of promoter strength and specificity. This approach aimed to fine-tune the promoter’s strength and minimize leakiness, ultimately identifying the optimal combination for blue light-induced bFGF expression while minimizing background expression in the absence of blue light stimulation. As presented in Figure 4C, the A3B5 variant of the promoter construct exhibited the lowest level of basal expression, indicative of a successful reduction in leakiness. However, the relative induction in bFGF expression under blue light illumination was modest, at only 8.1-fold. Notably, while the basal expression of the A4B3 group was significantly lower than that of A1B1 (the control PBLI) under dark conditions, a substantial 34.8-fold induction in bFGF expression was observed upon blue light illumination. These results underscored the critical role of PBLI promoter strength, as influenced by the specific combination of −35 and −10 hexamers, in achieving enhanced blue light-induced bFGF expression. Furthermore, we tried to optimize the efficiency of translation initiation by evaluating various ribosome binding sites (RBS) sequences positioned upstream of the bFGF gene [51]. A range of RBS with different predicted strengths was selected and tested to identify the optimal translation initiation rate that effectively balanced protein production with the allocation of cellular resources. As illustrated in Figure 4D, a stronger RBS variant (rbsD) was strategically positioned upstream of the bFGF coding sequence. Upon blue light induction, the strain DHELF-V12, which harbored the optimized RBS (T7g10), demonstrated the highest increase in bFGF protein expression among all tested strains, highlighting the efficacy of this optimization strategy. The result exhibited that the optimization of the PBLI promoter and the RBS sequences not only enhanced the inducibility of the bFGF gene but also ensured that the expression system remained quiescent in the absence of blue light stimulus, thereby minimizing the metabolic burden on the E. coli cell. These findings collectively demonstrated the importance of fine-tuning both transcriptional and translational control elements in maximizing the efficiency of blue light-induced bFGF expression.
Lastly, the EL222-binding region (C120) was identified as a critical element influencing the binding kinetics and cooperativity of blue light-activated EL222 dimers [39,40,43]. Acknowledging the vital role of this region on the overall performance of the EL222-based optogenetic system, we undertook a systematic optimization of both the length and copy number of C120 within the engineered PBLI promoter region. This optimization strategy was predicated on the hypothesis that fine-tuning the two parameters would enhance the system’s responsiveness to blue light stimulation, thereby potentially leading to improved control over bFGF expression. To elucidate the relationship between C120 length and PBLI promoter strength, we constructed a series of promoter variants with varying lengths of the C120 region. As illustrated in Figure 4E, the results demonstrated a non-linear relationship between the length of C120 and the resulting bFGF expression levels. Notably, a C120 length of 30 base pairs (bp) yielded the highest bFGF expression, suggesting an optimal spatial configuration for EL222 dimer binding and subsequent transcriptional activation. Subsequently, we investigated the impact of C120 copy number on promoter activity by generating a series of PBLI variants, each incorporating a different number of C120 repeats. Our findings revealed that the incorporation of five C120 copies resulted in maximal bFGF protein levels (Figure 4F). This observation implied that multiple EL222 binding sites might facilitate cooperative binding effects or create a more favorable local chromatin environment for transcriptional activation, thereby enhancing the overall efficiency of the EL222-based optogenetic system. These results collectively underscored the importance of fine-tuning C120 in EL222-based optogenetic systems and provided valuable insights for the rational design of blue light-inducible expression of the bFGF protein. Through the multifaceted optimization process, a highly responsive and tightly controlled optogenetic system for bFGF production was developed.
3.4. Fermentation of Blue Light-Induced bFGF Expression in E. coli
Building upon the successful optimization of the blue light-inducible bFGF expression strain, we engineered a novel plasmid, designated pEL222-bFGF-Opto, to enhance bFGF protein production in the resulting strain DHELF-Opto. This construct was designed to incorporate the key elements identified above as crucial for the enhancement of bFGF expression. These included BBa_J23106, the A4B3 variant, the T7g10 RBS, and five tandem copies of the C120-25bp element. To further augment bFGF protein yields, we undertook a systematic optimization of the fermentation process. This optimization was focused on critical parameters that were known to influence the efficiency of blue light induction and, by extension, the overall expression of the bFGF protein.
First, a pivotal factor in our investigation was the determination of the OD600 at which to initiate blue light induction. As illustrated in Figure 5A, we observed a non-linear relationship between the OD600 value at the time of induction and the subsequent bFGF protein yield. The production of bFGF protein initially increased with increasing OD600 values, reaching a maximum at an OD600 of 0.6 before declining at higher cell densities. This biphasic response suggested a complex interplay between cellular physiology and protein expression dynamics, indicative of a delicate balance between cellular growth and recombinant protein production. We hypothesized that at lower OD600 values, the blue light stimulus might preferentially promote cell division rather than protein production, resulting in suboptimal bFGF yields. Conversely, at higher OD600 values, the increased competition for cellular resources might constrain the efficiency of bFGF protein expression. The observed optimum at an OD600 of 0.6 likely represented a balance point where the metabolic capacity of the cells was sufficiently developed to support robust protein production yet not overwhelmed by the demands of high-density growth.
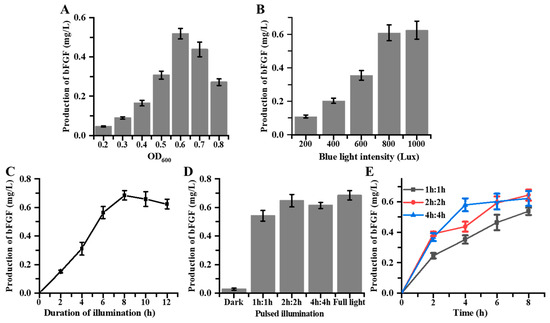
Figure 5.
Optimization of bFGF fermentation in the DHELF-Opto strain through modulation of blue light illumination parameters. (A) The optimization of initiate OD600 for blue light illumination initiation. (B) Dose–response relationship between blue light intensity and bFGF production. (C) Effect of blue light illumination duration on bFGF production. (D) Influence of intermediate light pulsing on bFGF production. (E) Temporal dynamics of bFGF accumulation at 2 h intervals under various blue light pulsing regimes.
In subsequent experiments, we conducted an in-depth analysis to elucidate the relationship between blue light intensity and the production of bFGF within E. coli cultures. The cultures, upon reaching an OD600 of 0.6, were exposed to a broad spectrum of blue light intensities, ranging from the absence of light (0 lux) to a maximum intensity of 1000 lux. The results, as delineated in Figure 5B, demonstrated a positive correlation between the intensity of blue light and the production of bFGF, indicative of a dose-dependent relationship. In the absence of blue light, bFGF levels remained below the detection threshold of our assay, affirming the stringent regulation of the optogenetic system and confirming negligible leaky expression under non-inducing conditions. As the blue light intensity increased, there was a concomitant and significant upregulation in bFGF expression. This positive correlation was sustained up to an illumination intensity of 800 lux, at which point the system achieved a maximum yield of 0.61 ± 0.05 mg/L of bFGF. Interestingly, further increments in light intensity to 1000 lux did not precipitate any additional enhancement in bFGF production, implying that the EL222-based optogenetic system had reached its saturation threshold. This saturation phenomenon might be attributed to factors such as the limited dynamic range of the EL222 dimers or potential phototoxicity effects at higher light intensities. These findings suggested that there was an optimal range of blue light intensity within which the optogenetic system operated most efficiently, beyond which no additional benefits were conferred in terms of bFGF production. Moreover, the observed dose–response relationship provided a foundation for implementing dynamic control strategies, potentially allowing for fine-tuned modulation of bFGF expression in response to varying fermentation processes.
Next, we conducted an in-depth temporal analysis to ascertain the dynamics of bFGF production in response to blue light stimulation within E. coli cultures. To figure out the impact of illumination duration on bFGF production, cultures were cultivated to an optimal OD600 of 0.6 and subsequently exposed to blue light at an intensity of 800 lux for varying durations: 0 (dark control), 2, 4, 6, 8, 10, and 12 h. The results demonstrated a time-dependent increase in bFGF production, which correlated positively with extended periods of illumination (Figure 5C). Significant bFGF expression was first detected after 2 h of exposure to blue light, with concentrations reaching 0.15 ± 0.01 mg/L. The most rapid increase in bFGF production was observed between 2 and 8 h of illumination, culminating in bFGF levels of 0.68 ± 0.03 mg/L at the 8 h time point. Notably, further extension of the illumination period beyond 8 h did not result in substantial increments in bFGF levels, with 10 h and 12 h illumination periods producing bFGF concentrations of 0.66 ± 0.05 mg/L and 0.62 ± 0.03 mg/L, respectively. This plateau in expression suggested saturation of the optogenetic system. The dark control (0 h) exhibited minimal bFGF production (0.03 ± 0.01 mg/L), confirming the blue light dependence of the engineered system. These results suggested that 8 h of blue light exposure at 800 lux provided optimal conditions for bFGF production in DHELF-Opto under the experimental parameters tested. Furthermore, to investigate whether continuous illumination was necessary for sustained bFGF production, we implemented a pulsed illumination strategy. Cultures were subjected to alternating periods of blue light (800 lux) and darkness in various ratios over a total duration of 8 h. Continuous illumination for 8 h served as a control. Interestingly, pulsed illumination maintained different levels of bFGF production across all tested regimes (Figure 5D). The 2h:2h pulsed illumination regime resulted in bFGF yields of 0.65 ± 0.04 mg/L, which was notably comparable to the continuous illumination. This pulsed regime achieved 94.6% of the yield obtained under full light conditions, demonstrating its efficiency in bFGF production. This finding suggested that intermittent light exposure was sufficient to sustain the optogenetic response and maintain high levels of bFGF production. To further characterize the system’s response to pulsed illumination, we monitored bFGF expression levels at 2 h intervals throughout the 8 h experiment (Figure 5E). The 2h:2h regime showed a stepwise increase in bFGF levels, with noticeable rises during light periods and plateaus during dark periods. In contrast, the 1h:1h regime displayed more frequent, smaller increments in bFGF levels, while the 4h:4h regime exhibited larger, less frequent increases. These observations provided insights into the kinetics of the optogenetic system and its ability to maintain activation during short dark periods. Collectively, the efficacy of pulsed illumination strategies suggested the potential for energy-efficient bioprocess designs in scaled-up production.
4. Conclusions
In summary, we have successfully developed and refined a blue light-inducible system for the precise modulation of bFGF expression in E. coli. By harnessing the blue light-responsive properties of the EL222 protein, we have achieved robust and tunable regulation of bFGF expression, demonstrating the potential of optogenetic systems in the production of bFGF protein. Our findings revealed that the bioactivity of bFGF protein induced by blue light was comparable to that produced by the conventional T7-expression system, validating the effectiveness of our optogenetic approach. Importantly, the blue light-responsive EL222 system provided stringent regulation of bFGF expression, allowing for precise temporal control without the need for chemical inducers. This novel system might not only address existing challenges in bFGF production but also offer substantial advantages for downstream purification processes.
Through a systematic optimization process, we fine-tuned the regulatory elements that govern bFGF expression in engineered strain, including promoter strength, RBS, and C120 within the PBLI promoter. Furthermore, our investigation into the fermentation process parameters, encompassing initial cell density, light intensity, illumination duration, and pulsed illumination patterns, provided valuable insights into the dynamics of the EL222-based optogenetic system. We determined that an initial OD600 of 0.6, a blue light intensity of 800 lux, and a total illumination duration of 8 h, with a pulsed illumination pattern of 2 h on and 2 h off, were optimal for achieving high bFGF expression levels in E. coli. This comprehensive characterization not only provided insights into the kinetics and efficiency of blue light-induced bFGF expression but also highlighted the potential for fine-tuned temporal control over protein production. The optogenetic system developed in this study offered a versatile and environmentally friendly approach to induce bFGF expression, with broad applicability in biotechnology and potential for scaling up in industrial bioprocesses. This strategy has the potential to be extended to the production of other high-value recombinant proteins, thereby providing a valuable tool for the biotechnology industry. Future studies may focus on further optimizing the system for large-scale production, exploring its applicability in other host organisms, and investigating its potential for the expression of other proteins.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation10120612/s1, Table S1: Bacterial strains and plasmids used in this study; Table S2: Primers used in this study; Figure S1: Structural predictions for bFGF using advanced bioinformatics tools. (A) Transmembrane topology prediction generated by the DeepTMHMM—1.0 algorithm. (B) Signal peptide analysis performed with SignalP—6.0.; Figure S2: The key genetic parts of pEL222-bFGF-Opto.
Author Contributions
F.M. and Z.X. co-first authors. F.M.: Methodology, Validation, Formal analysis, Investigation, Date Curation, Visualization, Writing—Original Draft. Z.X.: Methodology, Validation, Investigation, Data Curation, Visualization, Writing—Original Draft. X.F.: Validation, Formal analysis, Investigation, Visualization. Z.W.: Validation, Data Curation, Visualization. L.Z.: Conceptualization, Methodology, Project administration, Supervision, Resources, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number: 32101910).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Haraguchi, Y.; Asahi, T.; Kato, Y.; Kondo, A.; Hasunuma, T.; Shimizu, T. A serum-free culture medium production system by co-culture combining growth factor-secreting cells and L-lactate-assimilating cyanobacteria for sustainable cultured meat production. Sci. Rep. 2024, 14, 19578. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, S.; Post, M.J.; Moutsatsou, P. Towards resource-efficient and cost-efficient cultured meat. Curr. Opin. Food Sci. 2022, 47, 100885. [Google Scholar] [CrossRef]
- Zhang, M.C.; Zhao, X.Y.; Li, Y.; Ye, Q.H.; Wu, Y.W.; Niu, Q.Y.; Zhang, Y.; Fan, G.H.; Chen, T.X.; Xia, J.R.; et al. Advances in serum-free media for CHO cells: From traditional serum substitutes to microbial-derived substances. Biotechnol. J. 2024, 19, e2400251. [Google Scholar] [CrossRef]
- Geng, S.L.; Zou, Y.; Bai, Z.Y.; Zhang, M.; Wang, C.; Wang, T.Y. Serum-free medium for recombinant protein expression in insect cells. Biotechnol. Appl. Biochem. 2024, 1–15. [Google Scholar] [CrossRef]
- Romero, S.G.; Boyle, N. Systems biology and metabolic modeling for cultivated meat: A promising approach for cell culture media optimization and cost reduction. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3422–3443. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.J.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhao, X.R.; Li, X.L.; Du, G.C.; Zhou, J.W.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lin, J.X.; Li, J.; Mi, Y.L.; Zeng, W.D.; Zhang, C.Q. Basic fibroblast growth factor suppresses meiosis and promotes mitosis of ovarian germ cells in embryonic chickens. Gen. Comp. Endocrinol. 2012, 176, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhang, G.W.; Gu, T.X.; Li-Ling, J.; Wen, T.; Zhao, Y.; Wang, C.; Fang, Q.; Yu, L.; Liu, B. Exogenous basic fibroblast growth factor promotes cardiac stem cell-mediated myocardial regeneration after miniswine acute myocardial infarction. Coron. Artery Dis. 2011, 22, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nowwarote, N.; Sawangmake, C.; Pavasant, P.; Osathanon, T. Review of the role of basic fibroblast growth factor in dental tissue-derived mesenchymal stem cells. Asian Biomed. 2015, 9, 271–283. [Google Scholar]
- Ramasamy, R.; Tong, C.K.; Yip, W.K.; Vellasamy, S.; Tan, B.C.; Seow, H.F. Basic fibroblast growth factor modulates cell cycle of human umbilical cord-derived mesenchymal stem cells. Cell Prolif. 2012, 45, 132–139. [Google Scholar] [CrossRef]
- Garor, R.; Abir, R.; Erman, A.; Felz, C.; Nitke, S.; Fisch, B. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil. Steril. 2009, 91, 1967–1975. [Google Scholar] [CrossRef]
- de Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.J.; Han, M.H.; Yoon, N.K.; Kim, Y.C.; Ahn, J. Effective production of human growth factors in Escherichia coli by fusing with small protein 6HFh8. Microb. Cell Fact. 2021, 20, 9. [Google Scholar] [CrossRef]
- Gasparian, M.E.; Elistratov, P.A.; Drize, N.I.; Nifontova, I.N.; Dolgikh, D.A.; Kirpichnikov, M.P. Overexpression in Escherichia coli and purification of human fibroblast growth factor (FGF-2). Biochemistry 2009, 74, 221–225. [Google Scholar] [CrossRef]
- Mu, X.P.; Kong, N.; Chen, W.L.; Zhang, T.; Shen, M.; Yan, W.Q. High-level expression, purification, and characterization of recombinant human basic fibroblast growth factor in Pichia pastoris. Protein Expr. Purif. 2008, 59, 282–288. [Google Scholar] [CrossRef]
- Imsoonthornruksa, S.; Pruksananonda, K.; Parnpai, R.; Rungsiwiwut, R.; Ketudat-Cairns, M. Expression and purification of recombinant human basic fibroblast growth factor fusion proteins and their uses in human stem cell culture. J. Mol. Microbiol. Biotechnol. 2015, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Soleyman, M.R.; Khalili, M.; Khansarinejad, B.; Baazm, M. High-level expression and purification of active human FGF-2 in Escherichia coli by codon and culture condition optimization. Iran. Red Crescent Med. J. 2016, 18, e21615. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Mirzahoseini, H.; Abad, M.A.K.; Movahed, M.A. High level expression of human basic fibroblast growth factor in Escherichia coli: Evaluating the effect of the GC content and rare codons within the first 13 codons. Afr. J. Biotechnol. 2010, 9, 2456–2462. [Google Scholar]
- Masuda, A.; Xu, J.; Minamihata, K.; Kagawa, G.; Hamada, Y.; Morifuji, Y.; Yano, T.; Hino, M.; Morokuma, D.; Karasaki, N.; et al. Production of a biologically active human basic fibroblast growth factor using silkworm-baculovirus expression vector system. J. Asia-Pacif. Entomol. 2018, 21, 716–720. [Google Scholar] [CrossRef]
- Kurokawa, T.; Sasada, R.; Iwane, M.; Igarashi, K. Cloning and expression of cDNA encoding human basic fibroblast growth factor. FEBS Lett. 1987, 213, 189–194. [Google Scholar] [CrossRef]
- Cheng, T.; Cao, W.; Wen, R.; Steinberg, R.H.; LaVail, M.M. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Müller cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 581–591. [Google Scholar]
- Tripathi, N.K.; Shrivastava, A. Recent developments in bioprocessing of recombinant proteins: Expression hosts and process development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Chia, N.; Lee, S.Y.; Tong, Y. Optogenetic tools for microbial synthetic biology. Biotechnol. Adv. 2022, 59, 107953. [Google Scholar] [CrossRef]
- Muller, K.; Naumann, S.; Weber, W.; Zurbriggen, M.D. Optogenetics for gene expression in mammalian cells. Biol. Chem. 2015, 396, 145–152. [Google Scholar] [CrossRef]
- Omelina, E.S.; Yushkova, A.A.; Motorina, D.M.; Volegov, G.A.; Kozhevnikova, E.N.; Pindyurin, A.V. Optogenetic and chemical induction systems for regulation of transgene expression in plants: Use in basic and applied research. Int. J. Mol. Sci. 2022, 23, 1737. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, M.A.; Ip, S.S.; Carrasco-Lopez, C.; Day, C.; Zhao, E.M.; Kawabe, H.; Avalos, J.L. Optogenetic control of the lac operon for bacterial chemical and protein production. Nat. Chem. Biol. 2021, 17, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pouzet, S.; Banderas, A.; Le Bec, M.; Lautier, T.; Truan, G.; Hersen, P. The promise of optogenetics for bioproduction: Dynamic control strategies and scale-up instruments. Bioengineering 2020, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Motta-Mena, L.B.; Reade, A.; Mallory, M.J.; Glantz, S.; Weiner, O.D.; Lynch, K.W.; Gardner, K.H. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat. Chem. Biol. 2014, 10, 196–202. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, J.; Moser, F.; Song, M.; Voigt, C.A. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 2017, 13, 706–708. [Google Scholar] [CrossRef]
- Hennemann, J.; Iwasaki, R.S.; Grund, T.N.; Diensthuber, R.P.; Richter, F.; Moglich, A. Optogenetic control by pulsed illumination. ChemBioChem 2018, 19, 1296–1304. [Google Scholar] [CrossRef]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Luscher, C.; Mahn, M.; Pan, Z.H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2022, 2, 55. [Google Scholar] [CrossRef]
- Soffer, G.; Perry, J.M.; Shih, S.C.C. Real-time optogenetics system for controlling gene expression using a model-based design. Anal. Chem. 2021, 93, 3181–3188. [Google Scholar] [CrossRef]
- Nash, A.I.; McNulty, R.; Shillito, M.E.; Swartz, T.E.; Bogomolni, R.A.; Luecke, H.; Gardner, K.H. Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc. Natl. Acad. Sci. USA 2011, 108, 9449–9454. [Google Scholar] [CrossRef]
- Zoltowski, B.D.; Motta-Mena, L.B.; Gardner, K.H. Blue light-induced dimerization of a bacterial LOV-HTH DNA-binding protein. Biochemistry 2013, 52, 6653–6661. [Google Scholar] [CrossRef]
- Jayaraman, P.; Devarajan, K.; Chua, T.K.; Zhang, H.; Gunawan, E.; Poh, C.L. Blue light-mediated transcriptional activation and repression of gene expression in bacteria. Nucleic Acids Res. 2016, 44, 6994–7005. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, R.; Möglich, A. Light-regulated gene expression in bacteria: Fundamentals, advances, and perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1029403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Geng, F.; Shen, J.; Zhu, P.; Lu, Z.; Lu, F.; Zhou, L. Blue light-mediated gene expression as a promising strategy to reduce antibiotic resistance in Escherichia coli. Biotechnol. J. 2024, 19, 2400023. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz, A.; Savatier, P.; Cortay, V.; Kennedy, H.; Dehay, C. Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J. Neurosci. 2002, 22, 6610–6622. [Google Scholar] [CrossRef]
- Hung, J.H.; Weng, Z. Sequence alignment and homology search with BLAST and ClustalW. Cold Spring Harb. Protoc. 2016, 2016, 1016–1021. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022, bioRxiv:2022.04.08.487609. [Google Scholar]
- Nielsen, H.; Teufel, F.; Brunak, S.; von Heijne, G. SignalP: The Evolution of a Web Server. Methods Mol. Biol. 2024, 2836, 331–367. [Google Scholar]
- Ding, Q.; Ma, D.; Liu, G.Q.; Li, Y.; Guo, L.; Gao, C.; Hu, G.; Ye, C.; Liu, J.; Liu, L.; et al. Light-powered Escherichia coli cell division for chemical production. Nat. Commun. 2020, 11, 2262. [Google Scholar] [CrossRef]
- Stohr, A.M.; Ma, D.R.; Chen, W.L.; Blenner, M. Engineering conditional protein-protein interactions for dynamic cellular control. Biotechnol. Adv. 2024, 77, 108457. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Liu, C.; Guo, J.; Xu, X.; Zhang, H.; Nian, R.; Xian, M. Improvement of isoprene production in Escherichia coli by rational optimization of RBSs and key enzymes screening. Microb. Cell Fact. 2019, 18, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).