Probiotic SYNBIO® Blend’s Impact on Constipation in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
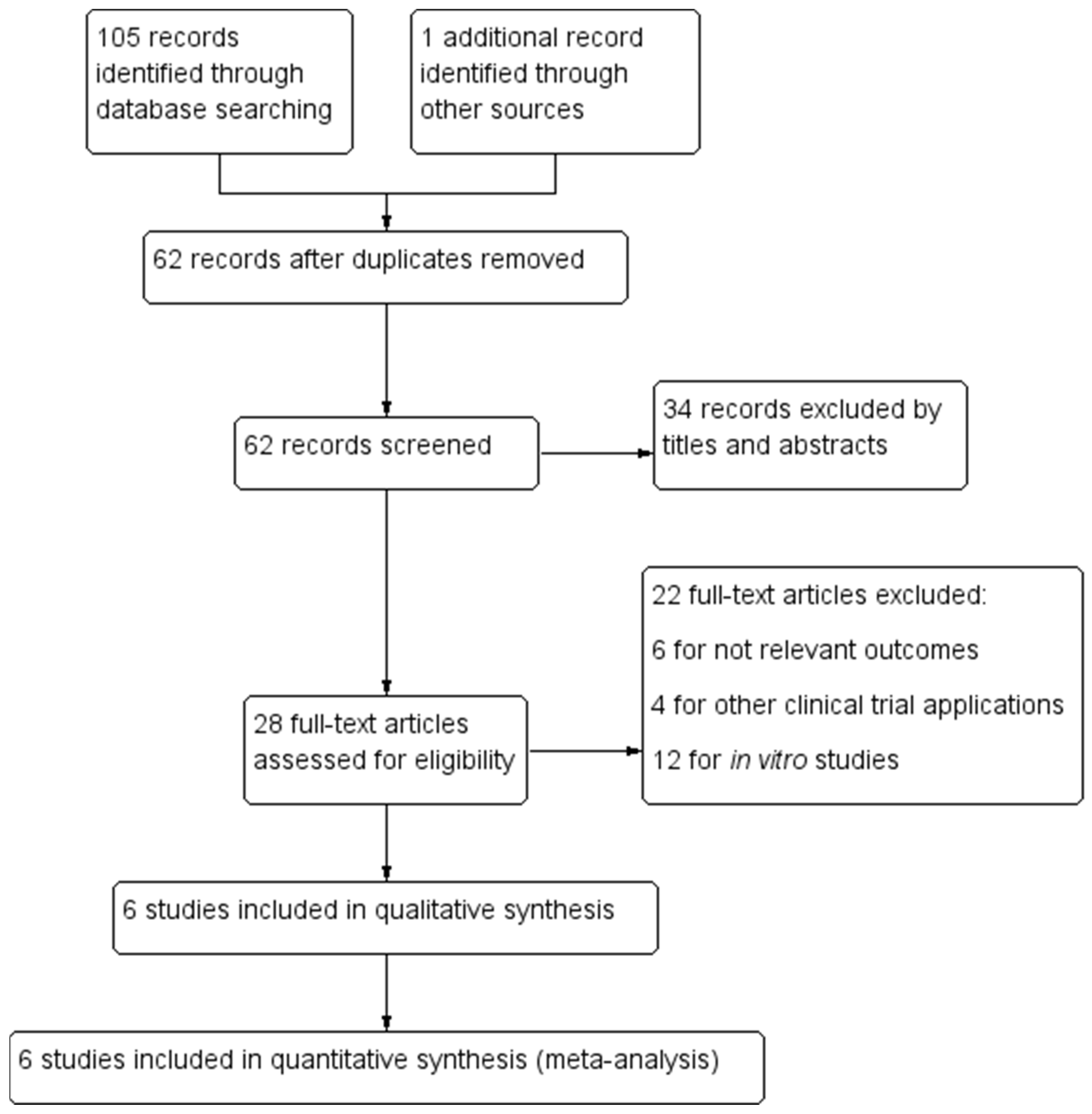
2.2. Strategy and Data Extraction
2.3. Quality Assessment and Assessment of Risk of Bias
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics, and Quality Assessment of Included Studies
3.2. Studies Using SYNBIO® Blend Pertinent to the Meta-Analyses
3.3. Effect of SYNBIO® Blend on Overall Constipation
3.4. Effect of SYNBIO® Blend on Intestinal Regularity
3.5. Effect of SYNBIO® Blend on Stool Volume and Consistency
3.6. Effect of SYNBIO® Blend on Other Outcomes Related to Constipation
3.7. Effect of SYNBIO® Blend on Psychological General Well-Being Index (PGWBI)
3.8. Response to SYNBIO® Blend Supplementation—The Effect of Dose, Form, and Duration
3.9. Adverse Events with Probiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Schoot, A.; Helander, C.; Whelan, K.; Dimidi, E. Probiotics and synbiotics in chronic constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2022, 41, 2759–2777. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Y.; Ma, L.; Ma, X.; Shen, L.; Ma, X.; Chen, Z.; Chen, H.; Li, D.; Su, Z.; et al. Constipation induced gut microbiota dysbiosis exacerbates experimental autoimmune encephalomyelitis in C57BL/6 mice. J. Transl. Med. 2021, 19, 317. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef] [PubMed]
- Šola, K.F.; Vladimir-Knežević, S.; Hrabač, P.; Mucalo, I.; Saso, L.; Verbanac, D. The effect of multistrain probiotics on functional constipation in the elderly: A randomized controlled trial. Eur. J. Clin. Nutr. 2022, 76, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Kashyap, P.C. Beyond phylotyping: Understanding the impact of gut microbiota on host biology. Neurogastroenterol. Motil. 2013, 25, 358–372. [Google Scholar] [CrossRef]
- Tierney, B.T.; Yang, Z.; Luber, J.M.; Beaudin, M.; Wibowo, M.C.; Baek, C.; Mehlenbacher, E.; Patel, C.J.; Kostic, A.D. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe 2019, 26, 283–295.e8. [Google Scholar] [CrossRef]
- Major, G.; Spiller, R. Irritable bowel syndrome, inflammatory bowel disease and the microbiome. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 15–21. [Google Scholar] [CrossRef]
- Bhat, M.I.; Sowmya, K.; Kapila, S.; Kapila, R. Potential Probiotic Lactobacillus rhamnosus (MTCC-5897) Inhibits Escherichia coli Impaired Intestinal Barrier Function by Modulating the Host Tight Junction Gene Response. Probiotics Antimicrob. Proteins 2020, 12, 1149–1160. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Orpianesi, C.; Cresci, A. Influence of a combination of two potential probiotic strains, Lactobacillus rhamnosus IMC 501® and Lactobacillus paracasei IMC 502® on bowel habits of healthy adults: Effect of probiotics on healthy humans. Lett. Appl. Microbiol. 2011, 52, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Orpianesi, C.; Cresci, A. Effect of probiotic combination Synbio on respiratory and gastrointestinal symptoms in athletes. Prebiotics Probiotics 2011, 13–16. [Google Scholar]
- Dupuy, H. The Psychological General Well-Being (PGWB) index. In Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies; Le Jacq Publishing: New York, NY, USA, 1984; pp. 170–183. [Google Scholar]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.D.; Wells, G.A.; Huët, C.; McAlister, F.A.; Salmi, L.R.; Fergusson, D.; Laupacis, A. Assessing the Quality of Randomized Trials: Reliability of the Jadad Scale. Control. Clin. Trials 1999, 20, 448–452. [Google Scholar] [CrossRef]
- RevMan. Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 27 November 2023).
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Akers, J.; Aguiar-Ibáñez, R.; Sari, A. CRD’s Guidance for Undertaking Reviews in Health Care; University of York: Heslington, UK, 2009. [Google Scholar]
- Silvi, S.; Verdenelli, M.C.; Cecchini, C.; Coman, M.M.; Bernabei, M.S.; Rosati, J.; De Leone, R.; Orpianesi, C.; Cresci, A. Probiotic-enriched foods and dietary supplement containing SYNBIO positively affects bowel habits in healthy adults: An assessment using standard statistical analysis and Support Vector Machines. Int. J. Food Sci. Nutr. 2014, 65, 994–1002. [Google Scholar] [CrossRef]
- Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Coman, M.M.; Orpianesi, C.; Pascucci, G.; Colizzi, V.; Cresci, A.; Filoni, R. Effects of synbiotics on house dust mite allergic symptoms: A baseline-controlled open-label study. Int. J. Probiotics Prebiotics 2016, 11, 117. [Google Scholar]
- Coman, M.M.; Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Gabbianelli, R.; Amadio, E.; Orpianesi, C.; Cresci, A. Knowledge and acceptance of functional foods: A preliminary study on influence of a synbiotic fermented milk on athlete health. Int. J. Probiotics Prebiotics 2017, 12, 33. [Google Scholar]
- Coman, M.M.; Micioni Di Bonaventura, M.V.; Cifani, C.; Silvi, S.; Verdenelli, M.C. SYNBIO® Probiotic and Antioxidant Dietary Supplementation: Clinical Trial Evaluation of Potential Effects on Airline Flight Crew Members’ Well-Being. Microorganisms 2023, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shang, X.; Zhou, L.; Li, X.; Guo, K.; Xu, M.; Hou, L.; Hui, X.; Li, S. Efficacy and Safety of Probiotics in Geriatric Patients with Constipation: Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2023, 27, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Hu, M.; Ding, Y.; Meng, Y.; Zhao, Y. Efficacy in bowel movement and change of gut microbiota on adult functional constipation patients treated with probiotics-containing products: A systematic review and meta-analysis. BMJ Open 2024, 14, e074557. [Google Scholar] [CrossRef]
- Wen, Y.; Li, J.; Long, Q.; Yue, C.-C.; He, B.; Tang, X.-G. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: A systematic review and meta-analysis based on seventeen randomized controlled trials. Int. J. Surg. 2020, 79, 111–119. [Google Scholar] [CrossRef]
- Johanson, J.F.; Kralstein, J. Chronic constipation: A survey of the patient perspective. Aliment. Pharmacol. Ther. 2007, 25, 599–608. [Google Scholar] [CrossRef]
- Jeong, J.J.; Ganesan, R.; Jin, Y.J.; Park, H.J.; Min, B.H.; Jeong, M.K.; Yoon, S.J.; Choi, M.R.; Choi, J.; Moon, J.H.; et al. Multi-strain probiotics alleviate loperamide-induced constipation by adjusting the microbiome, serotonin, and short-chain fatty acids in rats. Front. Microbiol. 2023, 14, 1174968. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Shi, Q.; Chen, Y.; Cao, L.; Jia, J.; Liu, C.; Zhang, J. Prevalence and Risk Factors of Functional Constipation According to the Rome Criteria in China: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 815156. [Google Scholar] [CrossRef]
- Dimidi, E.; Mark Scott, S.; Whelan, K. Probiotics and constipation: Mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals. Proc. Nutr. Soc. 2020, 79, 147–157. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of Probiotic Compounds in Relieving Constipation and Their Colonization in Gut Microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Salminen, S.; Salminen, E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand. J. Gastroenterol. Suppl. 1997, 222, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, M.; Zhao, W.; Kwok, L.-Y.; Zhang, W. Effects of probiotics and its fermented milk on constipation: A systematic review. Food Sci. Hum. Wellness 2023, 12, 2124–2134. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, H.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota–SCFA–hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Salvesi, C.; Silvi, S.; Fiorini, D.; Alessandroni, L.; Sagratini, G.; Palermo, F.A.; De Leone, R.; Egidi, N.; Cifani, C.; Di Bonaventura, M.V.M.; et al. Six-Month Synbio® Administration Affects Nutritional and Inflammatory Parameters of Older Adults Included in the PROBIOSENIOR Project. Microorganisms 2023, 11, 801. [Google Scholar] [CrossRef]
- Salvesi, C.; Silvi, S.; Fiorini, D.; Scortichini, S.; Sagratini, G.; Palermo, F.A.; De Leone, R.; Egidi, N.; Fatone, L.; Cifani, C.; et al. Impact of a probiotic diet on well-being of healthy senior: THE PROBIOSENIOR PROJECT. J. Appl. Microbiol. 2022, 133, 2941–2953. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Sun, S.; Si, Q.; Wang, L.; Zhang, Q.; Wu, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus rhamnosus Strains Relieve Loperamide-Induced Constipation via Different Pathways Independent of Short-Chain Fatty Acids. Front. Cell. Infect. Microbiol. 2020, 10, 423. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e3. [Google Scholar] [CrossRef]
- Araújo, M.M.; Botelho, P.B. Probiotics, prebiotics, and synbiotics in chronic constipation: Outstanding aspects to be considered for the current evidence. Front. Nutr. 2022, 9, 935830. [Google Scholar] [CrossRef]









| PICOS | Inclusion and Exclusion Criteria | Data Extraction |
|---|---|---|
| Patients | Adult populations aged ≥18 years, otherwise healthy. No restrictions for age, gender, or ethnicity. | Age, gender, location, inclusion and exclusion criteria, and number of subjects in the intervention and comparator group. |
| Interventions | SYNBIO® blend of two live probiotic strains. Probiotics may be administered as capsule or as enriched food products (as long as the control group is such that the effect of the probiotic alone can be isolated). | Combination of two probiotic strains. The dose and schedule of probiotic and duration of intervention period were also recorded. |
| Comparators | Trials were included if they used a placebo as a control. For trials in which the probiotic intervention was an enriched food product, an acceptable comparator was taken to be the food product without the probiotics. | Type and dose of comparator. |
| Outcomes | Reports of the clinical outcomes of constipation, stool frequency, stool consistency, other gastrointestinal symptoms (bloating, abdominal pain), or adverse effects/compliance. | Outcomes measured, their method of assessment, and endpoint values for the effect of the intervention on outcomes compared with the control group. |
| Study design | Randomized controlled trials only with ≥2 study groups, as long as it was possible to extract data only on probiotic and placebo groups. Both parallel and crossover studies were eligible. | Type of study design, fulfilment of intention-to-treat analysis, adequacy of randomization, and allocation concealment and blinding. |
| Questionnaires | Outcomes | Score Scale | References |
|---|---|---|---|
| Intestinal wellbeing | Constipation, intestinal regularity, stool frequency, stool volume, ease of defecation, bloating, abdominal pain, intestinal cramping | 10-point Likert scale: −5, 0, +5 (−5 means strong worsening, 0 means no changes, +5 means strong improvement) | [12,13] |
| Psychological General Wellbeing Index | 22 items for 6 dimensions: anxiety, depression, self-control, positive wellbeing, general wellbeing, and vitality | From 0 (worst) to 100 (best) | [14] |
| Bristol Stool Form Scale | Stool consistency | From type 1 to type 7 (type 1–2 indicates constipation, type 3–4 indicates ideal stools, type 5–7 indicates diarrhoea or severe diarrhoea) | [15] |
| Study, Year (Ref) | Study Design | Sample Size (% Female) | Age (Years) Mean ± SD | Intervention | Comparator (Dose) | Outcomes Included in Meta-Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Probiotic Group | Placebo Group | Probiotic Group | Placebo Group | Type | Dose (CFU/Daily) | Form | Duration (Weeks) | ||||
| Verdenelli et al., 2011a [12] | Double blind, randomized, parallel, placebo-controlled | 47 | 24 (60) | 23 (48) | 29.9 ± 7.8 | 30.2 ± 7.4 | SYNBIO® Blend | 1.0 × 109 | Probiotic food | 12 | Food without probiotics | Intestinal wellbeing and PGWBI |
| Verdenelli et al., 2011b [13] | Double blind, randomized, parallel, placebo-controlled | 153 | 77 (57) | 76 (55) | 35.4 ± 4.9 | 34.7 ± 8.3 | SYNBIO® Blend | 1.0 × 109 | Dietary supplement | 12 | Placebo capsules of maltodextrin | Intestinal wellbeing and PGWBI |
| Silvi et al., 2014a [21] | Double blind, randomized, parallel, placebo-controlled | 421 | 208 (56) | 213 (55) | 44.1 ± 1.4 | 44.0 ± 1.2 | SYNBIO® Blend | 1.0 × 109 | Dietary supplement | 12 | Placebo capsules of maltodextrin | Intestinal wellbeing and PGWBI |
| Silvi et al., 2014b [21] | Double blind, randomized, parallel, placebo-controlled | 427 | 217 (54) | 210 (57) | 45.0 ± 0.9 | 44.0 ± 0.9 | SYNBIO® Blend | 1.0 × 109 | Probiotic food | 12 | Food without probiotics | Intestinal wellbeing and PGWBI |
| Cecchini et al., 2016 [22] | Single arm, open label controlled towards the baseline | 30 | 30 (40) | 0 | 23.5 ± 8.7 | - | SYNBIO® Blend | 1.5 × 1010 | Dietary supplement | 24 | - | Intestinal wellbeing and PGWBI |
| Coman et al., 2017 [23] | Double blind, randomized, parallel, placebo-controlled | 10 | 5 (60) | 5 (80) | 30.0 ± 12.9 | 26.6 ± 4.2 | SYNBIO® Blend | 1.0 × 109 | Probiotic food | 4 | Food without probiotics | Intestinal wellbeing and PGWBI |
| Coman et al., 2023 [24] | Double blind, randomized, parallel, placebo-controlled | 37 | 19 (32) | 18 (39) | 46.6 ± 3.0 | 44.3 ± 1.8 | SYNBIO® Blend | 1.5 × 1010 | Dietary supplement | 4 | Placebo capsules of maltodextrin | Intestinal wellbeing and PGWBI |
| Studies Probiotic Groups | Overall Constipation | |||||||
|---|---|---|---|---|---|---|---|---|
| μPB | ϕPB+ | ϕPB= | ϕPB− | PM > 1 | ||||
| Mean | SD | N. of Subjects | CI | % Imp. | % Unc. | % Wor. | ||
| Verdenelli et al., 2011a [12] | 0.12 | 0.33 | 24 | [−0.02; 0.26] | 12 | 88 | 0 | 0.00 |
| Verdenelli et al., 2011b [13] | 0.68 | 1.25 | 77 | [0.39; 0.96] | 31 | 68 | 1 | 0.01 |
| Silvi et al., 2014a [21] | 1.30 | 2.06 | 208 | [1.02; 1.58] | 52 | 33 | 15 | 0.98 |
| Silvi et al., 2014b [21] | 1.00 | 1.95 | 217 | [0.74; 1.25] | 46 | 37 | 17 | 1.00 |
| Cecchini et al., 2016 [22] | 0.40 | 0.40 | 30 | [0.25; 0.54] | 20 | 80 | 0 | 0.00 |
| Coman et al., 2017 [23] | 0.85 | 0.50 | 5 | [0.41; 1.29] | 20 | 80 | 0 | 0.00 |
| Coman et al., 2023 [24] | 1.21 | 0.57 | 19 | [0.18; 1.31] | 35 | 65 | 0 | 0.18 |
| Constipation | Probiotic | Placebo | Differences of Proportions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | ϕPB+ | ϕPB= | ϕPB− | Number of Subjects | ϕPL+ | ϕPl= | ϕPL− | ϕPB+-ϕPL+ | ϕPB=-ϕPL= | ϕPB−-ϕPL− | ||||
| Imp. | Unc. | Wor. | Imp. | Unc. | Wor. | Imp. | CI | Unc. | CI | Wor. | CI | |||
| Verdenelli et al., 2011a [12] | 24 | 0.12 | 0.88 | 0.00 | 23 | 0.12 | 0.88 | 0.00 | 0.00 | [−0.18; 0.18] | 0.00 | [−0.18; 0.18] | 0.00 | [0.00; 0.00] |
| Verdenelli et al., 2011b [13] | 77 | 0.31 | 0.68 | 0.01 | 76 | 0.05 | 0.75 | 0.20 | 0.26 | [0.14; 0.37] | −0.07 | [−0.21; 0.07] | −0.19 | [−0.27; −0.09] |
| Silvi et al., 2014a [21] | 208 | 0.52 | 0.33 | 0.15 | 213 | 0.26 | 0.58 | 0.16 | 0.26 | [0.17; 0.35] | −0.25 | [−0.34; −0.15] | −0.01 | [−0.08; −0.06] |
| Silvi et al., 2014b [21] | 217 | 0.46 | 0.37 | 0.16 | 210 | 0.31 | 0.45 | 0.23 | 0.15 | [0.05; 0.23] | −0.08 | [−0.16; −0.02] | −0.07 | [−0.14; 0.01] |
| Cecchini et al., 2016 [22] | 30 | 0.20 | 0.80 | 0.00 | ||||||||||
| Coman et al., 2017 [23] | 5 | 0.20 | 0.80 | 0.00 | 5 | 0.20 | 0.80 | 0.00 | 0.00 | [−0.50; 0.50] | 0.00 | [−0.50; 0.50] | 0.00 | [0.00; 0.00] |
| Coman et al., 2023 [24] | 19 | 0.35 | 0.65 | 0.00 | 18 | 0.00 | 0.90 | 0.10 | 0.35 | [0.14; 0.56] | −0.25 | [−0.50; 0.00] | −0.10 | [−0.23; 0.03] |
| Studies Probiotic Groups | Intestinal Regularity | |||||||
|---|---|---|---|---|---|---|---|---|
| μPB | ϕPB+ | ϕPB= | ϕPB− | PM > 1 | ||||
| Mean | SD | Number of Subjects | CI | % Imp. | % Unc. | % Wor. | ||
| Verdenelli et al., 2011a [12] | 3.88 | 1.20 | 24 | [3.39; 4.37] | 100 | 0 | 0 | 1.00 |
| Verdenelli et al., 2011b [13] | 0.52 | 0.85 | 77 | [0.33; 0.71] | 32 | 68 | 0 | 0.00 |
| Silvi et al., 2014a [21] | 3.27 | 1.64 | 208 | [3.05; 3.50] | 91 | 8 | 1 | 1.00 |
| Silvi et al., 2014b [21] | 3.45 | 1.40 | 217 | [3.27; 3.64] | 94 | 6 | 0 | 1.00 |
| Cecchini et al., 2016 [22] | 1.90 | 0.50 | 30 | [1.72; 2.08] | 60 | 37 | 3 | 1.00 |
| Coman et al., 2017 [23] | 2.60 | 2.41 | 5 | [−12.3; 17.5] | 60 | 40 | 0 | 0.89 |
| Coman et al., 2023 [24] | 1.10 | 1.37 | 19 | [0.46; 1.74] | 45 | 55 | 0 | 0.63 |
| Intestinal Regularity | Probiotic | Placebo | Differences of Proportions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | ϕPB+ | ϕPB= | ϕPB− | Number of Subjects | ϕPL+ | ϕPl= | ϕPL− | ϕPB+-ϕPL+ | ϕPB=-ϕPL= | ϕPB−-ϕPL− | ||||
| Imp. | Unc. | Wor. | Imp. | Unc. | Wor. | Imp. | CI | Unc. | CI | Wor. | CI | |||
| Verdenelli et al., 2011a [12] | 24 | 1.00 | 0.00 | 0.00 | 23 | 0.16 | 0.84 | 0.00 | 0.84 | [0.70; 0.98] | −0.84 | [−0.98; 0.70] | 0.00 | [0.00; 0.00] |
| Verdenelli et al., 2011b [13] | 77 | 0.32 | 0.68 | 0.00 | 76 | 0.14 | 0.64 | 0.22 | 0.18 | [0.05; 0.31] | 0.04 | [−0.12; 0.18] | −0.22 | [−0.30; −0.12] |
| Silvi et al., 2014a [21] | 208 | 0.91 | 0.08 | 0.01 | 213 | 0.68 | 0.23 | 0.09 | 0.23 | [0.16; 0.31] | −0.15 | [−0.22; 0.09] | −0.08 | [−0.12; −0.04] |
| Silvi et al., 2014b [21] | 217 | 0.94 | 0.06 | 0.00 | 210 | 0.61 | 0.35 | 0.04 | 0.33 | [0.26; 0.41] | −0.29 | [−0.37; −0.23] | −0.04 | [−0.06; −0.01] |
| Cecchini et al., 2016 [22] | 30 | 0.60 | 0.37 | 0.03 | ||||||||||
| Coman et al., 2017 [23] | 5 | 0.60 | 0.40 | 0.00 | 5 | 0.80 | 0.20 | 0.00 | −0.20 | [−0.75; 0.35] | 0.20 | [−0.35; 0.75] | 0.00 | [0.00; 0.00] |
| Coman et al., 2023 [24] | 19 | 0.45 | 0.55 | 0.00 | 18 | 0.25 | 0.70 | 0.05 | 0.20 | [0.20; 0.49] | −0.15 | [−0.45; 0.14] | −0.05 | [−0.15; 0.05] |
| Outcomes | Number of Studies | Number of Subjects | Pooled Results | |||
|---|---|---|---|---|---|---|
| MD | 95% CI | p Value | AEM | |||
| Ease of defecation | 6 | 1095 | 1.20 | 0.64, 1.77 | <0.0001 | REM |
| Bloating | 5 | 1048 | 0.69 | 0.19, 1.18 | 0.006 | REM |
| Abdominal pain | 5 | 1048 | 0.35 | −0.02, 0.71 | 0.07 | REM |
| Intestinal cramping | 5 | 1048 | 0.13 | −0.03, 0.30 | 0.11 | REM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coman, M.M.; Egidi, N.; Silvi, S.; De Leone, R.; Verdenelli, M.C. Probiotic SYNBIO® Blend’s Impact on Constipation in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Fermentation 2024, 10, 518. https://doi.org/10.3390/fermentation10100518
Coman MM, Egidi N, Silvi S, De Leone R, Verdenelli MC. Probiotic SYNBIO® Blend’s Impact on Constipation in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Fermentation. 2024; 10(10):518. https://doi.org/10.3390/fermentation10100518
Chicago/Turabian StyleComan, Maria Magdalena, Nadaniela Egidi, Stefania Silvi, Renato De Leone, and Maria Cristina Verdenelli. 2024. "Probiotic SYNBIO® Blend’s Impact on Constipation in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Fermentation 10, no. 10: 518. https://doi.org/10.3390/fermentation10100518
APA StyleComan, M. M., Egidi, N., Silvi, S., De Leone, R., & Verdenelli, M. C. (2024). Probiotic SYNBIO® Blend’s Impact on Constipation in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Fermentation, 10(10), 518. https://doi.org/10.3390/fermentation10100518







