From Traditional to Exceptional: Impact of the Use of Dried Chicken Meat Powder on Sensory and Nutritional Quality of Tarhana
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
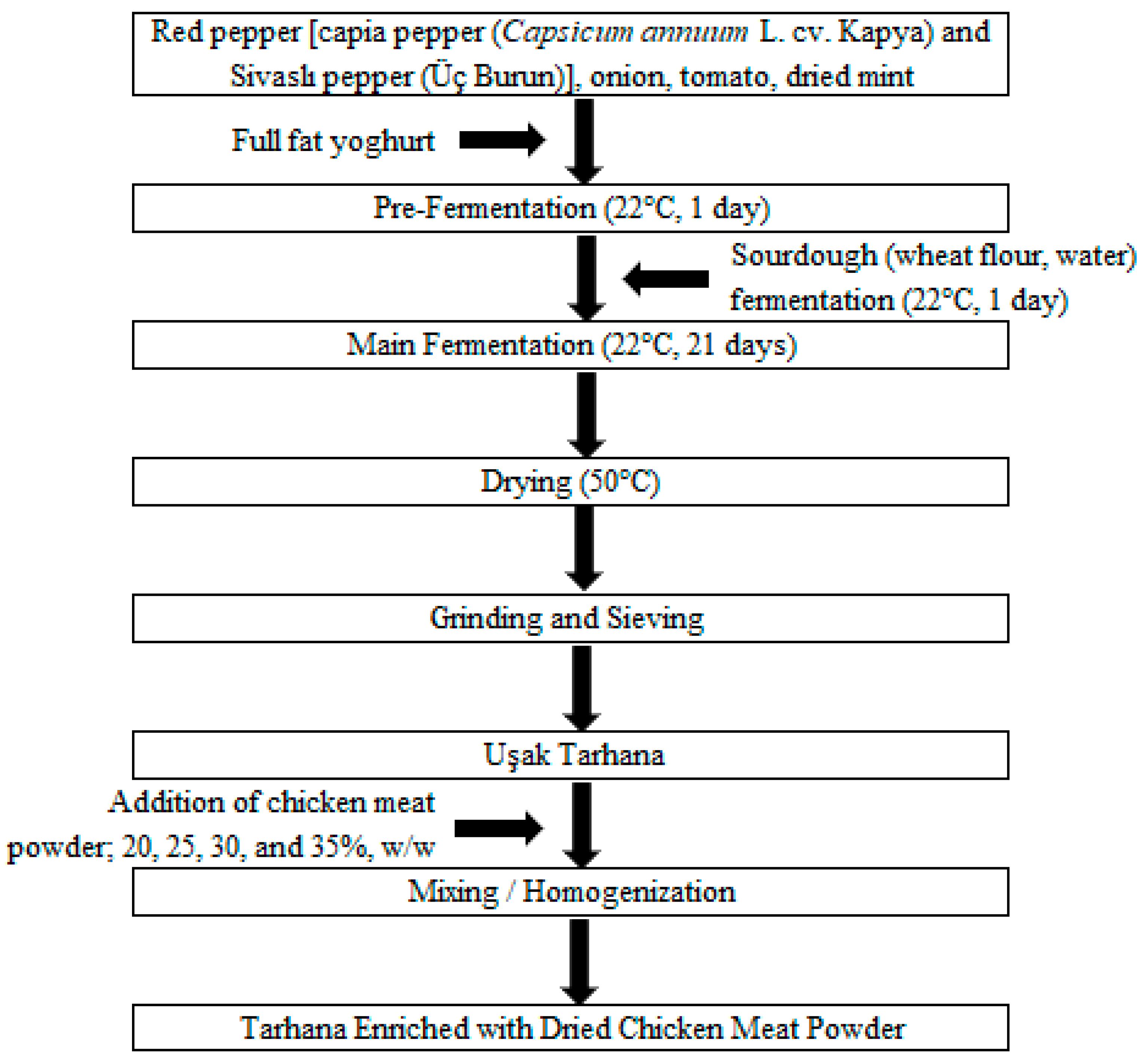
2.2. The Production of Tarhana Enriched with Dried Chicken Meat Powder
2.3. The Production of Tarhana Soup Enriched with Dried Chicken Meat Powder
2.4. Sensory Analyzes
2.5. Physicochemical Analyses
2.5.1. Moisture Content
2.5.2. Protein Content
2.5.3. Fat Content
2.5.4. Ash Content
2.5.5. Energy and Carbohydrate Content
2.5.6. Salt Content
2.5.7. Total Dietary Fiber and Water-İnsoluble Dietary Fiber Content
2.5.8. Sucrose Content
- Mobile gas flow mL min−1: 0.6
- Column Temperature °C: 80
- Column Type: A Carbosep Coregel 87p (Transgenomic, Omaha, Nebraska)
- Detector: Refractive Index
2.5.9. Color Measurement
2.5.10. Determination of Total Phenolic Compound Content and Antioxidant Value
2.5.11. Determination of Mineral Content
- Carrier gas flow L min−1: 1.2
- Plasma gas flow L min−1: 15
- Auxiliary gas flow L min−1: 1.0
- Spray chamber temperature °C: 2
- Mass resolution: 0.8
- Integration time points/ms: 3
- Replicates: 3
2.5.12. Determination of Fatty Acid Composition
- Column: DB-23 Fused silica capillary column (30 m, 0.2 mm ID, 0.25 μm film thickness)
- Column Temperature: 190 °C
- Detector: Flame Ionization Detector (FID)
- Detector Temperature: 240 °C
- Carrier Gas: Helium
- Flow Rate: 1.00 mL/min
- Injection Block Temperature: 230 °C
- Injection Amount: 1 µL
- Split Ratio: 1.80
2.6. Statistical Analyses
3. Results and Discussion
4. Conclusions and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daglioğlu, O. Tarhana as a traditional Turkish fermented cereal food. Its recipe, production and composition. Food/Nahrung 2000, 44, 85–88. [Google Scholar] [CrossRef]
- TS 2282; Tarhana Standardı. TSE: Ankara, Turkey. Available online: https://intweb.tse.org.tr/standard/standard/Standard.aspx?081118051115108051104119110104055047105102120088111043113104073099085078077109118073073119066066 (accessed on 11 November 2023).
- Şimşek, Ö.; Zehir, D. Bebek Beslenmesi için zenginleştirilmiş formülasyonla hazırlanan uşak tarhanası hamurunun fermantasyonunda mikrobiyolojik ve kimyasal değişimler. Akad. Gıda 2018, 16, 403–410. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Özel, S.; Çon, A.H. Comparison of lactic acid bacteria diversity during the fermentation of tarhana produced at home and on a commercial scale. Food Sci. Biotechnol. 2017, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.F.; Gadallah, M.G. Physico-chemical and sensory properties of tarhana prepared from different cereals and dairy ingredients. Curr. J. Appl. Sci. Technol. 2018, 29, 1–14. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Mutlu, C.; Candal, C.; Erbaş, M. Microbiological and chemical properties of wet tarhana produced by different dairy products. J. Food Sci. Technol. 2018, 55, 4770–4781. [Google Scholar] [CrossRef]
- Magala, M.; Kohajdová, Z.; Karovičová, J. Preparation of lactic acid bacteria fermented wheat-yoghurt mixtures. Acta Sci. Pol. Technol. Aliment. 2013, 12, 295–302. [Google Scholar]
- Köse, E.; Çağındı, Ö.S. An investigation into the use of different flours in tarhana. Int. J. Food Sci. Technol. 2002, 37, 219–222. [Google Scholar] [CrossRef]
- Türk Patent Enstitüsü. Coğrafi İşaret Belgesi. T.R. 209, Uşak Tarhanası, Uşak. Available online: https://ci.turkpatent.gov.tr/cografi-isaretler/detay/38156 (accessed on 11 November 2023).
- Koca, A.; Yazici, F.; Anil, M. Utilization of soy yoghurt in tarhana production. Eur. Food Res. Technol. 2002, 215, 293–297. [Google Scholar] [CrossRef]
- Ozboy-Ozbas, O.; Hancer, A.; Gokbulut, I. Utilization of sugarbeet fiber and brewers’ spent grain in the production of tarhana. Zuckerindustrie 2010, 135, 496–501. [Google Scholar]
- Kilci, A.; Gocmen, D. Phenolic acid composition, antioxidant activity and phenolic content of tarhana supplemented with oat flour. Food Chem. 2014, 151, 547–553. [Google Scholar] [CrossRef]
- Herken, E.N.; Aydin, N. Use of carob flour in the production of tarhana. Pol. J. Food Nutr. Sci. 2015, 65, 167–174. [Google Scholar] [CrossRef]
- Eker, T.; Bozuk, F. Effect of edible mushroom powder on antioxidant activity of tarhana. Indian J. Pharm. Educ. Res. 2017, 51, 268–270. [Google Scholar] [CrossRef]
- Isık, A.; Yapar, A. Effect of tomato seed supplementation on chemical and nutritional properties of tarhana. J. Food Meas. Charact. 2017, 11, 667–674. [Google Scholar] [CrossRef]
- Demirci, A.S.; Palabiyik, I.; Ozalp, S.; Tirpanci Sivri, G. Effect of using kefir in the formulation of traditional Tarhana. Food Sci. Technol. 2019, 39, 358–364. [Google Scholar] [CrossRef]
- Gulbandilar, A. Hops (Humulus lupulus L.): A novel ingredient in tarhana. J. Food Process. Preserv. 2021, 45, e15686. [Google Scholar] [CrossRef]
- Koten, M. Development of tef (Eragrostis tef (Zucc.) Trotter) based gluten-free tarhana. J. Food Process. Preserv. 2021, 45, e15133. [Google Scholar] [CrossRef]
- Atasoy, R.; Ertop, M.H. Assessment of nutritional and bioactive properties for gluten-free tarhana containing various legumes and cereals. J. Food Process. Preserv. 2021, 45, e15606. [Google Scholar] [CrossRef]
- Sensoy, E.; Tarakci, Z. Effect of almond pulp addition on physical. chemical and functional properties of tarhana. J. Tekirdağ Agric. Fac. 2023, 20, 620–630. [Google Scholar]
- Ogurlu, M.N.; Tarakci, Z. Effect of hazelnut pulp addition on physical and chemical properties of tarhana. KSU Tarim Ve Doga Derg.-KSU J. Agric. Nat. 2023, 26, 1358–1367. [Google Scholar] [CrossRef]
- Soguksu, S.; Kulcu, D.B. Determining some quality characteristics of vegan tarhana added with red beet (Beta vulgaris var. Cruenta) powder. Braz. Arch. Biol. Technol. 2023, 66, e23220844. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists; Cereals & Grains Association: Eagan, MN, USA, 1990. [Google Scholar]
- FAO. Food Energy: Methods of Analysis and Conversion Factors; Report of a Technical Workshop, FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-105014-9. Available online: https://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf (accessed on 6 August 2023).
- TS 3190; Hazır Kuru Çorbalık Standardı. TSE: Ankara, Turkey, 1995. Available online: https://intweb.tse.org.tr/Standard/Standard/Standard.aspx?081118051115108051104119110104055047105102120088111043113104073098086065114043052075088122097070 (accessed on 11 November 2023).
- AOAC Internatıonal. AOAC Internatıonal. AOAC Method 991.43. Total, soluble, and insoluble dietary fiber in foods. In 16th Edition of Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 1999. [Google Scholar]
- Chen, Y.; Yan, Y.; Xie, M.Y.; Nie, S.P.; Liu, W.; Gong, X.F.; Wang, Y.X. Development of a chromatographic fingerprint for the chloroform extracts of Ganoderma lucidum by HPLC and LC–MS. J. Pharm. Biomed. Anal. 2008, 47, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Erkan, H.; Çelik, S.; Bilgi, B.; Köksel, H. A new approach for the utilization of barley in food products: Barley tarhana. Food Chem. 2006, 97, 12–18. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Singh, P.; Kesharwani, R.K.; Keservani, R.K. Protein, Carbohydrates, and Fats: Energy Metabolism. In Sustained Energy for Enhanced Human Functions and Activity; Bagchi, D., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 103–115. [Google Scholar] [CrossRef]
- Nordic Committee on Food Analysis. NMKL Procedure No. 9: Evaluation of Results Derived from the Analysis of Certified Reference Materials; National Veterinary Institute: Oslo, Norway, 2001. [Google Scholar]
- AOAC International. AOAC International. AOAC Method 996.06 for Analysis of Fatty Acid in Meat By-products. In 19th Edition of Official Methods of Analysis; Association of Official Analytical Chemists: Rockville, MD, USA, 2012. [Google Scholar]
- Jiménez, J.J.; Bernal, J.L.; Nozal, M.; Martin, M.; Bernal, J. Sample preparation methods for beeswax chracterization by gas chromatography with flame ionization detection. J. Chromatogr. A 2006, 1129, 262–272. [Google Scholar] [CrossRef]
- Ertaş, N.; Sert, D.; Demir, M.K.; Elguen, A. Effect of whey concentrate addition on the chemical, nutritional and sensory properties of tarhana (a Turkish fermented cereal-based food). Food Sci. Technol. Res. 2009, 15, 51–58. [Google Scholar] [CrossRef]
- Kılıç Keskin, H. Glutensiz Tarhana Üretimi Üzerine Araştırmalar. Master’s Thesis, Necmettin Erbakan Üniversitesi Fen Bilimleri Enstitüsü, Konya, Türkiye, 2022. [Google Scholar]
- Nielsen, S.S. Determination of Moisture Content. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Food Science Texts Series; Springer: Boston, MA, USA, 2010; pp. 17–27. ISBN 978-1-4419-1463-7. [Google Scholar]
- Zambrano, M.V.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Aytunç, R.; Özsisli, B. Determination of some properties of the traditional maras tarhana produced by tempered corn addition. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1067–1073. [Google Scholar]
- Kirk, S.; Sawyer, R. Pearson’s Composition and Analysis of Foods; Longman Group Ltd.: Harlow, UK, 1991. [Google Scholar]
- Park, Y.W. Moisture and Ash Contents of Food. In Handbook of Food Analysis; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Bilgiçli, N. Tarhananın Fitik Asit İçeriği ve Bazı Besin Öğeleri Üzerine Maya, Malt ve Fitaz Katkılarının Etkiler. Ph.D. Thesis, Selçuk Üniversitesi Fen Bilimleri Enstitüsü, Konya, Türkiye, 2004. [Google Scholar]
- Meluzzi, A.; Sirri, F.; Castellini, C.; Roncarati, A.; Melotti, P.; Franchini, A. Influence of genotype and feeding on chemical composition of organic chicken meat. Ital. J. Anim. Sci. 2009, 8, 766–768. [Google Scholar] [CrossRef][Green Version]
- Ozdemir, S.; Gocmen, D.; Yildirim Kumral, A. A traditional Turkish fermented cereal food: Tarhana. Food Rev. Int. 2007, 23, 107–121. [Google Scholar] [CrossRef]
- Atar, Ş.; Özsisli, B. Determination of some characteristics of traditional Beyşehir Tarhana production with poppy seed substitution. Turk. J. Agric.-Food Sci. Technol. 2022, 10, 1293–1299. [Google Scholar]
- Cagindi, O.; Aksoylu, Z.; Savlak, N.Y.; Kose, E. Comparison of physicochemical and functional properties of domestic and commercial tarhana in Turkey. Bulg. J. Agric. Sci. 2016, 22, 324–330. [Google Scholar]
- Çalışkan Koç, G.; Özçıra, N. Chemical composition, functional, powder, and sensory properties of tarhana enriched with wheat germ. J. Food Sci. Technol. 2019, 56, 5204–5213. [Google Scholar] [CrossRef]
- Adebo, J.A.; Njobeh, P.B.; Gbashi, S.; Oyedeji, A.B.; Ogundele, O.M.; Oyeyinka, S.A.; Adebo, O.A. Fermentation of cereals and legumes: Impact on nutritional constituents and nutrient bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Herken, E.N.; Çon, A.H. Use of different lactic starter cultures in the production of tarhana. J. Food Process. Preserv. 2014, 38, 59–67. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Koh, W.Y.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H. Overview of fermentation process: Structure-function relationship on protein quality and non-nutritive compounds of plant-based proteins and carbohydrates. Crit. Rev. Food Sci. Nutr. 2022, 63, 7677–7691. [Google Scholar] [CrossRef]
- Gul, S.; Durante-Mangoni, E. Unraveling the puzzle: Health benefits of probiotics-a comprehensive review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Burdock, G.A. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef]
- Meier, R.F. Basics in clinical nutrition: Fibre and short chain fatty acids. E-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, 69–71. [Google Scholar] [CrossRef]
- Erbaş, M.; Certel, M.; Uslu, M.K. Yaş ve kuru tarhananın şeker içeriğine fermentasyon ve depolamanın etkisi. Gıda 2004, 29, 299–305. [Google Scholar]
- Tamer, C.E.; Kumral, A.; Aşan, M.; Şahin, İ. Chemical compositions of traditional tarhana having different formulations. J. Food Process. Preserv. 2007, 31, 116–126. [Google Scholar] [CrossRef]
- Wang, H.; Qin, X.; Li, X.; Wang, X.; Lei, Y.; Zhang, C. Effect of chilling methods on the surface color and water retention of yellow-feathered chickens. Poult. Sci. 2020, 99, 2246–2255. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.K.; Biswas, A.K.; Sahoo, J. Comparison study of chitosan, EDTA, eugenol and peppermint oil for antioxidant and antimicrobial potentials in chicken noodles and their effect on colour and oxidative stability at ambient temperature storage. LWT Food Sci. Technol. 2014, 55, 286–293. [Google Scholar] [CrossRef]
- Kilci, Z.; Cetin, R.U.; Ates, K.; Tutak, D. An innovative application developed to determine the blood output of chickens and its impact on the meat quality in poultry slaughtering. Poult. Sci. 2023, 102, 103080. [Google Scholar] [CrossRef]
- Karakaya, S.; Şimşek, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2013, 53, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Chin, N.L.; Yusof, Y.A. Phytochemicals and antioxidant activity of different cereals and their significance for a healthy diet. J. Cereal Sci. 2018, 80, 33–40. [Google Scholar]
- Tepe, T.K.; Otağ, F.B.; Ağan, C.; Kadakal, Ç.; Özer, Ç.; Batu, H.S. Comparatıve analysıs of anatolıan fermented food: Tarhana. In Proceedings of the 4th International Symposium on “Traditional Foods from Adriatic to Caucasus”, Kyrenia, Cyprus, 19–21 April 2018; p. 61. Available online: https://bhi.nku.edu.tr/basinyonetim/resim/images/editorresimleri/288/files/tffatc2018.pdf (accessed on 28 August 2023).
- Kiyak, S.N. Çitlembik (Celtis australis L.) ilave edilerek üretilmiş tarhananın kimyasal ve mikrobiyolojik özelliklerinin incelenmesi ve Gediz tarhanası ile karşılaştırılması. Master’s Thesis, Kütahya Dumlupınar Üniversitesi, Fen Bilimleri Enstitüsü, Kütahya, Türkiye, 2020. [Google Scholar]
- Gediz, Y. Vegan ve çölyak bireyler için geliştirilen tarhanaların bazı karakteristik özellikleri ile antioksidan potansiyonelinin belirlenmesi. Master’s Thesis, Manisa Celal Bayar Üniversitesi Fen Bilimleri Enstitüsü, Manisa, Türkiye, 2020. [Google Scholar]
- Sharma, P.; Gujral, H.S. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2010, 120, 673–678. [Google Scholar] [CrossRef]
- Şemşimoğlu, E. Çeşitli üzümsü meyvelerin ilavesi ile tarhana üretimi üzerine bir araştırma. Master’s Thesis, Afyon Kocatepe Üniversitesi, Fen Bilimleri Enstitüsü, Afyonkarahisar, Türkiye, 2019. [Google Scholar]
- Ghafoor, K.; Al-Juhaimi, F.; Özcan, M.M.; Babiker, E.E.; Ahmed, I.A.M.; Alsawmahi, O.N. Bioactive compounds, antioxidant activity and sensory properties of Tarhana, a traditional fermented food, enriched with pickling herb (Echinophora tenuifolia L.). Food Sci. Technol. 2021, 56, 3600–3606. [Google Scholar] [CrossRef]
- İstek, Ö.; Tomar, O.; Çağlar, A. Orman meyveli tarhananın fonksiyonel özellikleri. Avrupa Bilim Ve Teknol. Derg. 2021, 22, 118–127. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effects of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Bellumori, M.; Pucci, L.; Gabriele, M.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does fermentation really increase the phenolic content in cereals? a study on millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Işık, M.S.; Bilgin, R.; Gökırmaklı, Ç.; Şatır, G.; Güzel-Seydim, Z.B. Determination of the effects of different drying methods on tarhana with improved functional properties. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 460–469. [Google Scholar]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Reprod. Dev. Biol. 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, M.; Certel, M.; Uslu, M.K. Microbiological and chemical propertiesof Tarhana during fermentation and storage as wet-sensorial properties of Tarhana soup. LWT-Food Sci. Technol. 2005, 38, 409–416. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Aktaş, M. Grain foods as a source of minerals for the general population. Agric. Agric. Sci. Procedia 2015, 7, 7–13. [Google Scholar]
- Güldür, M.E.; Ceylan, I.; Pekşen, A. The mineral content of Turkish Tarhana as influenced by its ingredients. Int. J. Food Prop. 2016, 19, 432–440. [Google Scholar]
- Çekal, N.; Aslan, B. Gastronomik bir değer olarak tarhana ve coğrafi işaretlemede tarhananın yeri ve önemi. Güncel Tur. Araştırmaları Derg. 2017, 1, 124–135. [Google Scholar]
- Çalışkan Koç, G.; Tekgül, Y.; Erten, E.S.; Akdoğan, A. Mineral content, fatty acid composition, and volatile compounds of gluten-free tarhana formulated with different cereal and pulse flours. J. Food Sci. 2021, 86, 4376–4392. [Google Scholar] [CrossRef] [PubMed]
- Başlar, M.; Özçelik, G.; Çalışkan, H. A comprehensive review on Beyşehir Tarhana, a Turkish traditional food. Int. J. Gastron. Res. 2022, 1, 35–43. [Google Scholar] [CrossRef]
- Coşkun, F. Tarhana ve beslenme yönünden önemi. Gıda Ve Yem Bilim. Teknol. Derg. 2003, 3, 46–49. [Google Scholar]
- Angel, R.; Tamim, N.M.; Applegate, T.J.; Dhanndu, A.S.; Ellestad, L.E. Phytic acid chemistry: Influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 2002, 11, 47–480. [Google Scholar] [CrossRef]
- Vitali, D.; Dragojević, I.V.; Šebečić, B. Bioaccessibility of Ca, Mg, Mn and Cu from whole grain tea-biscuits: Impact of proteins, phytic acid and polyphenols. Food Chem. 2008, 110, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lydia Pramitha, J.; Subhalakshmi, V.K.I.; Jeeva, G.; Surendar, A. Small Millets Exploring the Hidden Potential; Scripown Publications: Delhi, India, 2021. [Google Scholar]
- Lydia Pramitha, J.; Jeeva, G.; Neethu, F.; Ravikesavan, R.; Thinakaran, J. Revitalization of small millets for nutritional and food security by advanced genetics and genomics approaches. Front. Genet. 2023, 13, 1007552. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, N. Effects of baker’s yeast additıon on some properties and phytic acid content of tarhana prepared with different cereal and legume products. Food Health 2017, 4, 9–18. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M. Mineral contents of some plants used as condiments in Turkey. Food Chem. 2011, 126, 1749–1755. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Consumption and Potassium Intake; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Akpinar, A. Transformations of Fatty Acids in Filamentous Fungi. Ph.D. Thesis, The University of Hull, Kingston upon Hull, UK, 1997. [Google Scholar]
- Akpınar-Bayizit, A. Doymamış yağ asitlerinin beslenme ve sağlık açısından önemi. Gıda Ve Yem Bilim.-Teknol. Derg. 2003, 2, 28–31. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of ω-6/ω-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An Increase in the omega-6/omega-3 fatty acid ratio ıncreases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Pollard, R.D.; Ferguson, D.S. Nutriential Hazards: Macronutrients: Essential Fatty Acids. In Encyclopedia of Food Safety; Yasmine, M., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 95–102. [Google Scholar]
- Jana, S.; Gandhi, A.; Jana, S. Nanotechnology in Bioactive Food Ingredients: Its Pharmaceutical and Biomedical Approaches. In Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 21–41. [Google Scholar]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dietary Recommendations on the Amount and Types of Fat for Adults; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ovando-Martinez, M.; Daglioglu, O.; Guner, K.G.; Gecgel, U.; Simsek, S. Analysis of the fatty acids and phenolic compounds in a cereal-based fermented food (Tarhana). Food Nutr. Sci. 2014, 5, 1177–1184. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. In Omega–6/Omega–3 Essential Fatty Acid Ratio: The Scientific Evidence; Simopoulos, A.P., Cleland, L.G., Eds.; World Review of Nutrition and Dietetics; Karger: Basel, Switzerland, 2003; Volume 92, pp. 1–22. [Google Scholar]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oilseeds Fats Crops Lipids 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Shojaei Saadi, H.A. trans-Fatty Acids. In Reference Module in Biomedical Sciences: Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 798–801. [Google Scholar]
- Eshak, E.S.; Yamagishi, K.; Iso, H. Dietary Fat and Risk of Cardiovascular Disease. In Encyclopedia of Cardiovascular Research and Medicine; Vasan, R.S., Sawyer, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 60–89. [Google Scholar]


| Ingredients | Amount (g) |
|---|---|
| Wheat Flour | 40 |
| Pepper | |
| Capia Pepper (Capsicum annuum L. cv. Kapya) | 17 |
| Sivaslı Pepper (Üç Burun) | 3 |
| Yogurt | 16 |
| Onion | 12 |
| Tomato | 10 |
| Salt | 1 |
| Mint | 0.5 |
| Sour Dough | 0.5 |
| Tarhana Samples | N | Appearance | Flavor | Odor | Taste | Texture | General Assessment |
|---|---|---|---|---|---|---|---|
| T (Control) | 12 | 4.67 ± 0.49 a | 4.67 ± 0.49 a | 4.67 ± 0.49 a | 4.33 ± 0.78 a | 4.33 ± 0.78 a | 4.33 ± 0.78 a |
| TCMP20 | 12 | 4.33 ± 0.49 a | 1.92 ± 0.67 c | 4.33 ± 0.49 a | 2.25 ± 1.54 b | 2.25 ± 1.54 b | 2.25 ± 1.54 b |
| TCMP25 | 12 | 2.25 ± 0.87 b | 2.67 ± 0.49 b | 2.58 ± 0.51 b | 2.75 ± 1.29 b | 2.75 ± 1.29 b | 2.75 ± 1.29 b |
| TCMP30 | 12 | 2.33 ± 0.65 b | 4.33 ± 0.49 a | 2.42 ± 0.51 b | 4.17 ± 0.83 a | 4.17 ± 0.83 a | 4.17 ± 0.83 a |
| TMCP35 | 12 | 1.42 ± 0.67 c | 1.42 ± 0.79 d | 1.17 ± 0.39 c | 2.50 ± 1.09 b | 2.50 ± 1.09 b | 2.50 ± 1.09 b |
| ANOVA | ** | ** | ** | ** | ** | ** | |
| Samples | Moisture (g/100 g) | Ash (g/100 g) | Protein (g/100 g) | Fat (g/100 g) | Carbohydrate (g/100 g) | Energy Value (kcal/100 g) |
|---|---|---|---|---|---|---|
| CMP | 29.18 a | 3.01 c | 62.65 a | 4.970 a | 0.003 c | 296.00 b |
| T | 9.89 c | 5.65 a | 12.46 c | 2.41 b | 63.17 a | 337.00 a |
| TCMP30 | 20.33 b | 4.62 b | 34.07 b | 3.60 | 31.56 b | 316.00 b |
| Statistical Significance | * | * | * | * | * | * |
| Samples | Cellulose (g/100 g) | Dietary Fiber (g/100 g) | Sucrose (g/100 g) | Total Salt (g/100 g) |
|---|---|---|---|---|
| CMP | 0.00 c | 0.00 c | 0.00 | 0.25 c |
| T | 1.78 a | 6.42 a | 0.00 | 4.40 a |
| TCMP30 | 1.59 b | 4.35 b | 0.00 | 1.33 b |
| Statistical Significance | * | * | * | * |
| Samples | Days | L* | a* | b* |
|---|---|---|---|---|
| CMP | 0 | 53.92 a | 2.57 f | 20.20 f |
| 14 | 52.74 b | 2.97 e | 20.32 e | |
| T | 0 | 44.09 c | 9.29 b | 22.97 b |
| 14 | 43.67 d | 9.42 a | 23.13 a | |
| TCMP30 | 0 | 36.97 e | 8.62 d | 21.75 d |
| 14 | 36.11 f | 8.98 c | 21.91 c | |
| Statistical Significance | ** | ** | * | |
| Samples | Days | Total Phenolic Content (mg GAE/100 g of Sample) | Total Antioxidant Capacity (μmol TE/g) |
|---|---|---|---|
| T | 0 | 71.50 a | 33.10.a |
| 14 | 34.38 d | 28.07.b | |
| TCMP30 | 0 | 51.34 c | 21.19.d |
| 14 | 58.37 b | 24.89.c | |
| Statistical Significance | * | * | |
| Samples | Calcium (mg/kg) | Copper (mg/kg) | Iron (mg/kg) | Potassium (mg/kg) | Magnesium (mg/kg) | Manganese (mg/kg) | Phosphorus (mg/kg) | Sodium (mg/kg) | Zinc (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| CMP | 71.67 ± 11.31 | 0.49 ± 0.02 | 8.73 ± 1.48 | 8909.37 ± 765.12 | 798.37 ± 151.74 | 0.00 ± 0.00 | 6212.13 ± 931.82 | 423.25 ± 68.33 | 14.32 ± 0.57 |
| T | 1065.25 ± 170.44 | 2.70 ± 0.11 | 42.32 ± 7.19 | 5304.28 ± 823.32 | 1147 ± 218.03 | 15.70 ± 0.00 | 2677.15 ± 401.57 | 1468.25 ± 896.51 | 14.10 ± 0.56 |
| TCMP30 | 428.06 ± 68.49 | 2.31 ± 0.09 | 37.53 ± 6.38 | 6007.02 ± 532.32 | 969 ± 184.30 | 11.30 ± 0.00 | 3179.96 ± 496.99 | 1125.68 ± 237.49 | 15.13 ± 0.61 |
| Fatty Acids (%) | CMP | T | TCMP30 |
|---|---|---|---|
| Saturated | |||
| Butyric Acid (C4H8O2) | <LOD | 0.41 | 0.28 |
| Caproic Acid (C6H12O2) | <LOD | 0.41 | 0.28 |
| Caprilic Acid (C8H16O2) | <LOD | 0.41 | 0.28 |
| Capric Acid (C10H20O2) | <LOD | 0.83 | 0.28 |
| Lauric Acid (C12H24O2) | 0.20 | 0.83 | 0.69 |
| Myristic Acid (C14H28O2) | 0.60 | 3.32 | 2.08 |
| Pentadecanoic Acid (C15H30O2) | <LOD | 0.41 | 0.28 |
| Palmitic Acid (C16H32O2) | 24.55 | 28.22 | 24.72 |
| Margaric Acid (C17H34O2) | 0.20 | 0.41 | 0.28 |
| Stearic Acid (C18H36O2) | 10.06 | 4.56 | 6.94 |
| Arachidic Acid (C20H40O2) | 0.20 | 0.41 | 0.28 |
| Behenic Acid (C22H44O2) | <LOD | <LOD | <LOD |
| Unsaturated | |||
| - Mono-Unsaturated | |||
| Myristoleic Acid (cis-9-tetradecenoic acid, C14H26O2) | <LOD | 0.41 | 0.28 |
| Palmitoleic Acid (cis-9-hexadecenoic acid, C16H30O2) | 2.01 | 0.41 | 1.39 |
| Margaroleic Acid (cis-9-heptadecenoic acid, C17H32O2) | 0.20 | 0.41 | 0.28 |
| Oleic Acid (cis-9-octadecenoic acid, C18H34O2) | 32.80 | 24.48 | 31.39 |
| Elaidic Acid (trans-9-octadecenoic acid, C18H34O2) | <LOD | 0.41 | 0.28 |
| Gadoleic Acid (cis-9-eicosenoic acid, C20H36O2) | 0.20 | 0.83 | 0.42 |
| - Poly-Unsaturated Fatty Acids | |||
| Linoleic Acid (cis-9,12-octadecadienoic acid, C18H32O2) | 26.76 | 31.12 | 27.78 |
| Linolelaidic Acid (trans,trans-9,12-octadecadienoic acid, C18H32O2) + trans-α-Linolenic Acid (all trans-9,12,15-octadecatrienoic acid, C18H30O2) | 0.17 | <LOD | 0.13 |
| α-Linolenic Acid (cis-9,12,15-octadecatrienoic acid, C18H30O2) | 2.01 | 1.45 | 1.59 |
| Trans Fatty Acids | |||
| Elaidic Acid (trans-9-octadecenoic acid, C18H34O2) | 0.17 | 0.41 | 0.28 |
| Linolelaidic Acid (trans,trans-9,12-octadecadienoic acid, C18H32O2) + trans-α-Linolenic Acid (all trans-9,12,15-octadecatrienoic acid, C18H30O2) | 0.20 | <LOD | 0.11 |
| Omega 3/6 Fatty Acids | |||
| Omega-3 Fatty Acids | 2.01 | 1.45 | 1.59 |
| Omega-6 Fatty Acids | 26.76 | 31.12 | 27.78 |
| Saturated Fatty Acids | 35.58 | 40.02 | 36.28 |
| Unsaturated Fatty Acids | 64.32 | 59.93 | 63.82 |
| - Mono-unsaturated Fatty Acids | 35.01 | 26.95 | 33.04 |
| - Poly-unsaturated Fatty Acids | 28.94 | 32.57 | 30.50 |
| Trans Fatty Acids | 0.37 | 0.41 | 0.28 |
| TOTAL | 99.90 | 99.95 | 100.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetin, R.U.; Kilci, Z.; Ates, K.; Kaya, D.; Akpinar-Bayizit, A. From Traditional to Exceptional: Impact of the Use of Dried Chicken Meat Powder on Sensory and Nutritional Quality of Tarhana. Fermentation 2024, 10, 501. https://doi.org/10.3390/fermentation10100501
Cetin RU, Kilci Z, Ates K, Kaya D, Akpinar-Bayizit A. From Traditional to Exceptional: Impact of the Use of Dried Chicken Meat Powder on Sensory and Nutritional Quality of Tarhana. Fermentation. 2024; 10(10):501. https://doi.org/10.3390/fermentation10100501
Chicago/Turabian StyleCetin, Ramazan Ulku, Zeynep Kilci, Kivilcim Ates, Dogan Kaya, and Arzu Akpinar-Bayizit. 2024. "From Traditional to Exceptional: Impact of the Use of Dried Chicken Meat Powder on Sensory and Nutritional Quality of Tarhana" Fermentation 10, no. 10: 501. https://doi.org/10.3390/fermentation10100501
APA StyleCetin, R. U., Kilci, Z., Ates, K., Kaya, D., & Akpinar-Bayizit, A. (2024). From Traditional to Exceptional: Impact of the Use of Dried Chicken Meat Powder on Sensory and Nutritional Quality of Tarhana. Fermentation, 10(10), 501. https://doi.org/10.3390/fermentation10100501






