Cassava Bagasse as a Low-Cost Substrate for Cellulase and Organic Acid Production Using Co-Cultivated Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Preparation of Avicel Phosphoric Acid Swollen Cellulose (PASC)
2.3. Cellulase Production
2.3.1. Screening of Cellulase-Producing Fungi
2.3.2. BGL Optimisation
2.4. Cassava Bagasse Valorisation
2.4.1. Sample Preparation
2.4.2. Submerged Fermentation
2.5. Assays
2.5.1. Cellulase Activity
2.5.2. Fourier-Transform Infrared (FTIR) Analysis
2.5.3. Glucose, Ethanol, and Organic Acid Analysis
3. Results
3.1. Cellulase Activity
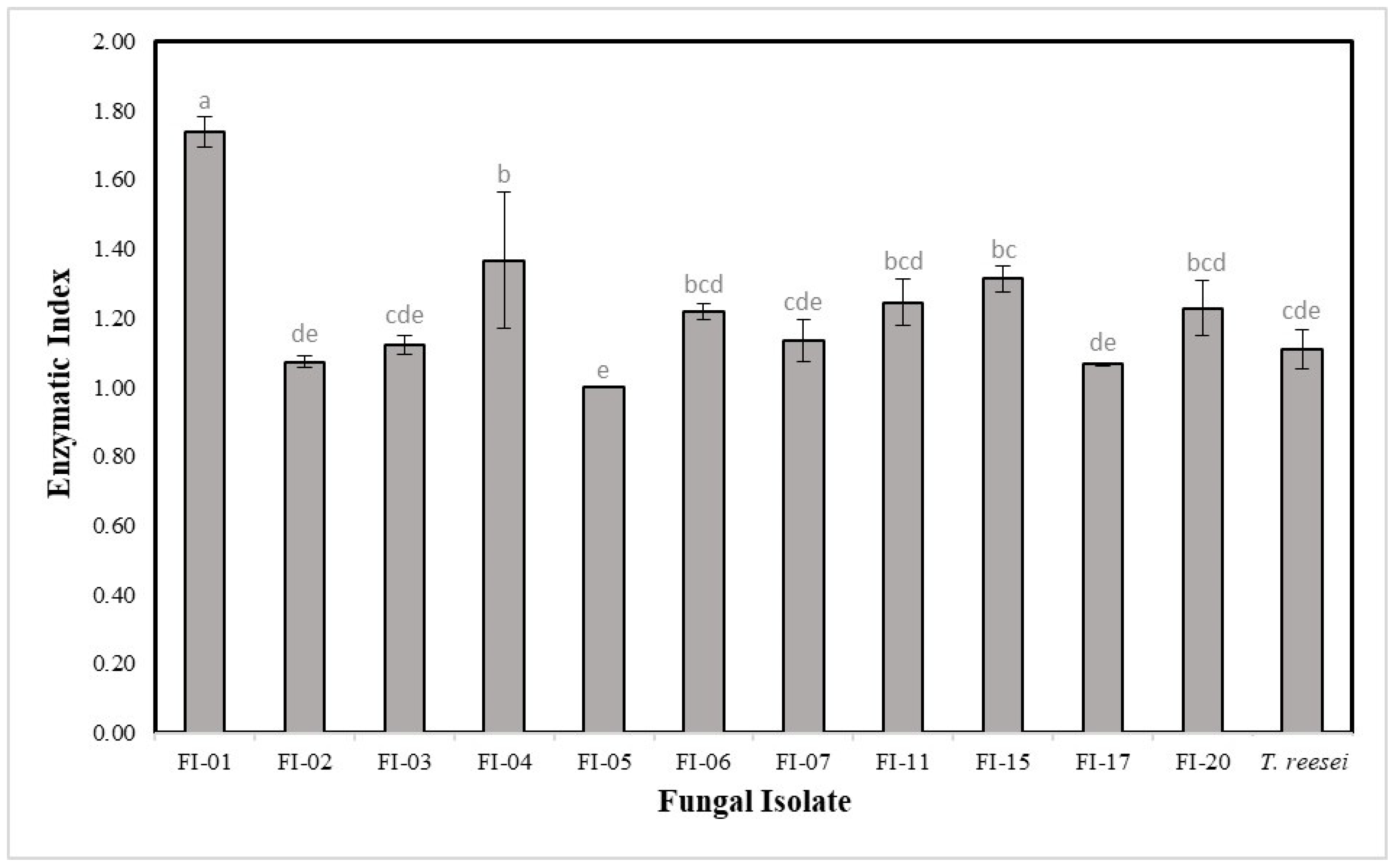
3.1.1. Congo Red Staining
3.1.2. BGL Optimisation
3.1.3. Cellulase Activity of A. violaceofuscus and T. reesei RUT-C30 in Monoculture and Mixed Culture
3.2. Cassava Bagasse Valorisation
3.2.1. Cellulose, Hemicellulose, and Lignin Analysis
3.2.2. Cellulase Profiling on Cassava Bagasse and Avicel PASC
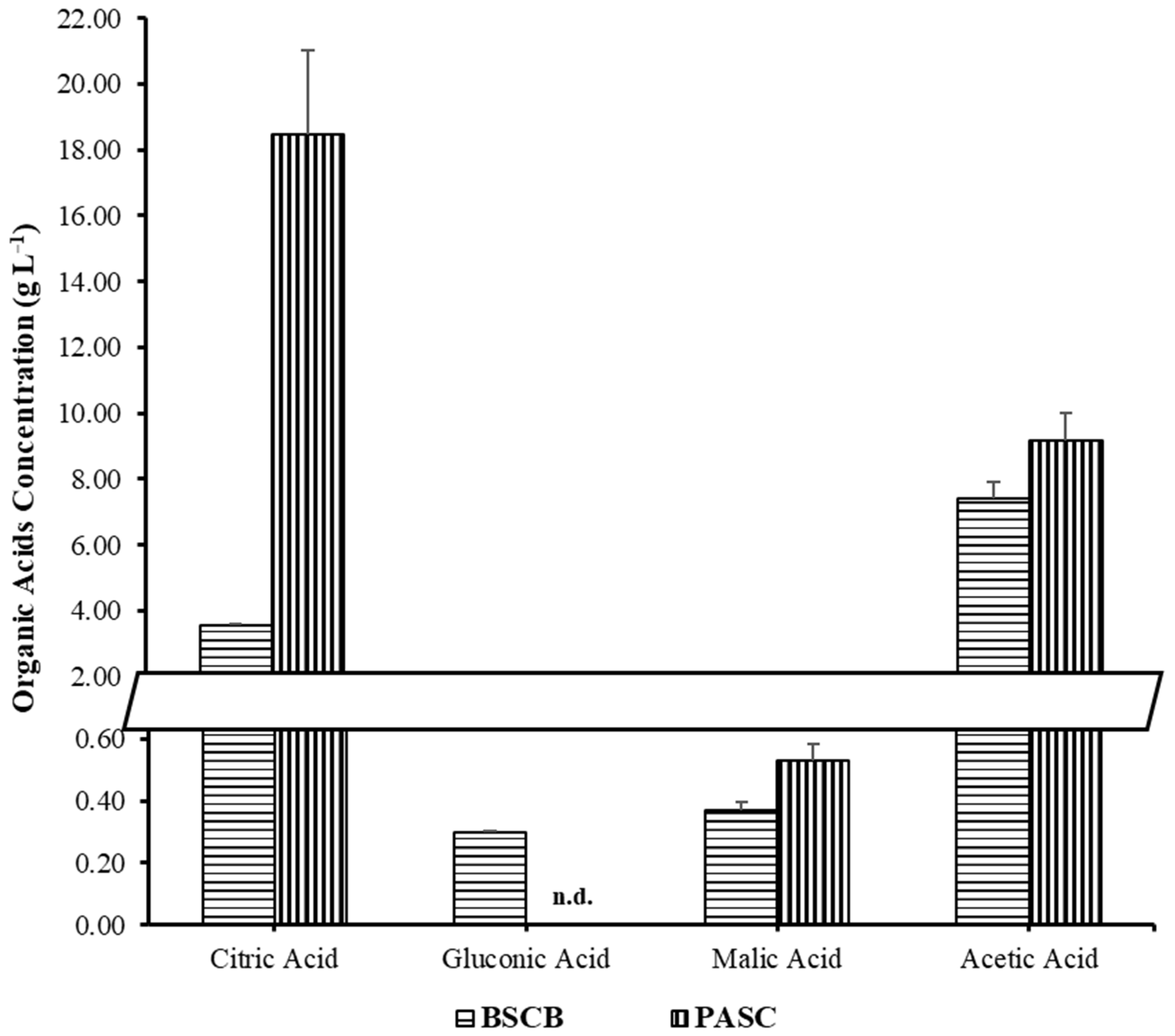
3.2.3. Organic Acid Production on BSCB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kshirsagar, S.; Waghmare, P.; Saratale, G.; Saratale, R.; Kurade, M.; Jeon, B.-H.; Govindwar, S. omposition of Synthesized Cellulolytic Enzymes Varied with the Usage of Agricultural Substrates and Microorganisms. Appl. Biochem. Biotechnol. 2020, 191, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Behera, S.; Kumar, S. Bioprospecting Thermophilic/Thermotolerant Microbes for Production of Lignocellulosic Ethanol: A Future Perspective. Renew. Sustain. Energy Rev. 2015, 51, 699–717. [Google Scholar] [CrossRef]
- Sørensen, A.; Lübeck, M.; Lübeck, P.S.; Ahring, B.K. Fungal Beta-Glucosidases: A Bottleneck in Industrial Use of Lignocellulosic Materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Bohlin, C.; Baumann, M.J.; Olsen, S.N.; Sørensen, T.H.; Anderson, L.; Borch, K.; Westh, P. Product Inhibition of Five Hypocrea jecorina Cellulases. Enzym. Microb. Technol. 2013, 52, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ellilä, S.; Fonseca, L.; Uchima, C.; Cota, J.; Goldman, G.H.; Saloheimo, M.; Sacon, V.; Siika-Aho, M. Development of a Low-Cost Cellulase Production Process Using Trichoderma reesei for Brazilian Biorefineries. Biotechnol. Biofuels 2017, 10, 30. [Google Scholar] [CrossRef]
- Lodha, A.; Pawar, S.; Rathod, V. Optimised Cellulase Production from Fungal Co-Culture of Trichoderma reesei NCIM 1186 and Penicillium citrinum NCIM 768 under Solid State Fermentation. J. Environ. Chem. Eng. 2020, 8, 103958. [Google Scholar] [CrossRef]
- Shen, L.; Su, Y.; Sun, Y.; Wang, G.; Chen, H.; Yu, X.; Zhang, S.; Chen, G. Establishment of a Highly Efficient and Low Cost Mixed Cellulase System for Bioconversion of Corn Stover by Trichoderma reesei and Aspergillus niger. Biocatal. Agric. Biotechnol. 2021, 32, 101849. [Google Scholar] [CrossRef]
- Pham, T.A.; Tran, L.N.; Dam, T.H.; To, K.A. Valorization of Cassava Bagasse Using Co-Culture of Aspergillus oryzae VS1 and Sporidiobolus pararoseus O1 in Solid-State Fermentation. Waste Biomass Valorization 2022, 13, 3003–3012. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, L.; Fang, H. Mixed Culture of Recombinant Trichoderma reesei and Aspergillus niger for Cellulase Production to Increase the Cellulose Degrading Capability. Biomass Bioenergy 2018, 112, 93–98. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Mulla, S.I.; Ninnekar, H.Z. Optimization of Endoglucanase Production from Trichoderma harzianum Strain HZN11 by Central Composite Design under Response Surface Methodology. Biomass Convers. Biorefin. 2018, 8, 305–316. [Google Scholar] [CrossRef]
- Maguinsay, C.S.; Teves, F.G. b-Glucosidase production by Aspergillus niger van Tieghem Using Submerged Fermentation of Pineapple Waste. AES Bioflux 2013, 5, 23–29. [Google Scholar]
- Kumar, A.K.; Parikh, B.S. Cellulose-Degrading Enzymes from Aspergillus terreus D34 and Enzymatic Saccharification of Mild-Alkali and Dilute-Acid Pretreated Lignocellulosic Biomass Residues. Bioresour. Bioprocess. 2015, 2, 7. [Google Scholar] [CrossRef][Green Version]
- Imran, M.; Hussain, A.; Anwar, Z.; Irshad, M.; Jabeen, F. Beta-Glucosidase Production Optimization from Newly Isolated Aspergillus tubingensis IMMIS2 Using Taguchi Statistical Design. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 701–707. [Google Scholar] [CrossRef]
- Liu, P.; Lin, A.; Zhang, G.; Zhang, J.; Chen, Y.; Shen, T.; Zhao, J.; Wei, D.; Wang, W. Enhancement of Cellulase Production in Trichoderma reesei RUT-C30 by Comparative Genomic Screening. Microb. Cell Fact. 2019, 18, 81. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Parreiras, L.S.; Murakami, M.T. Rational Engineering of the Trichoderma reesei RUT-C30 Strain into an Industrially Relevant Platform for Cellulase Production. Biotechnol. Biofuels 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.J.; Sit, N. Optimization of the Culture Conditions for Cellulase Production from Suitable Food Waste Using Fungal Strain Isolated from Different Soils. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Sowcharoensuk, C. Thailand Industry Outlook 2022–2024 (Cassava Industry); Krungsri: Bangkok, Thailand, 2021. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.S.; Mohan, R. Biotechnological Potential of Agroindustrial residues: II: Cassava Bagasse. Bioreous Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Baladi, S.; Bosma, E.F.; Angelidaki, I. Fermentative Production of Lactic Acid as a Sustainable Approach to Valorize Household Bio-Waste. Front. Sustain. 2020, 1, 4. [Google Scholar] [CrossRef]
- Ratnadewi, A.A.; Rahma, M.T.; Nurhayati, N.; Santoso, A.B.; Senjarini, K.; Labes, A.; Reza, M. Production of Xylooligosaccharide from Cassava Pulp’s Waste by Endo-b-1,4-D-Xylanase and Characterization of Its Prebiotic Effect by Fermentation of Lactobacillus acidophilus. Fermentation 2022, 8, 488. [Google Scholar] [CrossRef]
- Rajendran, N.; Han, J. Integrated Polylactic Acid and Biodiesel Production from Food Waste: Process Synthesis and Economics. Bioresour. Technol. 2022, 343, 126119. [Google Scholar] [CrossRef]
- Turner, T.L.; Zhang, G.C.; Kim, S.R.; Subramaniam, V.; Steffen, D.; Skory, C.D.; Jang, J.Y.; Yu, B.J.; Jin, Y.S. Lactic Acid Production from Xylose by Engineered Saccharomyces cerevisiae without PDC or ADH Deletion. Appl. Microbiol. Biotechnol. 2015, 99, 8023–8033. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Zhang, G.C.; Oh, E.J.; Subramaniam, V.; Adiputra, A.; Subramaniam, V.; Skory, C.D.; Jang, J.Y.; Yu, B.J.; Park, I.; et al. Lactic Acid Production from Cellobiose and Xylose by Engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2016, 113, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, T.; Mosier, N.S.; Han, L.; Xiao, W. Cellulose Modification by Recyclable Swelling Solvents. Biotechnol. Biofuels 2018, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.; Vermette, P. Effect of Culture Medium Composition on Trichoderma reesei’s Morphology and Cellulase Production. Bioresour. Technol. 2009, 100, 5979–5987. [Google Scholar] [CrossRef]
- Ahamed, A.; Vermette, P. Culture-Based Strategies to Enhance Cellulase Enzyme Production from Trichoderma reesei RUT-C30 in Bioreactor Culture Conditions. Biochem. Eng. J. 2008, 40, 399–407. [Google Scholar] [CrossRef]
- Sazci, A.; Erenler, K.; Radford, A. Detection of Cellulolytic Fungi by Using Congo Red as an Indicator: A Comparative Study with the Dinitrosalicyclic Acid Reagent Method. J. Appl. Bacteriol. 1986, 61, 559–562. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Combining Autoclaving With Mild Alkaline Solution As a Pretreatment Technique To Enhance Glucose Recovery From the Invasive Weed Chloris barbata. Biomolecules 2019, 9, 120. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008; 17p.
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Cui, Y.; Cheng, Q.; Zhang, Z.; Lu, J.H.; Meng, Q.; Teng, L.; Ren, X. Measurement of Filter Paper Activities of Cellulase with Microplate-Based Assay. Saudi J. Biol. Sci. 2016, 23, S93–S98. [Google Scholar] [CrossRef]
- Lokapirnasari, W.P.; Nazar, D.S.; Nurhajati, T.; Supranianondo, K.; Yulianto, A.B. Production and Assay of Cellulolytic Enzyme Activity of Enterobacter cloacae WPL 214 Isolated from Bovine Rumen Fluid Waste of Surabaya Abbatoir, Indonesia. Vet. World 2015, 8, 367–371. [Google Scholar] [CrossRef]
- Han, S.J.; Yoo, Y.J.; Kang, S.H. Characterization of a Bifunctional Cellulase and Its Structural Gene: The Cel Gene of Bacillus sp. D04 Has Exo- and Endoglucanase Activity. J. Biol. Chem. 1995, 270, 26012–26019. [Google Scholar] [CrossRef]
- Yoon, J.H.; Park, J.E.; Suh, D.Y.; Hong, S.B.; Ko, S.J.; Kim, S.H. Comparison of Dyes for Easy Detection of Extracellular Cellulases in Fungi. Mycobiology 2007, 35, 21. [Google Scholar] [CrossRef]
- Florencio, C.; Couri, S.; Farinas, C.S. Correlation between Agar Plate Screening and Solid-State Fermentation for the Prediction of Cellulase Production by Trichoderma Strains. Enzym. Res. 2012, 2012, 793708. [Google Scholar] [CrossRef]
- Castrillo, M.L. A Combination of Solid Mandels Medium, CMC, and Congo Red Technique for Rapid, Sensitive and Reproducible Screening of Cellulase-Producing Fungi. Biog. Sci. Res. 2020, 5, 130. [Google Scholar] [CrossRef]
- Sohail, M.; Siddiqi, R.; Ahmad, A.; Khan, S.A. Cellulase Production from Aspergillus niger MS82: Effect of Temperature and PH. N. Biotechnol. 2009, 25, 437–441. [Google Scholar] [CrossRef]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Khan, J.; Awasthi, M.K.; Sarsaiya, S. Optimization for the Production of Cellulase Enzyme from Municipal Solid Waste Residue by Two Novel Cellulolytic Fungi. Biotechnol. Res. Int. 2011, 2011, 810425. [Google Scholar] [CrossRef]
- Abd Elrsoul, R.M.M.A.; Bakhiet, S.E.A. Optimization of Factors Influencing Cellulase Production by Some Indigenous Isolated Fungal Species. Jordan J. Biol. Sci. 2018, 11, 31–36. [Google Scholar]
- Blessing Adebola, A.; Sarafadeen, K.O.; Idowu, A.A.; Monilola, W.S. Optimization of Cellulase Enzyme from Sorghum Straw by Yeasts Isolated from Plant Feeding−termite Zonocerus variegatus. Food Appl. Biosci. J. 2019, 7, 81–99. [Google Scholar]
- Barapatre, S.; Rastogi, M.; Nandal, M. Isolation of Fungi and Optimization of pH and Temperature for Cellulase Production. Nat. Environ. Pollut. Technol. 2020, 19, 1729–1735. [Google Scholar] [CrossRef]
- Kaar, W.E.; Holtzapple, M.T. Benefits from Tween during Enzymic Hydrolysis of Corn Stover. Biotechnol. Bioeng. 1998, 59, 419–427. [Google Scholar] [CrossRef]
- Jin, W.; Chen, L.; Hu, M.; Sun, D.; Li, A.; Li, Y.; Hu, Z.; Zhou, S.; Tu, Y.; Xia, T.; et al. Tween-80 Is Effective for Enhancing Steam-Exploded Biomass Enzymatic Saccharification and Ethanol Production by Specifically Lessening Cellulase Absorption with Lignin in Common Reed. Appl. Energy 2016, 175, 82–90. [Google Scholar] [CrossRef]
- Xin, D.; Yang, M.; Chen, X.; Zhang, Y.; Ma, L.; Zhang, J. Improving the Hydrolytic Action of Cellulases by Tween 80: Offsetting the Lost Activity of Cellobiohydrolase Cel7A. ACS Sustain. Chem. Eng. 2017, 5, 11339–11345. [Google Scholar] [CrossRef]
- Wattanachaisaereekul, S.; Tachaleat, A.; Punya, J.; Haritakun, R.; Boonlarppradab, C.; Cheevadhanarak, S. Assessing Medium Constituents for Optimal Heterologous Production of Anhydromevalonolactone in Recombinant Aspergillus oryzae. AMB Express 2014, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Kolasa, M.; Ahring, B.K.; Lübeck, P.S.; Lübeck, M. Co-Cultivation of Trichoderma reesei RutC30 with Three Black Aspergillus Strains Facilitates Efficient Hydrolysis of Pretreated Wheat Straw and Shows Promises for on-Site Enzyme Production. Bioresour. Technol. 2014, 169, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of Hemicellulose, Cellulose, Holocellulose and Lignin Content Using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. and Guazuma crinita Lam. PLoS ONE 2021, 16, 0256559. [Google Scholar] [CrossRef] [PubMed]
- Bouramdane, Y.; Fellak, S.; Mansouri, F. El Impact of Natural Degradation on the Aged Lignocellulose Fibers of Moroccan Cedar Softwood: Structural Elucidation by Infrared Spectroscopy (ATR-FTIR) and X-ray Diffraction (XRD). Fermentation 2022, 8, 698. [Google Scholar] [CrossRef]
- Pancholi, M.J.; Khristi, A.; Bagchi, D. Comparative Analysis of Lignocellulose Agricultural Waste and Pre-Treatment Conditions with FTIR and Machine Learning Modeling. Bioenergy Res. 2022, 16, 123–137. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical Composition, Structure, Physicochemical and Functional Properties of Rice Bran Dietary Fiber Modified by Cellulase Treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Qi, B.; Chen, X.; Shen, F.; Su, Y.; Wan, Y. Optimization of Enzymatic Hydrolysis of Wheat Straw Pretreated by Alkaline Peroxide Using Response Surface Methodology. Ind. Eng. Chem. Res. 2009, 48, 7346–7353. [Google Scholar] [CrossRef]
- Jia, H.; Sun, W.; Li, X.; Zhao, J. Cellulose Induced Protein 1 (Cip1) from Trichoderma reesei Enhances the Enzymatic Hydrolysis of Pretreated Lignocellulose. Microb. Cell Fact. 2021, 20, 136. [Google Scholar] [CrossRef]
- Kobakhidze, A.; Asatiani, M.; Kachlishvili, E.; Elisashvili, V. Induction and Catabolite Repression of Cellulase and Xylanase Synthesis in the Selected White-Rot Basidiomycetes. Ann. Agrar. Sci. 2016, 14, 169–176. [Google Scholar] [CrossRef]
- Datsomor, O.; Yan, Q.; Opoku-Mensah, L.; Zhao, G.; Miao, L. Effect of Different Inducer Sources on Cellulase Enzyme Production by White-Rot Basidiomycetes Pleurotus ostreatus and Phanerochaete chrysosporium under Submerged Fermentation. Fermentation 2022, 8, 561. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, A.; Dutt, D. Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover. Fermentation 2016, 2, 12. [Google Scholar] [CrossRef]
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a Versatile Cell Factory for Organic Acid Production. Fungal Biol. Rev. 2017, 31, 33–49. [Google Scholar] [CrossRef]
- Karaffa, L.; Sándor, E.; Fekete, E.; Szentirmai, A. The Biochemistry of Citric Acid Accumulation by Aspergillus niger (A Review). Acta Microbiol. Immunol. Hung. 2001, 48, 429–440. [Google Scholar] [CrossRef]
- Lu, F.; Ping, K.; Wen, L.; Zhao, W.; Wang, Z.; Chu, J.; Zhuang, Y. Enhancing Gluconic Acid Production by Controlling the Morphology of Aspergillus niger in Submerged Fermentation. Process Biochem. 2015, 50, 1342–1348. [Google Scholar] [CrossRef]


| Factor | Coding Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: pH | 4.5 | 5 | 5.5 |
| B: Spore concentration (spores·mL−1) | 1 × 105 | 5.01 × 107 | 1 × 108 |
| C: Tween 80 (%) | 0.01 | 0.03 | 0.05 |
| Run No. | A | B | C | BGL (IU·gds−1) |
|---|---|---|---|---|
| 1 | 4.50 (−1) | 5.01 × 107 (0) | 0.05 (+1) | 31.460 |
| 2 | 4.50 (−1) | 1 × 105 (−1) | 0.03 (0) | 20.071 |
| 3 | 5.00 (0) | 1 × 105 (−1) | 0.01 (−1) | 6.513 |
| 4 | 5.00 (0) | 1 × 108 (+1) | 0.01 (−1) | 9.316 |
| 5 | 5.00 (0) | 5.01 × 107 (0) | 0.03 (0) | 11.238 |
| 6 | 5.50 (+1) | 1 × 105 (−1) | 0.03 (0) | 4.416 |
| 7 | 5.00 (0) | 1 × 105 (−1) | 0.05 (+1) | 9.860 |
| 8 | 5.50 (+1) | 5.01 × 107 (0) | 0.01 (−1) | 7.631 |
| 9 | 5.00 (0) | 1 × 108 (+1) | 0.05 (+1) | 10.476 |
| 10 | 4.50 (−1) | 5.01 × 107 (0) | 0.01 (−1) | 27.426 |
| 11 | 5.50 (+1) | 1 × 108 (+1) | 0.03 (0) | 11.089 |
| 12 | 5.00 (0) | 5.01 × 107 (0) | 0.03 (0) | 8.357 |
| 13 | 5.50 (+1) | 5.01 × 107 (0) | 0.05 (+1) | 12.101 |
| 14 | 5.00 (0) | 5.01 × 107 (0) | 0.03 (0) | 11.545 |
| 15 | 4.50 (−1) | 1 × 108 (+1) | 0.03 (0) | 28.207 |
| Model | Sum of Squares | DF | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A (pH) | 646.687 | 1 | 646.687 | 109.650 | 0.000 |
| B (spore concentration) | 41.533 | 1 | 41.533 | 7.040 | 0.045 |
| C (tween 80 concentration) | 21.161 | 1 | 21.161 | 3.590 | 0.117 |
| AA | 241.625 | 1 | 241.625 | 40.970 | 0.001 |
| AB | 0.535 | 1 | 0.535 | 0.090 | 0.775 |
| AC | 0.048 | 1 | 0.048 | 0.010 | 0.932 |
| BB | 23.518 | 1 | 23.518 | 3.990 | 0.102 |
| BC | 1.196 | 1 | 1.196 | 0.200 | 0.671 |
| CC | 5.185 | 1 | 5.185 | 0.880 | 0.392 |
| R-squared = 97.1127% |
| Organism | 1 BGL (IU·gds−1) | 1 CBH (IU·gds−1) | 1 EG (IU·gds−1) |
|---|---|---|---|
| T. reesei RUT C-30 (TR) | 1.83 ± 0.142 | 1.28 ± 0.028 | 0.010 ± 0.003 |
| A. violaceofuscus (AV) | 28.48 ± 0.295 | 4.42 ± 0.853 | 0.023 ± 0.005 |
| Mixed culture (TR + AV) | 39.73 ± 3.910 | 3.89 ± 0.380 | 0.017 ± 0.002 |
| Biomass | Cellulose (% w/w) | Hemicellulose (% w/w) | Acid Soluble Lignin (% w/w) | Acid Insoluble Lignin (% w/w) |
|---|---|---|---|---|
| ASCB | 58.01 ± 1.10 | 1.29 ± 0.02 | 2.77 ± 0.10 | 15.65 ± 0.34 |
| BSCB | 72.91 ± 0.77 | 0.90 ± 0.02 | 2.44 ± 0.03 | 6.41 ± 0.23 |
| SCB | 66.77 ± 1.57 | 2.17 ± 0.10 | 2.54 ± 0.07 | 4.48 ± 0.21 |
| UCB | 63.82 ± 0.83 | 2.18 ± 0.02 | 2.53 ± 0.08 | 4.92 ± 0.49 |
| Substrate | 1 BGL (IU·gds−1) | 1 CBH (IU·gds−1) | 1 EG (IU·gds−1) |
|---|---|---|---|
| Avicel PASC | 39.73 ± 3.91 | 3.89 ± 0.38 | 0.017 ± 0.002 |
| BSCB | 68.30 ± 2.22 | 11.01 ± 1.07 | 0.048 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farniga, A.; Khaokhajorn, P.; Wattanachaisaereekul, S. Cassava Bagasse as a Low-Cost Substrate for Cellulase and Organic Acid Production Using Co-Cultivated Fungi. Fermentation 2024, 10, 14. https://doi.org/10.3390/fermentation10010014
Farniga A, Khaokhajorn P, Wattanachaisaereekul S. Cassava Bagasse as a Low-Cost Substrate for Cellulase and Organic Acid Production Using Co-Cultivated Fungi. Fermentation. 2024; 10(1):14. https://doi.org/10.3390/fermentation10010014
Chicago/Turabian StyleFarniga, Analdi, Phimrak Khaokhajorn, and Songsak Wattanachaisaereekul. 2024. "Cassava Bagasse as a Low-Cost Substrate for Cellulase and Organic Acid Production Using Co-Cultivated Fungi" Fermentation 10, no. 1: 14. https://doi.org/10.3390/fermentation10010014
APA StyleFarniga, A., Khaokhajorn, P., & Wattanachaisaereekul, S. (2024). Cassava Bagasse as a Low-Cost Substrate for Cellulase and Organic Acid Production Using Co-Cultivated Fungi. Fermentation, 10(1), 14. https://doi.org/10.3390/fermentation10010014






