Chemical Activation of Apricot Pit-Derived Carbon Sorbents for the Effective Removal of Dyes in Environmental Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
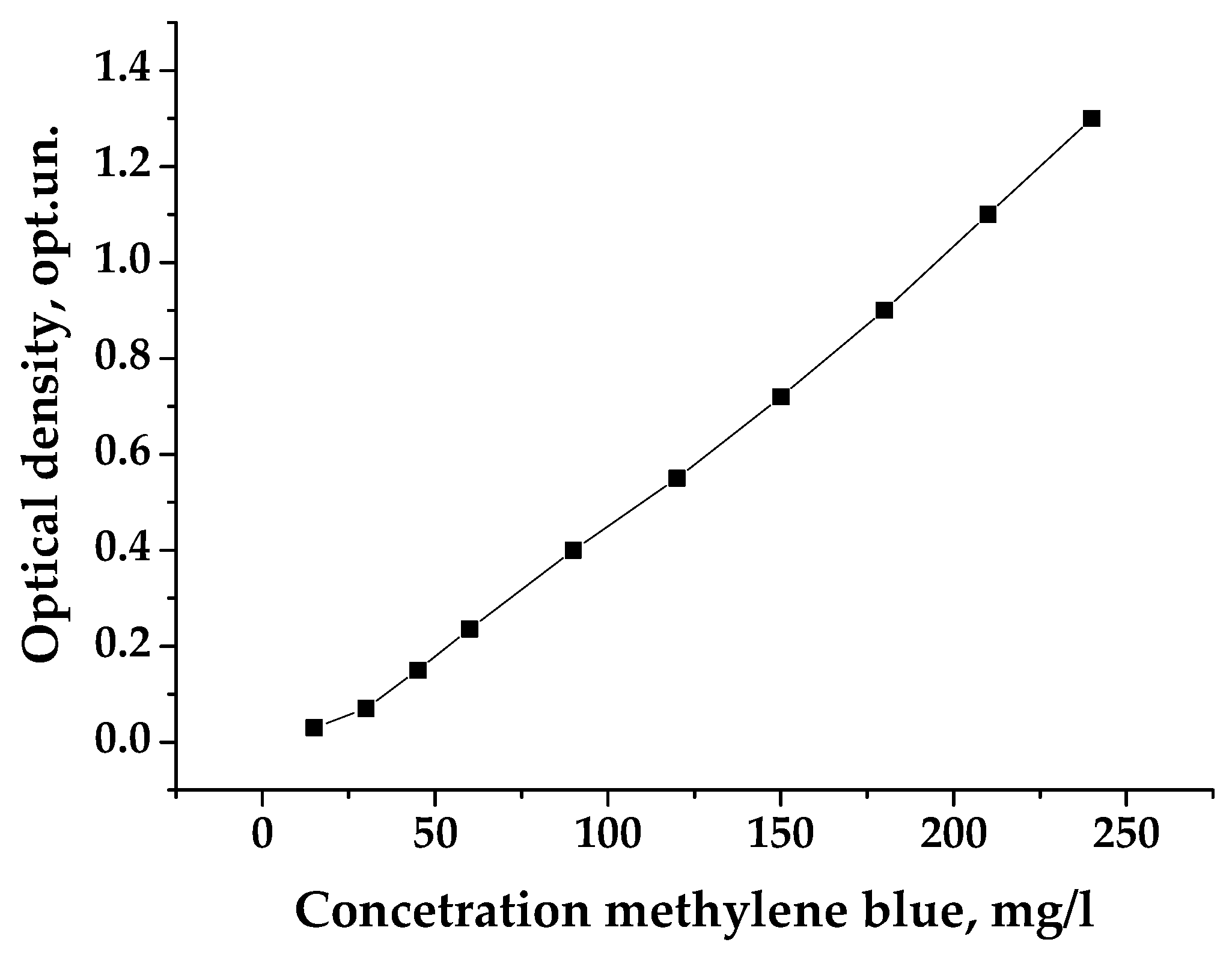
2.2. Characterization of the Samples
3. Results and Discussion
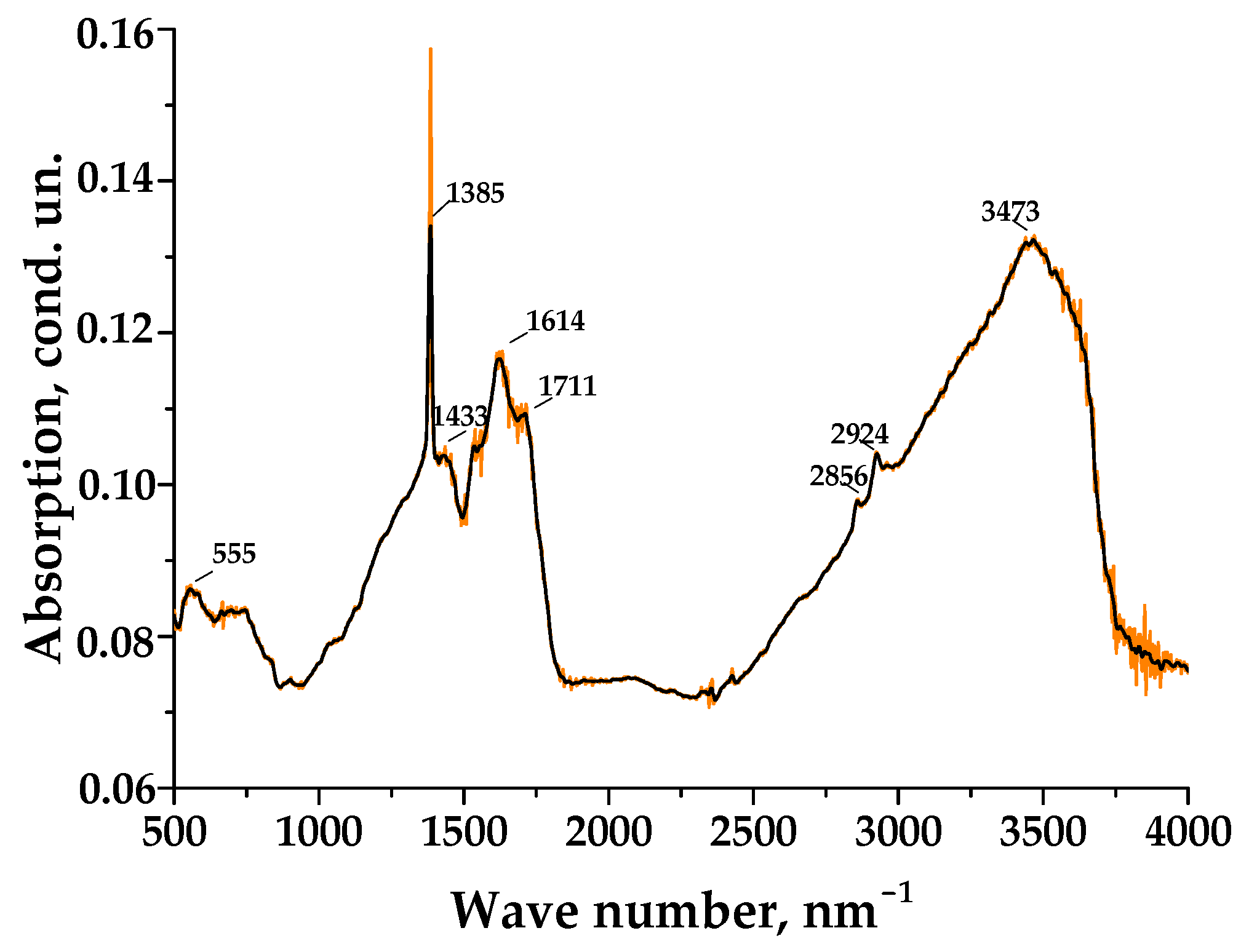
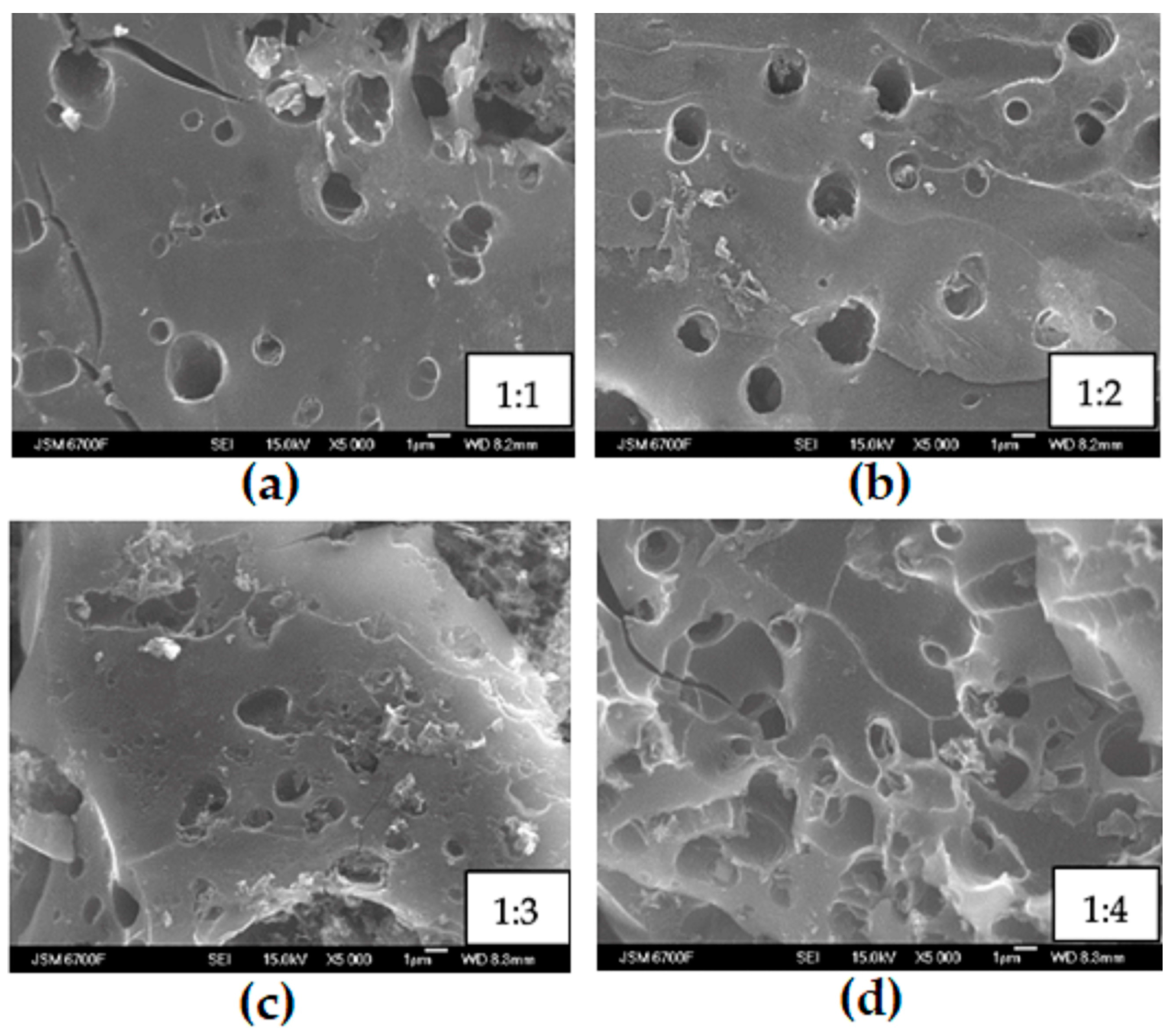
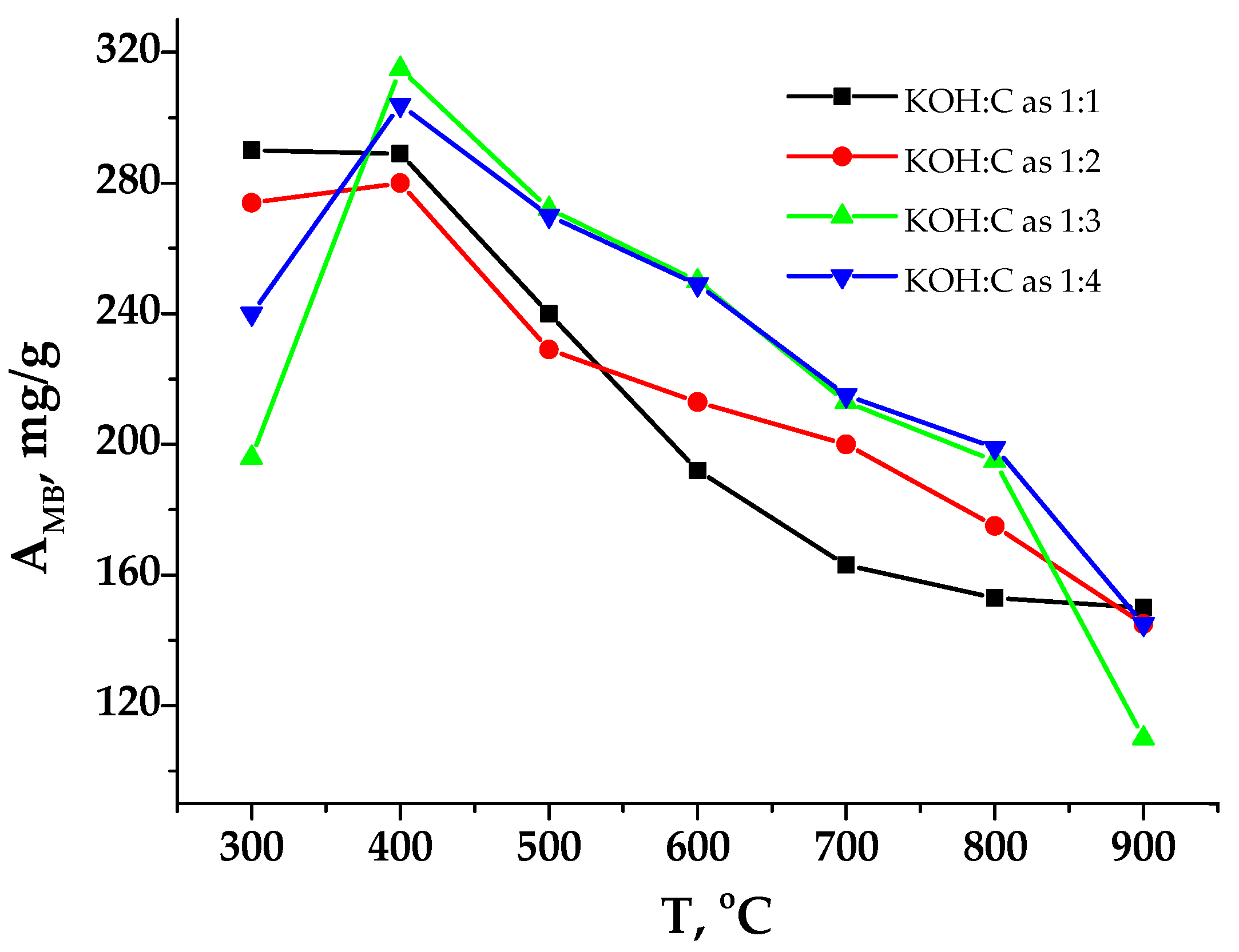
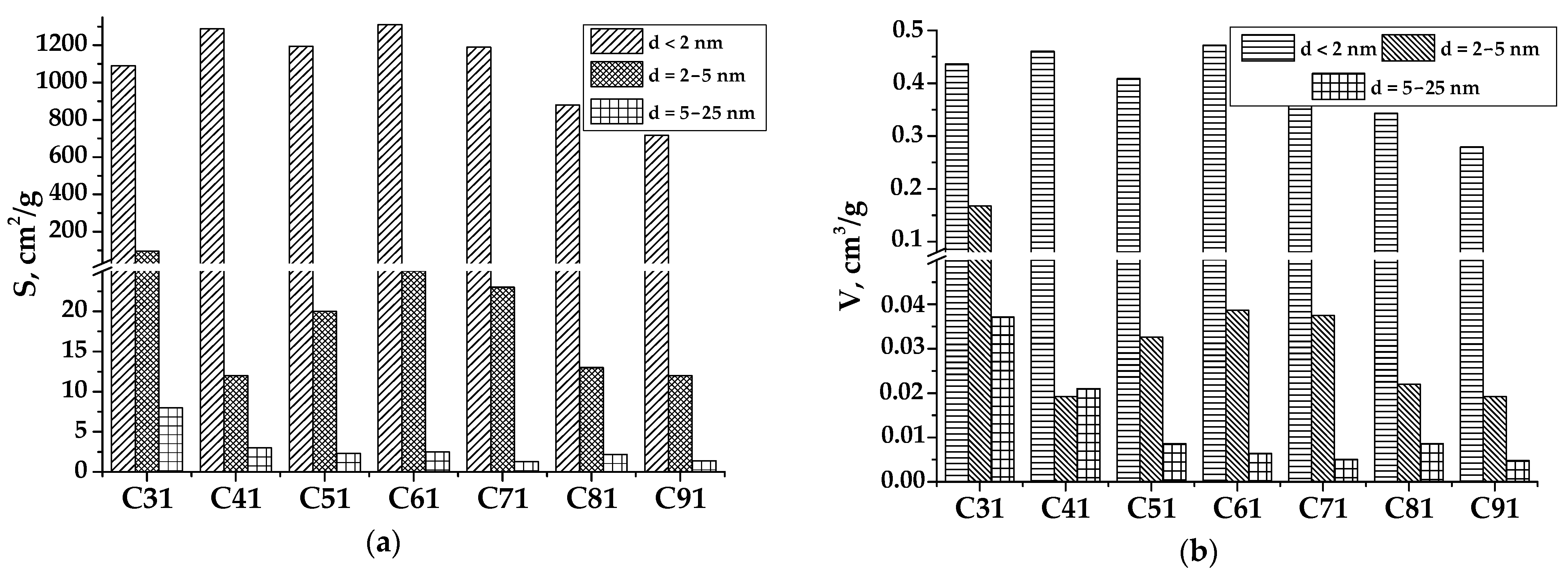
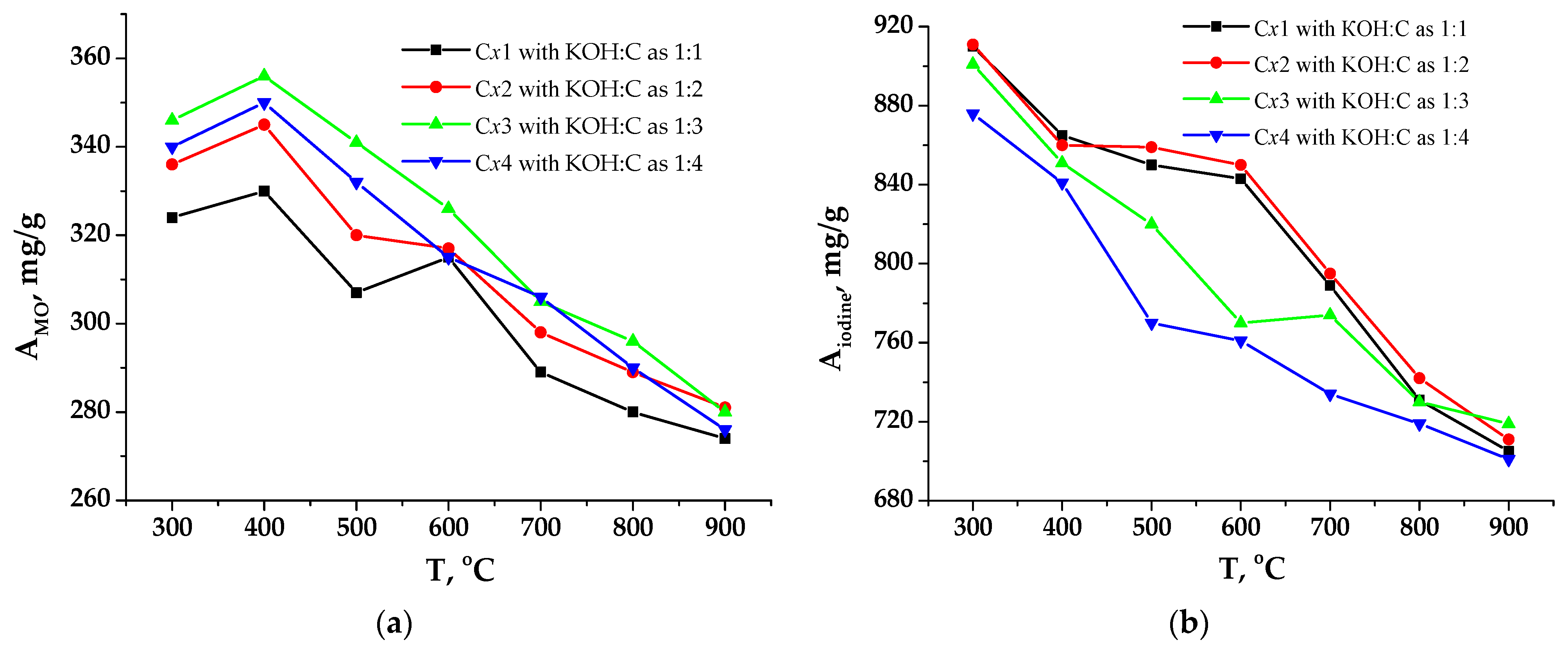
3.1. Porous Carbon Material (PCM) and Its Activation
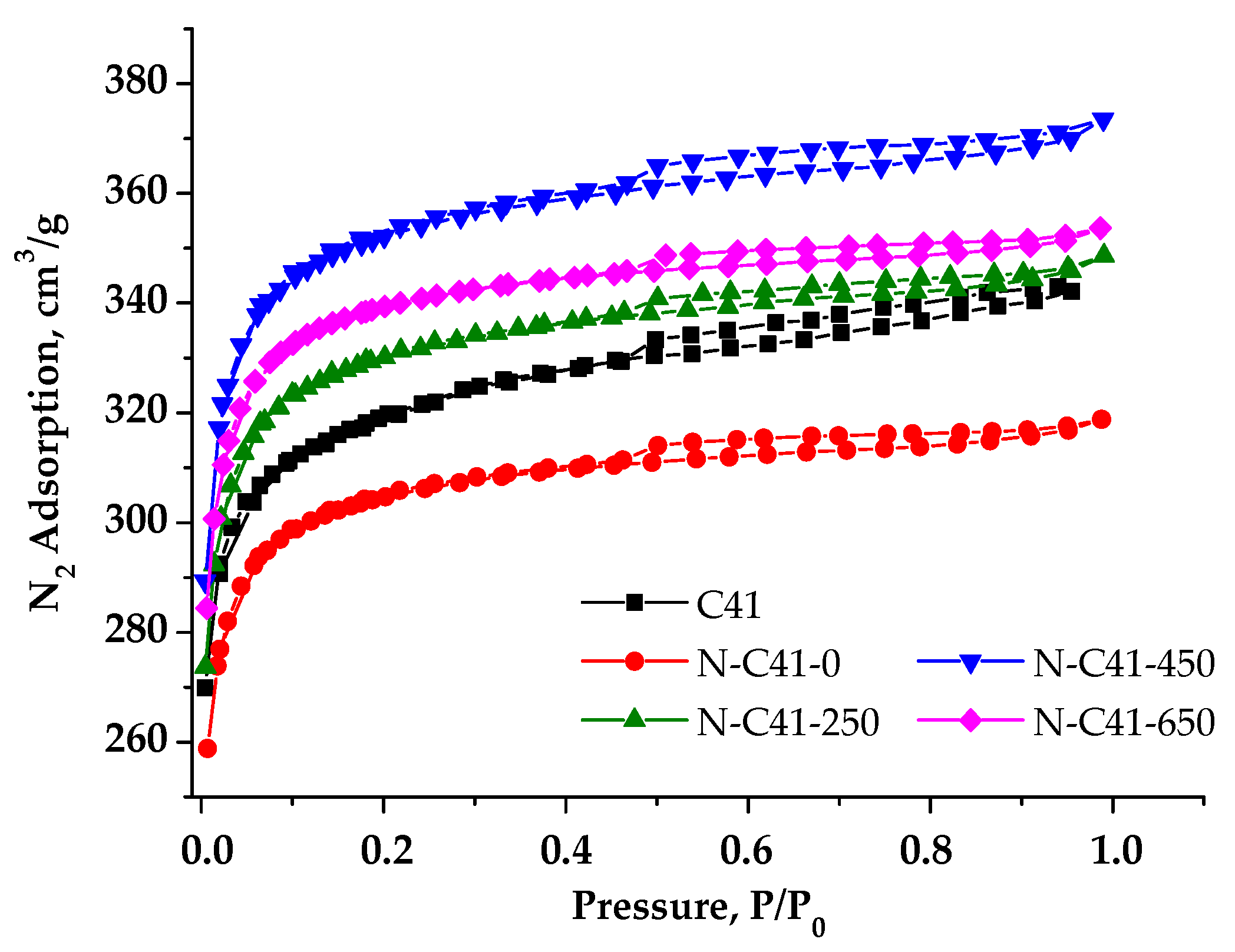
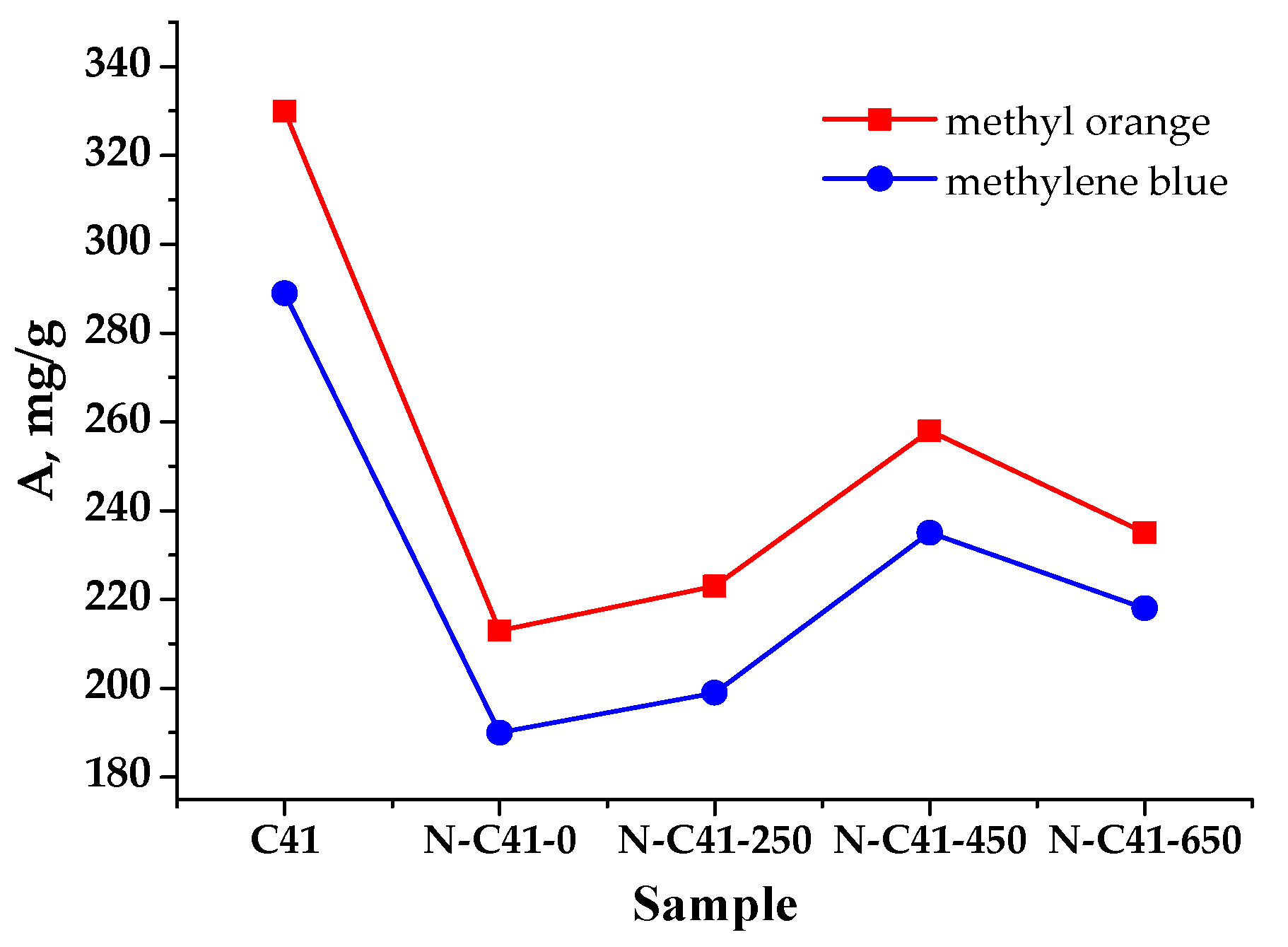
3.2. Nitrogen (N)-Enriched Samples of PCM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Pyrzynska, K. Application of carbon sorbents for the concentration and separation of metal ions. Anal. Sci. 2007, 23, 631–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seitkhan, A. Synthesis of carbonized nano mesoporous sorbents based on vegetable raw materials. J. Nanosci. Nanoeng. 2003, 1, 41–44. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, 04691. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-based adsorbents for removal of dyes from wastewater: A review. Front. Environ. Sci. 2021, 9, 558. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Boychuk, T.Y.; Budzulyak, I.M.; Ivanichok, N.Y.; Lisovskiy, R.P.; Rachiy, B.I. Electrochemical properties of hybrid supercapacitors formed from nanosized spinel LiMn1.5Fe0.5O4. J. Nano Electron. Phys. 2015, 7, 1019. [Google Scholar]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 6, 106716. [Google Scholar] [CrossRef]
- Ketabchi, M.R.; Babamohammadi, S.; Davies, W.G.; Gorbounov, M.; Soltani, S.M. Latest advances and challenges in carbon capture using bio-based sorbents: A state-of-the-art review. Carbon. Capture Sci. Technol. 2023, 6, 100087. [Google Scholar] [CrossRef]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Mandzyuk, V.I.; Lisovskyy, R.P. Electrochemical characteristics of capacitor systems formed on chemically modified carbon base. Nanosistemi Nanomater. Nanotehnologii 2008, 6, 1207–1217. [Google Scholar]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Lisovska, S.A.; Ilnytskyy, R.V.; Lisovskyy, R.P.; Ivanichok, N.Y.; Bandura, K.V.; Rachiy, B.I. Structural and sorption properties of nanoporous carbon materials obtained from walnut shells. Phys. Chem. Solid. State 2023, 24, 348–353. [Google Scholar] [CrossRef]
- Sklepova, S.V.; Gasyuk, I.M.; Ivanichok, N.Y.; Kolkovskyi, P.I.; Kotsyubynsky, V.O.; Rachiy, B.I. The porous structure of activated carbon-based on waste coffee grounds. Phys. Chem. Solid State 2022, 23, 484–490. [Google Scholar] [CrossRef]
- Laine, J.; Calafat, A.; Labady, M. Preparation and characterization of activated carbons from coconut shell impregnated with phosphoric acid. Carbon 1989, 27, 191–195. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Linares-Solano, A.; Gonzalez, L.J.d.D.; Sabio, M.M. Active carbons from almond shells as adsorbents in gas and liquid phases. J. Chem. Technol. Biotech. 1980, 30, 65–72. [Google Scholar] [CrossRef]
- Bevla, F.R.; Rico, D.P.; Gomis, A.F.M. Activated carbon from almond shells. Chemical activation. 2. Zinc chloride activation temperature influence. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 269–271. [Google Scholar]
- Balcı, S.; Doğu, T.; Yücel, H. Characterization of activated carbon produced from almond shell and hazelnut shell. J. Chem. Technol. Biotechnol. 1994, 60, 419–426. [Google Scholar] [CrossRef]
- Toles, C.A.; Marshall, W.E.; Johns, M.M.; Wartelle, L.H.; McAloon, A. Acid-activated carbons from almond shells: Physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 71, 87–92. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Zhu, G.; Duan, J.; Zhao, H.; Liu, M.; Li, F. Apricot shell: A potential high-quality raw material for activated carbon. Adv. Mater. Res. 2013, 798, 3–7. [Google Scholar] [CrossRef]
- Abbas, M. Experimental investigation of activated carbon prepared from apricot stones material (ASM) adsorbent for removal of malachite green (MG) from aqueous solution. Adsorpt. Sci. Technol. 2020, 38, 24–45. [Google Scholar] [CrossRef]
- Abd Ali, K.M. Synthesis of activated carbon by chemical activation of apricot stone with adsorption kinetics. J. Mater. Environ. Sci. 2021, 12, 887–898. [Google Scholar]
- Tan, H.; Tall, O.E.; Liu, Z.; Wei, N.; Yapici, T.; Zhan, T.; Han, Y. Selective oxidation of glycerol to glyceric acid in base-free aqueous solution at room temperature catalyzed by platinum supported on carbon activated with potassium hydroxide. ChemCatChem 2016, 8, 1699–1707. [Google Scholar] [CrossRef]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Rachiy, B.I.; Vashchynsky, V.M.; Mandzyuk, V.I.; Lisovsky, R.P.; Shyyko, L.O. Thermochemical activated carbon as an electrode material for supercapacitors. Nanoscale Res. Lett. 2015, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhu, M. New type nitrogen-doped carbon material applied to deep adsorption desulfurization. Energy Fuels 2020, 34, 9320–9327. [Google Scholar] [CrossRef]
- Sing, K.S. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76, 3–11. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Aktaş, Ö.; Çeçen, F. Effect of type of carbon activation on adsorption and its reversibility. J. Chem. Technol. Biotechnol. 2006, 81, 94–101. [Google Scholar] [CrossRef]
- Lang, J.W.; Yan, X.B.; Liu, W.W.; Wang, R.T.; Xue, Q.J. Influence of nitric acid modification of ordered mesoporous carbon materials on their capacitive performances in different aqueous electrolyte. J. Power Sources 2012, 24, 220–229. [Google Scholar] [CrossRef]
- Su, F.; Poh, C.K.; Chen, J.S.; Xu, G.; Wang, D.; Li, Q.; Lin, J.; Lou, X.W. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. [Google Scholar] [CrossRef]
- Zhu, M.; Weber, C.J.; Yang, Y.; Konuta, M.; Starke, U.; Kern, K.; Bittner, A.M. Chemical and electrochemical ageing of carbon materials used in supercapacitor electrodes. Carbon 2008, 46, 1829–1840. [Google Scholar] [CrossRef]
- Huck, C.W. Advances of infrared spectroscopy in natural product research. Phytochem. Lett. 2015, 11, 384–393. [Google Scholar] [CrossRef]
- Marsh, H.; Yan, D.S.; O’Grady, T.M.; Wenneerberg, A. Formation of active carbons from cokes using potassium hydroxide. Carbon 1984, 22, 603–611. [Google Scholar] [CrossRef]
- Kucherenko, V.A.; Tamarkina, Y.V.; Raenko, G.F.; Chernyshova, M.I. Thermolysis of brown coal in the presence of alkali metal hydroxides. Solid Fuel Chem. 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Rachiy, B.I.; Budzulyak, I.M.; Vashchynsky, V.M.; Ivanichok, N.Y.; Nykoliuk, M.O. Electrochemical properties of nanoporous carbon material in aqueous electrolytes. Nanoscale Res. Lett. 2016, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Vashchynskyi, V.M.; Semkiv, I.V.; Kashuba, A.I.; Petrus’, R.Y. Influence of carbonization conditions on porous structure of carbon materials. Khimiya Fiz. Tekhnologiya Poverhni 2022, 13, 349–357. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, T.; Pu, F.; Chen, H.; Wang, Z.; Zhang, H.; Guan, S. Graphene/carbon-coated Si nanoparticle hybrids as high-performance anode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3449–3455. [Google Scholar] [CrossRef]
- Mandzyuk, V.I.; Nagirna, N.I.; Strelchuk, V.V.; Budzulyak, S.I.; Budzulyak, I.M.; Rachiy, B.I. Electrical and optical properties of porous carbon material. Phys. Chem. Solid State 2012, 13, 94–101. [Google Scholar]
- Zubrik, A.; Matik, M.; Hredzák, S.; Lovás, M.; Danková, Z.; Kováčová, M.; Briančin, J. Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J. Clean. Prod. 2017, 143, 643–653. [Google Scholar] [CrossRef]
- Budzulyak, I.M.; Vashchynsky, V.M.; Rachiy, B.I. Adsorption properties of porous carbon materials obtained by chemical activation. JSPE 2015, 13, 84–90. [Google Scholar]
- Şentorun-Shalaby, Ç.; Uçak-Astarlıoglu, M.G.; Artok, L.; Sarıcı, Ç. Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater. 2006, 88, 126–134. [Google Scholar] [CrossRef]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Rachiy, B.I.; Kuzyshyn, M.M.; Vashchynskyi, V.M.; Mykyteichuk, P.M.; Merena, R.I. Adsorption properties of carbon activated with orthophosphoric acid. Khimiya Fiz. Tekhnologiya Poverhni 2014, 5, 204–209. [Google Scholar]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue. Desalination 2011, 275, 302–305. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption studies of coconut shell carbons prepared by KOH activation for removal of lead (II) from aqueous solutions. Sustainability 2014, 6, 86–98. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhai, K.; He, C.; Li, Q.; Guo, P. Methylene blue adsorption from aqueous solution by loofah sponge-based porous carbons. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 28–35. [Google Scholar] [CrossRef]
- Namal, O.O.; Kalipci, E. Adsorption kinetics of methylene blue removal from aqueous solutions using potassium hydroxide (KOH) modified apricot kernel shells. Int. J. Environ. Anal. Chem. 2020, 100, 1549–1565. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]



| Designation | Carbonization Temperature, °C | Ratio of KOH and C |
|---|---|---|
| C31, C41, C51, C61, C71, C81, C91 | 300, 400, 500, 600, 700, 800, 900, respectively | 1:1 in all samples |
| C32, C42, C52, C62, C72, C82, C92 | 300, 400, 500, 600, 700, 800, 900, respectively | 1:2 in all samples |
| C33, C43, C53, C63, C73, C83, C93 | 300, 400, 500, 600, 700, 800, 900, respectively | 1:3 in all samples |
| C34, C44, C54, C64, C74, C84, C94 | 300, 400, 500, 600, 700, 800, 900, respectively | 1:4 in all samples |
| Designation | Carbonization Temperature, °C | Ratio KOH:C | Activation by HNO3 | Annealing in Ar, °C |
|---|---|---|---|---|
| N-C41-0 | 400 | 1:1 | Yes | - |
| N-C41-250 | 400 | 1:1 | Yes | 250 |
| N-C41-450 | 400 | 1:1 | Yes | 450 |
| N-C41-650 | 400 | 1:1 | Yes | 650 |
| Sample | SBET, m2/g | SDFT, m2/g | Smicro, m2/g | Smeso, m2/g | Vmicro, cm3/g | Vtotal, cm3/g |
|---|---|---|---|---|---|---|
| C31 | 1313 | 1196 | 1067 | 246 | 0.438 | 0.684 |
| C41 | 1188 | 1303 | 1148 | 40 | 0.470 | 0.521 |
| C51 | 1068 | 1216 | 1018 | 50 | 0.416 | 0.470 |
| C61 | 1213 | 1332 | 1160 | 53 | 0.477 | 0.555 |
| C71 | 1042 | 1214 | 984 | 58 | 0.398 | 0.466 |
| C81 | 837 | 894 | 804 | 33 | 0.330 | 0.404 |
| C91 | 721 | 731 | 692 | 29 | 0.273 | 0.317 |
| Sample | Stotal, m2/g | Smicro, m2/g | Smeso, m2/g | Vtotal, cm3/g | Vmicro, cm3/g |
|---|---|---|---|---|---|
| C41 | 1188 | 1148 | 40 | 0.521 | 0.469 |
| N-C41-0 | 1158 | 1130 | 27 | 0.493 | 0.453 |
| N-C41-250 | 1251 | 1219 | 31 | 0.539 | 0.491 |
| N-C41-450 | 1339 | 1303 | 36 | 0.577 | 0.523 |
| N-C41-650 | 1292 | 1261 | 31 | 0.547 | 0.504 |
| Starting Material | Processing Temperature, °C/ Time, Hours | Used Acid for Activation Process | Surface Area, m2/g | Adsorption Capacity, mg/g/Type of Dye | Refs. |
|---|---|---|---|---|---|
| apricot kernel shells | 105/24 | KOH | 359 | 33.67/methylene blue | [49] |
| apricot stone | 200/24 | H2SO4 | 642 | metal ions 27.21/Ni(II) 30.07/Co(II) 33.57/Cd(II) 24.21/Cu(II) 22.85/Pb(II) 29.47/Cr(III) 7.86/Cr(VI) | [20] |
| apricot stones | 250/4 | H3PO4 | 88 | 23.94/malachite green | [22] |
| apricot stones | 600–700/2–3 | H3PO4 | 1115 | <80/reactive blue | [23] |
| apricot stones | 700/1 | H3PO4-HNO3 | 359 | 98/methylene blue 81/methyl orange | [50] |
| apricot shell | 700–900/<1 | -(by moisture and CO2) | 866 | -/methylene blue | [21] |
| apricot pits (sample N-C41-450) | 400–450/1 | KOH-HNO3 | 1339 | 235/methylene blue 260/methyl orange | current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashchynskyi, V.; Okhay, O.; Boychuk, T. Chemical Activation of Apricot Pit-Derived Carbon Sorbents for the Effective Removal of Dyes in Environmental Remediation. C 2023, 9, 93. https://doi.org/10.3390/c9040093
Vashchynskyi V, Okhay O, Boychuk T. Chemical Activation of Apricot Pit-Derived Carbon Sorbents for the Effective Removal of Dyes in Environmental Remediation. C. 2023; 9(4):93. https://doi.org/10.3390/c9040093
Chicago/Turabian StyleVashchynskyi, Vitalii, Olena Okhay, and Tetiana Boychuk. 2023. "Chemical Activation of Apricot Pit-Derived Carbon Sorbents for the Effective Removal of Dyes in Environmental Remediation" C 9, no. 4: 93. https://doi.org/10.3390/c9040093
APA StyleVashchynskyi, V., Okhay, O., & Boychuk, T. (2023). Chemical Activation of Apricot Pit-Derived Carbon Sorbents for the Effective Removal of Dyes in Environmental Remediation. C, 9(4), 93. https://doi.org/10.3390/c9040093







