Abstract
MnO2/nitrogen-containing graphene (x-NGM) composites with varying contents of Mn were used as the electrode materials for flexible asymmetric solid-state supercapacitors. The MnO2 was a two-phase mixture of γ- and α-MnO2. The combination of nitrogen-containing graphene and MnO2 improved reversible Faraday reactions and charge transfer. However, excessive MnO2 reduced conductivity, hindering ion diffusion and charge transfer. Overloading the electrode with active materials also negatively affected conductivity. Both the mass loading and MnO2 content were crucial to electrochemical performance. x-NGM composites served as cathode materials, while graphene acted as the anode material. Operating by two charge storage mechanisms enabled a synergistic effect, resulting in better charge storage purposes. Among the supercapacitors, the 3-NGM1//G1 exhibited the highest conductivity, efficient charge transfer, and superior capacitive characteristics. It showed a superior specific capacitance of 579 F·g−1, leading to high energy density and power density. Flexible solid-state supercapacitors using x-NGM composites demonstrated good cycle stability, with a high capacitance retention rate of 86.7% after 2000 bending cycles.
1. Introduction
The fast evolution of portable and wearable consumer electronics has triggered a demand for high-performance power storage devices. The escalating concerns about energy and the environment have further underscored the significance of developing stable energy storage systems [1,2,3,4,5,6,7,8]. Over the past two decades, supercapacitors have garnered considerable attention because of their exceptional attributes, including high charge-discharge rates, high power density, excellent cycle stability, and cost-effective manufacturing and maintenance [1,2,3,4,5,6,7,8]. Regarded as one of the foremost electrochemical energy storage devices, supercapacitors depend on the electrostatic interactions among electrolyte ions to store and discharge electrical energy [9,10,11]. They are categorized into two primary types based on the storage mechanisms: double-layer capacitors and pseudocapacitors. The former entails energy storage between two electrodes, while the latter involves electrochemical adsorption on the electrode surface through redox reactions. Optimal electrode materials for supercapacitors should possess a large surface area, favorable wettability, and conductivity. However, the existing electrode materials currently exhibit relatively low specific capacitance (ranging from 80 F·g−1 to 120 F·g−1), preventing supercapacitors from meeting the requirements of commercial energy storage [12,13].
The energy (E) stored in a capacitor is determined by its capacitance (C) and operation potential window (V), as shown by the formula E = CV2⁄2 [14,15,16,17]. Electrode materials play an essential role in determining the capacitance and energy density of supercapacitors. Extensive efforts have been dedicated to developing active electrode materials with high specific capacitances. Materials with excellent electrochemical performance for positive electrodes can be matched with appropriate counterparties for negative electrodes. By leveraging the electrolyte decomposition overpotential at both electrodes [18,19,20,21], the battery-like Faraday electrode acts as the energy source, while the capacitor electrode functions as the power source [22]. Thus, the operating potential range can be extended by the complementary potentials on both sides, and the energy density can be further enhanced for more diverse applications [23]. The selection and design of a material system that combines the benefits of each component is a strategic approach to asymmetric supercapacitors [24]. Using solid electrolytes when assembling asymmetric supercapacitors can expand the operation potential window. Compared to conventional liquid electrolyte supercapacitors, solid-state supercapacitors (SSCs) are anticipated to offer higher energy density, rate capability, safety, and cycle stability [11,25,26,27,28,29].
Active electrode materials can be chosen from various materials such as carbon fibers, activated carbon, carbon nanotubes, graphene (G), transition metal oxides, conductive polymers, and their derivatives/composites [30]. Among them, manganese dioxide (MnO2) has gained significant attention due to its low toxicity, affordability, ease of preparation, and high theoretical specific capacitance (approximately 1300 F·g−1). Consequently, MnO2 has been widely utilized as a positive electrode material [31]. The pseudocapacitance behavior of MnO2 can be attributed to the single-electron transfer in the Mn4+/Mn3+ redox system [32]. However, it is limited by some inherent drawbacks, such as poor conductivity, a smaller specific surface area, larger volume changes, and high ion/charge transport resistances, leading to rapid capacitance decay during the charge/discharge processes. This makes it difficult to fully discharge and obtain high capacitive performance, which hinders practical application. To address these issues, incorporating conductive materials with MnO2 has proven to be an effective solution. By doping or formation of composites, different morphologies and crystalline structures can be obtained to tailor the electrochemical characteristics [18,33,34]. The combination of MnO2 nanostructures and modified carbonaceous materials may result in higher specific capacitances in the composites.
Liu et al. developed a simple and cost-effective method to synthesize nitrogen (N)-doped G/MnO2 nanosheet composites with good electrochemical properties. The N-doped G was utilized as the template to grow layered δ-MnO2 nanosheets. At a scan rate of 5 mV·s−1, the highest specific capacitance was 305 F·g−1. The composites were then used as the cathode material in flexible asymmetric solid-state supercapacitors (ASSCs) with activated carbon as the anode material. The ASSCs could work reversibly over the potential range of 0 V–1.8 V and demonstrate high energy density and good cycle stability. The maximum energy density reached 3.5 mWh·cm−3 at a power density of 0.019 W·cm−3 [30]. Zhu et al. used N-doped carbon nanotubes (N-CNTs) derived from polypyrrole as a support material for MnO2 nanoparticles in the preparation of N-CNTs/MnO2 composites. By optimizing the mass ratio between N-CNTs and MnO2, an electrode with superior electrochemical performance was achieved. The capacitance reached 366.5 F·g−1 at a current density of 0.5 A·g−1 and remained at 245.5 F·g−1 as the current density increased to 25 A·g−1. The asymmetric supercapacitors using N-CNTs in the negative electrode and N-CNTs/MnO2 composites in the positive electrode had a high energy density of 20.9 Wh·kg−1 when operating within a potential window of 1.8 V. After 5000 cycles, the capacitance retention rate was 91.6% [13]. Du et al. employed yeast cells as a template to prepare N-doped porous hollow carbon spheres (HCS) and in-situ deposited MnO2 nanowires. Varied morphologies and electrochemical properties were achieved by controlling reaction parameters. A high specific capacitance of 255 F·g−1 was obtained at a current density of 1 A·g−1 utilizing a liquid electrolyte. The asymmetric supercapacitor was constructed using the MnO2/HCS composite in the positive electrode and HCS in the negative electrode. Operating at a power density of 500 W·kg−1 within a potential window of 2.0 V, the maximum energy density reached 41.4 Wh·kg−1. Even at a higher power density of 7901 W·kg−1, the energy density remained at 23.0 Wh·kg−1. After 5000 cycles, the capacitance retention rate was 93.9%. This work showcased an environmentally friendly approach that combines biomass and energy for the preparation of electrode materials [35].
Despite the progress made in MnO2-based asymmetric supercapacitors, their energy density still lags behind lead-acid batteries. The power density would inevitably be reduced due to the differences in kinetics and specific capacitances between the two electrodes. Thus, maintaining high power density while increasing specific capacitance and energy density is a key challenge for MnO2-based supercapacitors. One approach to improve conductivity is the incorporation of conductive carbonaceous materials into MnO2 composites. In this study, we address the challenge by utilizing G as the support for growing MnO2 nanostructures, aiming to elevate the electrochemical performance of supercapacitors. As demonstrated in previous studies [36,37,38], N doping can increase wettability and active sites, promoting the accessibility of electrolyte ions and carrier density by enriching free charges on the electrode surface. To exploit this, we fabricated positive electrodes based on MnO2/N-containing G composites, with G serving as the active material for the negative electrode, to produce flexible ASSCs. N-containing G enhanced conductivity and reduced interfacial impedance. Equilibrium conditions where both electrodes exhibited optimal capacitance were achieved by using identical mass loading on both sides. Mixed-phase MnO2 has been demonstrated to exhibit superior performance over δ- MnO2 [30]. By optimizing the MnO2 content and mass loading in the electrode, a high specific capacitance of 110 F·g−1 was achieved at a current density of 1.0 A·g−1. Operating at a power density of 4400 W·kg−1 over a wide potential window of 3.9 V, a peak energy density of 73.6 Wh·kg−1 was achieved. After 2000 bending cycles, the capacitance retention rate was 86.7% at the current density of 1.0 A·g−1. The results have revealed that our materials processing strategy improved not only the capacitive behavior of individual electrodes but also the overall performance of ASSCs. The synergistic effect arising from the combination of N-containing G and MnO2 nanostructures led to higher specific capacitances and good capacitance retention at high charge/discharge rates.
2. Experimental
2.1. Preparation of G
Graphite oxide (GO) was synthesized using the modified Hummers’ method with graphite powder [39]. Prior to GO synthesis, a pre-oxidation procedure was carried out [40,41]. Four grams of graphite powder (UniRegion Bio-Tech, New Taipei City, Taiwan, <20 μm) was added to a solution composed of 2 g of potassium persulfate (K2S2O8, J. T. Baker, 99%), 2 g of phosphorus pentoxide (P2O5, J. T. Baker, 99%), and 30 mL of concentrated sulfuric acid (H2SO4, Sigma Aldrich, 95 vol%–97 vol%). The mixture was heated to 80 °C and stirred continuously for 6 h. After cooling to room temperature, it was rinsed repeatedly with deionized (DI) water through centrifugation until a neutral pH level was reached. Subsequently, 4 g of pre-oxidized graphite powder was added to 100 mL of concentrated H2SO4 solution in an ice bath. Then, 12 g of potassium permanganate (KMnO4, J. T. Baker, 99%) was slowly added at 35 °C, and the stirring continued for 2 h until the color turned dark brown. Following this, a solution containing 200 mL of DI water and 40 mL of hydrogen peroxide (H2O2, 30 vol% in water) was slowly added, resulting in a vigorous chemical reaction. Once the reaction was complete, a yellow-brown intermediate was produced and placed in a dilute aqueous hydrochloric acid (HCl, Showa, 36 vol%) solution to remove metal ions. After 1 h of ultrasonication, it was repeatedly rinsed with DI water through centrifugation until a neutral pH level was reached, resulting in the obtained GO powder.
To prepare the GO solution, 20 mg of GO powder was added to 100 mL of DI water. The mixture underwent ultrasonication for 2 h to ensure proper dispersion. Subsequently, the suspension was transferred to an autoclave and subjected to a 200 °C hydrothermal process for 2 h in a furnace. After cooling to room temperature, the product was collected by filtration and dried at 80 °C for 12 h. Finally, the dried product was ground to obtain G powder [42].
2.2. Preparation of N-Doped G (NG) Composites
To prepare the NG powder, 55 mg of G was combined with 8.6 mL of ammonia hydroxide solution (28 vol%–30 vol%) in 70 mL of DI water. The mixture underwent ultrasonication for 2 h to ensure proper dispersion. Subsequently, the suspension was transferred to an autoclave and subjected to a 140 °C hydrothermal process for 6 h in a furnace. After cooling to room temperature, the product was rinsed repeatedly with DI water until a neutral pH level was reached and then collected by centrifugation. After being dried at 80 °C for 12 h, the collected product was ground to obtain NG powder [30].
2.3. Preparation of NG/MnO2 (NGM) Composites
G (55 mg) was mixed with 8.6 mL of ammonia hydroxide solution (28 vol%–30 vol%) in 70 mL of DI water. After ultrasonication for 2 h to ensure a better G dispersion, the suspension was transferred to an autoclave placed in a furnace for a 140 °C hydrothermal process for 6 h. Upon cooling to room temperature, it was divided into five portions. Different weights of KMnO4 were added to each portion to create solutions with concentrations of 8.9 mM, 17.8 mM, 26.7 mM, 35.6 mM, and 44.5 mM of KMnO4, respectively.
Each mixture underwent ultrasonication for 30 min and was then transferred to the autoclave for another hydrothermal process at 160 °C for 2 h. After cooling to room temperature, each was rinsed repeatedly with DI water until the neutral pH level was reached and then collected by centrifugation. The collected products were dried at 80 °C for 12 h, followed by grinding. This resulted in five NGM composites with varying amounts of Mn [30], which were named x-NGM, where x is 1, 2, 3, 4, and 5, respectively, corresponding to the abovementioned five KMnO4 concentrations.
2.4. Fabrication of Electrodes
G, NG, and x-NGM composites (100 mg) were mixed with 12.5 mg of carbon black (UniRegion Bio-Tech, New Taipei City, Taiwan) in 2 mL of absolute ethanol (Shimakyu, Samut Sakhon, Thailand, 99.5 vol%), respectively. After ultrasonication for 10 min to improve dispersion, 0.5 g of ethyl cellulose (Aldrich, St. Louis, MI, USA) and 1 mL of terpineol (Aldrich, St. Louis, MI, USA) were added to each suspension. Another ultrasonication for 10 min ensured thorough mixing. Subsequently, stirring for 10 min was performed to allow ethanol to evaporate and achieve the desired consistencies of the G, NG, and x-NGM slurries for electrode fabrication.
A 3.5 cm × 2.5 cm polyimide (PI) tape was affixed to graphite paper to create a flexible PI/graphite substrate (Figure 1a,b). Meanwhile, a square hole measuring 1.6 cm in length was created at the center of a transparency sheet, which was placed above the flexible substrate and secured with 3M tape (Figure 1c). The transparency adhered closely to the substrate, defining the effective area. Next, an even layer of the G, NG, and x-NGM slurries was uniformly coated within the square hole on the flexible substrate using the doctor-blade method (Figure 1c). After allowing it to stand overnight at room temperature, the transparency was removed, leaving behind the electrodes (Figure 1d). They were then subjected to calcination at 200 °C for 1 h to remove organic residues. Three different mass loadings were employed for coating the active materials. The resulting electrodes were named Gy, NGy, and x-NGMy, where y represents the mass loadings of 1 mg, 2 mg, and 3 mg, respectively.
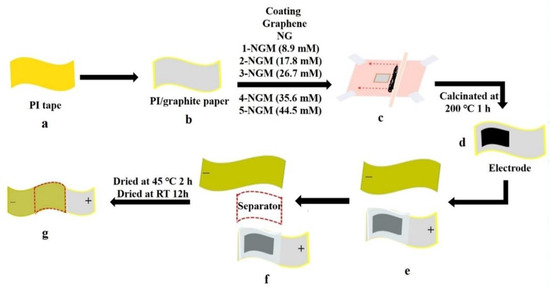
Figure 1.
Fabrication flow chart of the ASSCs: (a, b) creation of a flexible PI/graphite substrate; (c) defining the effective area and spreading electrode materials; (d) formation of positive electrodes; (e) formation of the negative electrode; (f) placement of the separator between two electrodes; (g) assembling flexible ASSCs with a sandwich architecture.
2.5. Fabrication of ASSCs
A 10.6 g portion of lithium chloride (LiCl, Alfa Aesar, 99%) was mixed with 5.0 g of polyvinyl alcohol (PVA, Aldrich, 87–89%) in 50 mL of DI water to prepare a 5 M PVA/LiCl solution, which was stirred at 85 °C for 3 h until it transformed into a clear gel. After cooling to room temperature, a piece of filter paper cut to the desired size was immersed in the gel for 15 min. After removal from the gel, the PVA/LiCl gel membrane serving as the solid electrolyte was obtained [30].
The positive electrodes were fabricated using the three groups of active materials: Gy, NGy, and x-NGMy (Figure 1d). Only G1 was used to fabricate the negative electrode (Figure 1e). As a result, the ASSCs were designated as Gy//G1, NGy//G1, and x-NGMy//G1. A total of 21 flexible devices were obtained. The PVA/LiCl gel membrane also served as the separator between two electrodes (Figure 1f). To secure and position each component, two slide glasses were placed on the top and bottom of the devices, respectively. After baking at 45 °C for 2 h and air drying for 12 h, the slide glasses were removed, resulting in lightweight and flexible ASSCs with a sandwich architecture (Figure 1g) [39,43,44,45].
2.6. Characterization
The surface morphologies of the active materials were examined using field-emission gun scanning electron microscopes (SEM, JEOL JSM-7610F, Tokyo, Japan). The microstructures and lattice fringes were investigated using a high-resolution transmission electron microscope (HRTEM, JEOL JEM-ARM200F, Tokyo, Japan). Elemental mappings were obtained through energy-dispersive X-ray spectroscopy (EDS). The chemical compositions were examined using an X-ray photoelectron spectrometer (XPS, Thermo VG-Scientific, Sigma Probe, Waltham, MA, USA). The chemical states of the elements were determined based on the binding energies of emitted photoelectrons. Raman spectroscopy (Horiba Jobin Yvon, iHR320, East Kilbride, UK) was employed to identify molecular vibrational modes in the range of 400 cm−1 to 2000 cm−1, allowing for determination of the chemical compositions, bonding configurations, and molecular structures of the active materials.
2.7. Electrochemical Measurements
The electrochemical properties were characterized by cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS) using a potentiostat/galvanostat (CH Instruments, CHI627C, Austin, TX, USA). For measurements on electrodes coated with active materials, a 5 M LiCl solution was used as the electrolyte, and a three-electrode configuration was employed with a platinum (Pt) wire auxiliary electrode and a silver chloride (Ag/AgCl) reference electrode. For the corresponding ASSCs, a 5 M PVA/LiCl gel electrolyte was utilized, and a two-electrode configuration was adopted, with the G1 counter electrode serving as both the reference and auxiliary electrodes.
The capacitance characteristics of the electrodes and ASSCs were determined by the areas inside the CV curves at different scan rates and the symmetry of the GCD curves at different current densities. The gravimetric specific capacitances, CCV and CC-DC of individual electrodes, and ASSCs were calculated from the CV and GCD curves using Equations (1) and (2), respectively [46]:
where k represents the electrode constant, typically 2 for a single electrode and 4 for a pair of electrodes. The variables i, ∫i, m, s, ∆t, and ∆V correspond to the discharging current, integral area of a CV curve, mass of electrode materials, scan rate (100 mV·s−1 in this study), discharging time, and potential window subtracting the initial potential drop, respectively. For individual electrodes and ASSCs in this study, the potential windows were set as −1.9 V to 1.0 V and −2.9 V to 1.0 V, respectively. By substituting CCV into Equations (3) and (4) and CC-DC into Equations (5) and (6), the energy densities of electrodes (EEL) and corresponding capacitors (ECell) were calculated. Subsequently, by substituting EEL into Equation (7) and ECell into Equation (8), the power densities of electrodes (PEL) and corresponding capacitors (PCell) were obtained [46].
EIS was used to investigate electronic and ionic transports at the interface of an electrode-active material. The frequency range for EIS was 10–2 Hz to 105 Hz, with an AC amplitude of 10 mV applied between two electrodes. To demonstrate the flexibility of our ASSCs, bending tests were conducted on 3-NGM1//G1, which exhibited superior electrochemical performance. The device was cycled between diameters of 10 mm, 5 mm, and 2 mm before returning to its original unbent state. A total of 2000 cycles were completed. The capacitance parameters were recorded every 50 cycles using the GCD method, with a current density of 1 A·g−1, allowing for comparing the cycle stability of the ASSCs.
3. Results and Discussion
Ultrathin sheet-like structures were discovered in graphite oxide, G, and NG. Fine needle-like structures were observed on the surface of NG. The morphology and structure of x-NGM composites were significantly influenced by the Mn content [47]. Higher concentrations of KMnO4 during preparation resulted in the formation of larger needle-like structures. To demonstrate the presence and uniform distribution of carbon (C), oxygen (O), N, and Mn elements in x-NGM, elemental mappings were again conducted, as displayed in Figure 2. This serves as one of the pieces of evidence for the successful N doping and preparation of x-NGM composites. NG played a dual role in enhancing conductivity and acting as a template for inducing the growth of MnO2 nanostructures [47]. In comparison to the literature [48], the NG template acted as a stronger reducing agent, reacting more readily with KMnO4 and facilitating MnO2 growth. The sparser distribution of the N element, as revealed in Figure 2f, is attributed to its relatively lower proportion in the composite. TEM microstructures used to confirm further the deposition of MnO2 nanostructures on the surface of NG have been presented elsewhere [47]. High-resolution images revealed that the MnO2 in x-NGM composites was a mixture of γ- and α-MnO2 [47]. The XRD patterns presented in Figure 3 further substantiate the presence of both phases. Specifically, the diffraction peaks at 12.5°, 18.1°, 26.1°, 28.9°, 37.2°, 42.8°, 51.2°, 56.4°, 60.1°, and 65.6° correspond to the (110), (200), (220), (310), (211), (301), (411), (600), (521), and (002) planes of α-MnO2, respectively [49]. Additionally, the diffraction peaks at 21.2°, 32.4°, 36.1°, 38.1°, 44.6°, 55.1°, 58.6°, and 64.7° can be attributed to the (120), (031), (131), (230), (300), (160), (401), and (421) planes of γ-MnO2, respectively [50]. Notably, the peak intensities of both phases in 4-NGM and 5-NGM are reduced. The reduction is a consequence of the excess MnO2 content, which adversely affects crystallinity. The combined results from XRD, Raman, and XPS have validated the successful preparation of the x-NGM composites containing NG and MnO2 using the hydrothermal method [47].
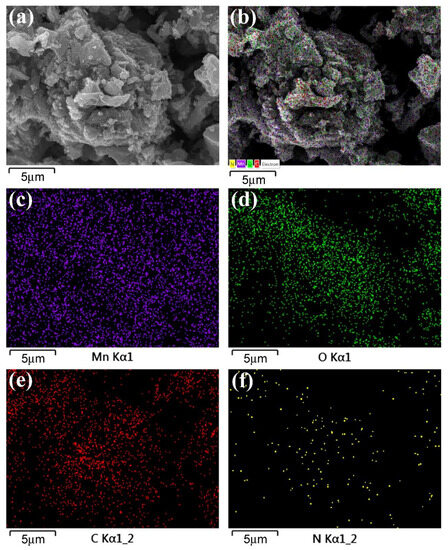
Figure 2.
(a) SEM and (b) EDS layered images of 3-NGM. Elemental mappings of 3-NGM: (c) Mn, (d) O, (e) C, and (f) N.

Figure 3.
XRD patterns of G, NG, and x-NGM composites.
Based on the EIS results of the 21 different active materials on the PI/graphite flexible substrate, it was concluded that the optimal mass loading was 1 mg. Given the stable potential windows and the high specific capacitances exhibited by x-NGM composites, they served as excellent cathode candidates for ASSCs. By pairing G as the anode material with x-NGM as the cathode material, the operating voltage of the capacitor was extended from 2.9 V to 3.9 V. Figure 4 depicts the Nyquist plots of the supercapacitors. The equivalent circuit used for EIS analysis is similar to that reported earlier and consists of the following components [47]: (1) charge transfer impedance at the electrode/electrolyte interface in the high-frequency region (RCT), (2) solution resistance (RS) indicating the contact series resistance between the substrate and current collector, (3) Warburg impedance (W) representing the diffusion resistance of ions in the electrolyte and related to the line tail slope in the low-frequency region, (4) electric double-layer capacitance (C1), and (5) Faraday pseudocapacitance (C2) [51,52,53]. The RCT values obtained by simulation using the equivalent circuit are listed in Table 1.

Figure 4.
Nyquist plots of the 21 supercapacitors with Gy, NGy, and x-NGMy composites. Insets are enlargements when the mass loading is (a) 1 mg, (b) 2 mg, and (c) 3 mg.

Table 1.
RCT values of the 21 supercapacitors with Gy, NGy, and x-NGMy composites obtained by EIS simulation.
As depicted in the inset (a) of Figure 4, when the mass loading of active materials is 1 mg, the Nyquist plots of the G1//G1, NG1//G1, 1-NGM1//G1, 2-NGM1//G1, 3-NGM1//G1, 4-NGM1//G1, and 5-NGM1//G1 devices exhibit smaller semicircles in the high-frequency region, resulting in the following RCT values: 3.46 Ω, 2.68 Ω, 2.35 Ω, 1.43 Ω, 1.24 Ω, 9.14 Ω, and 9.87 Ω, respectively. Table 1 demonstrates that the addition of N to G decreases the RCT from 3.46 Ω to 2.68 Ω. A further reduction to 2.35 Ω is obtained with the addition of MnO2. The combination of NG and MnO2 enhances the reversible Faraday reactions on the surface of active materials and improves charge transfer capability, leading to a more linear response in the low-frequency region of the Nyquist plot. A steeper slope indicates lower diffusion resistance, faster ion transport, and better capacitive properties [51,54]. Among the 21 supercapacitors, the 3-NGM1//G1 device exhibits the smallest RCT, implying the best conductivity. It also shows the steepest slope in the Nyquist plot’s low-frequency region, suggesting rapid charge transfer and excellent capacitive characteristics. However, the RCT value significantly increases in the 4-NGM1//G1 and 5-NGM1//G1 devices with higher MnO2 content. Consistent with the results observed in active material electrodes [47], an excess of MnO2 in the 4-NGM1 and 5-NGM1 electrodes led to larger RCT and poorer conductivity due to fewer contacts. Herein, excess MnO2 is observed to result in a smaller slope in the Nyquist plot’s low-frequency region, which hampers ion diffusion and Faraday charge transfer.
As displayed in the inset (b) of Figure 4, when the mass loading of active materials is 2 mg, the Nyquist plots of the G2//G1, NG2//G1, 1-NGM2//G1, 2-NGM2//G1, 3-NGM2//G1, 4-NGM2//G1, and 5-NGM2//G1 devices exhibit larger semicircles in the high-frequency region, resulting in the corresponding RCT values of 3.74 Ω, 3.41 Ω, 2.59 Ω, 1.74 Ω, 1.32 Ω, 9.43 Ω, and 10.24 Ω. Both insets (a) and (b) demonstrate a decrease in electrochemical impedance due to N doping. Moreover, it is observed that the RCT increases with higher mass loading. Similarly, as shown in the inset (c) of Figure 4, when the mass loading of active materials is 3 mg, the Nyquist plots of the G3//G1, NG3//G1, 1-NGM3//G1, 2-NGM3//G1, 3-NGM3//G1, 4-NGM3//G1, and 5-NGM3//G1 devices exhibit even larger semicircles in the high-frequency region, resulting in larger corresponding RCT values of 15.40 Ω, 9.46 Ω, 8.97 Ω, 2.24 Ω, 1.89 Ω, 9.74 Ω, and 13.60 Ω. This suggests that an excessive mass of active materials on flexible electrodes is unfavorable for conductivity improvement. As proven, 1 mg is the most appropriate mass loading. Amounts of 2 mg and 3 mg are excessive and cause reduced specific capacitance, energy density, and power density. The results once again demonstrate that both the mass loading of active materials and the content of MnO2 impact the conductivity in the ASSCs. Consistent with the EIS results, better charge transfer efficiency can only be achieved when the most appropriate MnO2 content is used in an x-NGM composite. Therefore, in this study, x-NGM composites and G were utilized as the active materials for cathodes and anodes, respectively, to manufacture ASSCs operating based on both (pseudocapacitance and electric double-layer capacitor) mechanisms simultaneously. The synergistic effect of the two types of materials enabled improved charge storage.
Table 2 presents the capacitor parameters obtained from the CV results for the 21 flexible ASSCs using Gy, NGy, and x-NGMy composites. Among the nine capacitors using Gy, NGy, and 1-NGMy composites, the 1-NGM1//G1 had the largest integral area within its CV curve loop, resulting in a high specific capacitance of 209 F·g−1. Its energy density and power density were 441.9 Wh·kg−1 and 1744.3 W·kg−1, respectively. The capacitive characteristics of the 12 x-NGMy//G1 capacitors with varying MnO2 content and three mass loadings of active materials for each MnO2 content were also explored and compared. Although their CV curves deviated from rectangles due to the synergistic effect of the two charge storage mechanisms, they remained symmetric, indicating a faster and reversible charge/discharge process. Consistent with the findings reported in the literature [30,35], the specific capacitance decreased significantly with increasing mass loading. Among the 15 capacitors using x-NGMy composites, the 3-NGM1//G1 had the largest CV curve loop, implying the highest conductivity. The charge transfer path was shortened, facilitating electrolyte ions to reach the electrode surface and enabling faster electron transfer [32]. It exhibited the highest specific capacitance of 579 F·g−1, resulting in corresponding high energy density and power density of 1223.3 Wh·kg−1 and 73,153.6 W·kg−1, respectively, as listed in Table 2. Previous results have shown that the 3-NGM1 electrode exhibited excellent capacitance performance [47]. Therefore, x = 3 and y =1 were identified as the optimal parameters for x-NGMy materials. The utilization of the 3-NGM1 composite in ASSCs facilitated reversible redox (pseudocapacitive) reactions on the electrode surface. When combined with the fast charge/discharge property of the electric double-layer capacitor material, the overall performance of the 3-NGM1//G1 capacitor was enhanced by the synergistic effect of the two charge storage mechanisms. However, when x = 4 and 5, there was an excess of MnO2 in the active materials, leading to the growth of nanorod structures that impeded ion diffusion in the electrolyte and the functioning of the pseudocapacitive storage mechanism. As a result, the synergistic effect could not be effectively achieved. The excessive mass loading of active materials on electrodes resulted in a reduction in specific capacitance, energy density, and power density. The CV results of ASSCs were consistent with the findings for the electrodes [47]. Figure 5a shows the Ragone plot of the supercapacitors obtained by plotting energy density vs. power density calculated using Equations (4) and (8). Again, it is evident that the 3-NGM1//G1 capacitor exhibits the best capacitance characteristic among the ASSCs.

Table 2.
Capacitance parameters obtained from the CV results of the 21 supercapacitors with Gy, NGy, and x-NGMy composites.
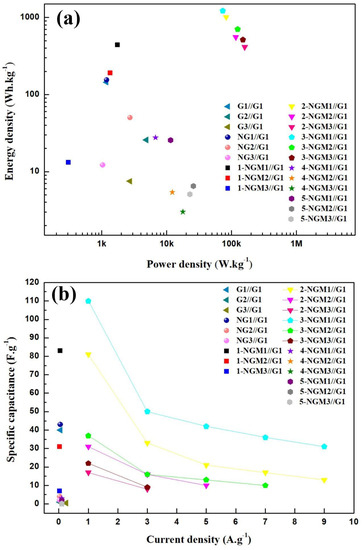
Figure 5.
(a) Ragone plot obtained from the CV results. (b) Plots of specific capacitance vs. current density were obtained from the GCD results of the Gy//G1, NGy//G1, and x-NGMy//G1 supercapacitors.
Figure 5b shows the plots of specific capacitance vs. current density obtained from the GCD curves of three Gy//G1 symmetric capacitors and 18 NGy//G1 and x-NGMy//G1 asymmetric capacitors. Evidently, the Gy//G1, NGy//G1, 1-NGMy//G1, 4-NGMy//G1, and 5-NGMy//G1 devices are unable to sustain current densities exceeding 1 A·g−1. The 2-NGM2//G1 capacitor can only withstand current densities of 1 A·g−1, 3 A·g−1, and 5 A·g−1. In contrast, the 3-NGM2//G1 capacitor can endure current densities of 1 A·g−1, 3 A·g−1, 5 A·g−1, and 7 A·g−1, while the 2-NGM1//G1 and 3-NGM1//G1 capacitors can handle all current densities. Among them, the 3-NGM1//G1 capacitor stands out for its exceptional endurance and sustainability, indicating that an optimal Mn content in an NGM composite leads to superior performance. Table 3 shows the specific capacitance of the 3-NGM1//G1 capacitor under a current density of 1 A·g−1, which is 110 F·g−1. This corresponds to the energy density and power density of 73.6 Wh·kg−1 and 4400.0 W·kg−1, respectively, across a wide potential window of 3.9 V. When compared to the results in Ref. [30], it can be inferred that mixed-phase MnO2 nanostructures may offer better overall capacitive characteristics than δ-MnO2. Additionally, the GCD results indicate an inverse relationship between charge/discharge time and current density. Supercapacitors operate at higher current density charges and discharge faster but have lower specific capacitance due to limited time for redox reactions [55]. Conversely, those operated at lower current density charges discharge more slowly but have higher specific capacitance due to sufficient time for redox reactions. This is consistent with the observation that electrodes performed more efficiently at low current densities. The GCD results of the ASSCs reveal a similar trend to those of the electrodes [47]. Combining the pseudocapacitive material 3-NGM1 with the fast charge/discharge electric double-layer capacitor material G1 produced a synergistic effect, enhancing the performance of the 3-NGM1//G1 capacitor.

Table 3.
Capacitance parameters obtained from the GCD results of the supercapacitors with Gy, NGy, and x-NGMy composites.
Bending tests were conducted to evaluate the flexibility and cycle stability of the solid-state ASSCs. The devices were bent to diameters of 10 mm, 5 mm, and 2 mm, and then returned to their nonbending state to complete a cycle. Figure 6a illustrates the CV curves of the 3-NGM1//G1 capacitor obtained from the bending test at a scan rate of 100 mV·s−1. After one cycle, the CV curve closely resembles the original curve. Figure 6b presents the GCD curves of the same capacitor obtained from the bending test at the current density of 1 A·g−1. The curves exhibit slight distortion from the ideal triangular shape due to the pseudocapacitive contribution from MnO2. However, after one cycle, the GCD curve closely matches the original curve, confirming the excellent flexibility and high charge/discharge stability of the solid-state ASSCs utilizing x-NGMy active materials. Figure 6c displays the GCD curves obtained after subjecting the 3-NGM1//G1 capacitor to 2000 bending cycles at the current density of 1 A·g−1. Data were recorded every 50 cycles. The capacitance parameters (specific capacitance, energy density, and power density) after 50, 100, 200, 400, 800, 1600, and 2000 cycles, as listed in Table 4, are compared with the results from the first cycle. With an increasing cycle count, the shape and position of the GCD plot exhibit no significant deviations compared to the first cycle. As depicted in Figure 6d, the capacitance retention rate after 2000 bending cycles is 86.7%, confirming the excellent cycle stability of the flexible ASSCs using x-NGMy composites. Figure 6e shows the Ragone plot, comparing the energy density of the 3-NGM1//G1 capacitor calculated from the GCD results at different current densities with those in the literature [45,56,57,58,59,60,61,62,63,64,65,66,67]. The performance achieved in this study surpasses that of most results reported elsewhere, highlighting the potential of x-NGMy composites as electrode-active materials for flexible supercapacitors.
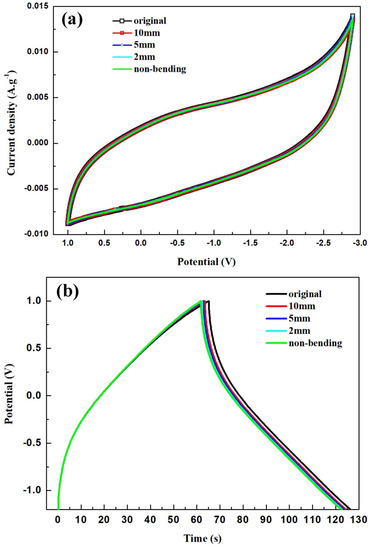
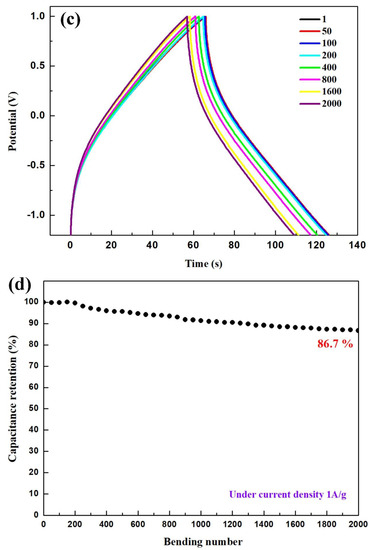
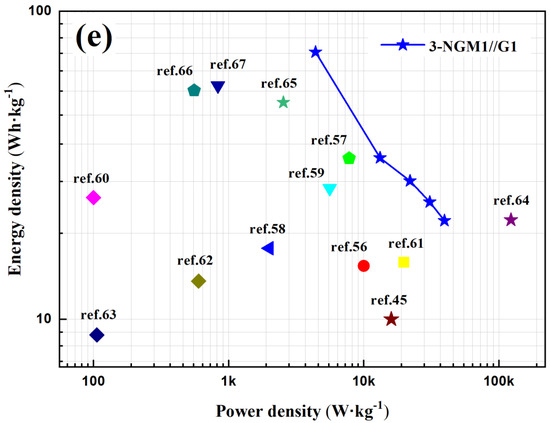
Figure 6.
3-NGM1//G1 supercapacitor: (a) CV and (b) GCD curves obtained by one bending cycle; (c) GCD curves and (d) retention rate of specific capacitance obtained by 2000 bending cycles; (e) Ragone plot, which compares the energy density calculated by GCD results with those reported in the literature [45,56,57,58,59,60,61,62,63,64,65,66,67].

Table 4.
Capacitance parameters obtained from the GCD results of the 3-NGM1//G1 capacitor after 50, 100, 200, 400, 800, 1600, and 2000 bending cycles.
4. Conclusions
Hydrothermal synthesis produced x-NGM composites comprising NG and MnO2 with varying Mn content for use in ASSCs as active electrode materials. SEM, EDS mappings, XPS, and Raman results confirmed the presence of NG and MnO2, as well as the successful preparation of the composites. TEM analysis revealed a two-phase mixture of γ- and α-MnO2 in x-NGM composites. The combination of NG and MnO2 enhanced reversible Faraday reactions and charge transfer capability. Excessive MnO2 reduced conductivity and the Nyquist plot’s slope in the low-frequency region, hindering ion diffusion and charge transfer. Optimum charge transfer efficiency requires the appropriate MnO2 content. Overloading the flexible electrode with active materials also showed a negative impact on conductivity. A mass loading of 1 mg was the most suitable, while 2 mg and 3 mg reduced specific capacitance, energy density, and power density. Both the mass loading and the MnO2 content played crucial roles in determining capacitor performance.
By utilizing x-NGM composites as cathode materials and G as the anode material, the simultaneous operation of two charge-storage mechanisms was enabled, leading to improved charge storage. Among the supercapacitors, the 3-NGM1//G1 device showed superior conductivity and the steepest slope in the Nyquist plot’s low-frequency region, indicating its efficient charge transfer and best capacitive properties. It demonstrated a high specific capacitance of 579 F·g−1, giving rise to the high energy density (1223.3 Wh·kg−1) and power density (73,153.6 W·kg−1). Furthermore, the flexible ASSCs using x-NGMy composites exhibited good cycle stability. The capacitance retention rate was 86.7% after 2000 bending cycles. The exceptional performance in this study surpassed most literature results, highlighting the potential of x-NGMy composites as promising electrode materials for supercapacitors.
Author Contributions
Conceptualization, H.-Y.C. and C.-P.C.; methodology, H.-Y.C. and C.-P.C.; software, H.-Y.C. and C.-P.C.; validation, H.-Y.C. and C.-P.C.; formal analysis, H.-Y.C. and C.-P.C.; investigation, H.-Y.C. and C.-P.C.; resources, C.-P.C.; data curation, H.-Y.C. and C.-P.C.; writing—original draft preparation, C.-P.C.; writing—review and editing, C.-P.C.; visualization, H.-Y.C. and C.-P.C.; supervision, C.-P.C.; project administration, C.-P.C.; funding acquisition, C.-P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science and Technology Council, Taiwan under the grant number of MOST 109-2221-E-260-009-. The APC was funded by National Chi Nan University.
Data Availability Statement
Not applicable.
Acknowledgments
Supports from the National Science and Technology Council, Taiwan and National Chi Nan University are gratefully appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Du, X.; Wang, J.; Ma, H.; Jing, X. Polypyrrole composites with carbon materials for supercapacitors. Chem. Pap. 2017, 71, 293. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-performance supercapacitors: A comprehensive review on paradigm shift of conventional energy storage devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Gholami, M.; Naseri, N. Inkjet printing of MnO2 nanoflowers on surface-modified A4 paper for flexible all-solid-state microsupercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 3894. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.G.; Yan, X.H.; Cao, Y.; Gao, H.L.; Luo, H.W.; Gao, K.Z.; Xue, S.C.; Jing, X. Recent advances and perspectives on graphene-based gels for superior flexible all-solid-state supercapacitors. J. Power Sources 2023, 565, 232916. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Hu, J.; Wei, L.; Liu, J.; Zheng, M. Facile synthesis of MnO2 grown on nitrogen-doped carbon nanotubes for asymmetric supercapacitors with enhanced electrochemical performance. J. Power Sources 2018, 393, 135. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Wu, H.B.; Lin, J.; Shen, Z.; Lou, X.W. High-performance flexible asymmetric supercapacitors based on a new graphene foam/carbon nanotube hybrid film. Energy Environ. Sci. 2014, 7, 3709. [Google Scholar] [CrossRef]
- Ning, P.; Duan, X.; Ju, X.; Lin, X.; Tong, X.; Pan, X.; Wang, T.; Li, Q. Facile synthesis of carbon nanofibers/MnO2 nanosheets as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 2016, 210, 754. [Google Scholar] [CrossRef]
- Ghosh, K.; Yue, C.Y.; Sk, M.M.; Jena, R.K. Development of 3D urchin-shaped coaxial manganese dioxide@polyaniline (MnO2@PANI) composite and self-assembled 3D pillared graphene foam for asymmetric all-solid-state flexible supercapacitor application. ACS Appl. Mater. Interfaces 2017, 9, 15350. [Google Scholar] [CrossRef]
- Kong, S.; Cheng, K.; Ouyang, T.; Gao, Y.; Ye, K.; Wang, G.; Cao, D. Facile dip coating processed 3D MnO2-graphene nanosheets/MWNT-Ni foam composites for electrochemical supercapacitors. Electrochim. Acta 2017, 226, 29. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.S.; Lu, X.; Lee, P.S. Dodecyl sulfate-induced fast faradic process in nickel cobalt oxide-reduced graphite oxide composite material and its application for asymmetric supercapacitor device. J. Mater. Chem. 2012, 22, 23114. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Mirfakhrai, T.; Chen, Z.; Casalongue, H.S.; Dai, H. Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 2011, 4, 729. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Wu, H.; Duan, J.; Zhang, Y. Hydrothermal self-assembly of manganese dioxide/manganese carbonate/reduced graphene oxide aerogel for asymmetric supercapacitors. Electrochim. Acta 2015, 164, 154. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Zhai, T.; Xie, S.; Ling, Y.; Tong, Y.; Li, Y. H-TiO2@MnO2//H-TiO2@C core-shell nanowires for high performance and flexible asymmetric supercapacitors. Adv. Mater. 2013, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Fernández-Garcia, L.; González, Z.; Granda, M.; Menendez, R.; Gryglewicz, G. MnO2/thermally reduced graphene oxide composites for high-voltage asymmetric supercapacitors. Electrochim. Acta 2017, 240, 53. [Google Scholar] [CrossRef]
- Tseng, L.H.; Hsiao, C.H.; Nguyen, D.D.; Hsieh, P.Y.; Lee, C.Y.; Tai, N.H. Activated carbon sandwiched manganese dioxide/graphene ternary composites for supercapacitor electrodes. Electrochim. Acta 2018, 266, 284. [Google Scholar] [CrossRef]
- Niu, Z.; Dong, H.; Zhu, B.; Li, J.; Hng, H.H.; Zhou, W.; Chen, X.; Xie, S. Highly stretchable, integrated supercapacitors based on single-walled carbon nanotube films with continuous reticulate architecture. Adv. Mater. 2013, 25, 1058. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Zhai, T.; Wang, G.; Xie, S.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 2013, 13, 2628. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ding, T.; Yuan, L.; Shen, Y.; Zhong, Q.; Zhang, X.; Cao, Y.; Hu, B.; Zhai, T.; Gong, L. WO3-x/MoO3-x core/shell nanowires on carbon fabric as an anode for all-solid-state asymmetric supercapacitors. Adv. Energy Mater. 2012, 2, 1328. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, K.H.; Lin, M.C.; Wu, Y.T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 2006, 6, 2690. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly flexible and all-solid-state paper like polymer supercapacitors. Nano Lett. 2010, 10, 4025. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, X.; Fang, J.; Zhang, X.; Chen, S.; Li, W.; Feng, W.; Chen, Y.; Wang, W.; Zhang, Y. Layered-MnO2 nanosheet grown on nitrogen-doped graphene template as a composite cathode for flexible solid-state asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 5251. [Google Scholar] [CrossRef]
- Liu, M.; Gan, L.; Xiong, W.; Xu, Z.; Zhu, D.; Chen, L. Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes. J. Mater. Chem. 2014, 2, 2555. [Google Scholar] [CrossRef]
- Mu, B.; Zhang, W.; Xu, W.; Wang, A. Hollowed-out tubular carbon@MnO2 hybrid composites with controlled morphology derived from kapok fibers for supercapacitor electrode materials. Electrochim. Acta 2015, 178, 709. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Zhan, J.; Sun, X.; Kang, L.; Jiang, F.; Zhang, X.; Shao, Q.; Dong, M.; Liu, H.; et al. Biological cell template synthesis of nitrogen-doped porous hollow carbon spheres/MnO2 composites for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 296, 907. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y.; et al. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771. [Google Scholar] [CrossRef]
- Wen, Y.; Ding, H.; Shan, Y. Preparation and visible light photocatalytic activity of Ag/TiO2/graphene nanocomposite. Nanoscale 2011, 3, 4411. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Guo, Y.; Wang, S.; Liu, Y. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl. Mater. Interfaces 2013, 5, 2227. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Cao, C.B.; Tao, S.; Chu, W.S.; Wu, Z.Y.; Li, Y.D. Ultrathin nickel hydroxide and oxide nanosheets: Synthesis, characterizations and excellent supercapacitor performances. Sci. Rep. 2015, 4, 5787. [Google Scholar] [CrossRef]
- Zeiger, M.; Jackel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172. [Google Scholar] [CrossRef]
- Brousse, T.; Taberna, P.L.; Crosnier, O.; Dugas, R.; Guillemet, P.; Scudeller, Y.; Zhou, Y.; Favier, F.; Bélanger, D.; Simon, P. Long-term cycling behavior of asymmetric activated carbon/MnO2 aqueous electrochemical supercapacitor. J. Power Sources 2007, 173, 633. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Huang, N.M.; Harrison, I.; Andou, Y. Flexible graphene-based supercapacitors: A review. J. Phys. Chem. C 2016, 120, 4153. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Cho, C.P. Mixed-phase MnO2/N-Containing graphene composites applied as electrode active materials for flexible asymmetric solid-state supercapacitors. Nanomaterials 2018, 8, 924. [Google Scholar] [CrossRef]
- Liu, L.; Su, L.; Lang, J.; Hu, B.; Xu, S.; Yan, X. Controllable synthesis of Mn3O4 nanodots@nitrogen-doped graphene and its application for high energy density supercapacitors. J. Mater. Chem. A 2017, 5, 5523. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, N.; Shi, C.; Liu, E.; He, C. Improve the supercapacity performance of MnO2-decorated graphene by controlling the oxidization extent of graphene. J. Phys. Chem. C 2012, 116, 25226. [Google Scholar] [CrossRef]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 2008, 112, 4406. [Google Scholar] [CrossRef]
- Li, Z.; Mi, Y.; Liu, X.; Liu, S.; Yang, S.; Wang, J. Flexible graphene/MnO2 composite papers for supercapacitor electrodes. J. Mater. Chem. 2011, 21, 14706. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Wu, C.W.; Chen, I.W.P.; Chang, H.T.; Lin, C.H.; Huang, C.C. Carbon dot-mediated synthesis of manganese oxide decorated graphene nanosheets for supercapacitor application. ACS Sustain. Chem. Eng. 2016, 4, 3008. [Google Scholar] [CrossRef]
- Du, D.; Li, P.; Ouyang, J. Nitrogen-doped reduced graphene oxide prepared by simultaneous thermal reduction and nitrogen doping of graphene oxide in air and its application as an electrocatalyst. ACS Appl. Mater. Interfaces 2015, 7, 26952. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, B.; Liu, J.; Long, Y.; Li, N.; Wen, Z.; Jiang, Y. Graphene/ MnO2 composite prepared by a simple method for high performance supercapacitor. Mater. Res. Innov. 2016, 20, 92. [Google Scholar] [CrossRef]
- Xiao, W.; Zhou, W.; Yu, H.; Pu, Y.; Zhang, Y.; Hu, C. Template synthesis of hierarchical mesoporous δ-MnO2 hollow microspheres as electrode material for high-performance symmetric supercapacitor. Electrochim. Acta 2018, 264, 1. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. High-performance asymmetric supercapacitor based on graphene hydrogel and nanostructured MnO2. ACS Appl. Mater. Interfaces 2012, 4, 2801. [Google Scholar] [CrossRef]
- Yang, M.; Kim, D.S.; Hong, S.B.; Sim, J.W.; Kim, J.; Kim, S.S.; Choi, B.G. MnO2 nanowire/biomass-derived carbon from hemp stem for high-performance supercapacitors. Langmuir 2017, 33, 5140. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020. [Google Scholar] [CrossRef]
- Cheng, H.; Duon, H.M. Three dimensional manganese oxide on carbon nanotube hydrogels for asymmetric supercapacitors. RSC Adv. 2016, 6, 36954. [Google Scholar] [CrossRef]
- Śliwak, A.; Gryglewicz, G. High-voltage asymmetric supercapacitors based on carbon and manganese oxide/oxidized carbon nanofiber composite electrodes. Energy Technol. 2014, 2, 819. [Google Scholar] [CrossRef]
- Chou, T.C.; Doong, R.A.; Hu, C.C.; Zhang, B.; Su, D.S. Hierarchically porous carbon with manganese oxides as highly efficient electrode for asymmetric supercapacitors. ChemSusChem 2014, 7, 841. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability. J. Phys. Chem. C 2014, 118, 22865. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Zhang, H.; Varanasi, C.V.; Liu, J. Synergistic effects from graphene and carbon nanotubes enable flexible and robust electrodes for high-performance supercapacitors. Nano Lett. 2012, 12, 4206. [Google Scholar] [CrossRef]
- Khomenko, V.; Pinero, E.R.; Beguin, F. Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2V in aqueous medium. J. Power Sources 2006, 153, 183. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Yang, D.; Li, J. High performance MnO2 supercapacitor material prepared by modified electrodeposition method with different electrodeposition voltages. J. Energy Storage 2020, 29, 101363. [Google Scholar] [CrossRef]
- Wu, J.; Raza, W.; Wang, P.; Hussain, A.; Ding, Y.; Yu, J.; Wu, Y.; Zhao, J. Zn-doped MnO2 ultrathin nanosheets with rich defects for high performance aqueous supercapacitors. Electrochim. Acta 2022, 418, 140339. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on carbon fibers modified with carbon nanotubes for ultrafast flexible supercapacitors in ionic liquid electrolytes with wide voltage windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).