Abstract
The aim of this work is to study the properties of carbon materials prepared from apricot stones by carbonization at 300–900 °C and chemical activation by KOH with different ratios between components. It was found that increasing the carbonization temperature to 800–900 °C leads to the degradation of narrow micropores and the carbon matrix. The adsorbent materials were characterized with FTIR and SEM, and a specific surface area was calculated. Moreover, additional activation by HNO3 and annealing at 450 °C led to an increase in surface area up to 1300 m2/g. The obtained N-enriched sorbents show adsorption activities of 190–235 mg/g for methylene blue and 210–260 mg/g for methyl orange. The results of this study can be useful for future scale-up using the apricot material as a low-cost adsorbent for the removal of dyes in environmental remediation production.
1. Introduction
In the modern world, one of the key problems that concern humanity is environmental pollution, which has become a growing problem and is of serious negative effects. In the modern world, one of the key problems that concern humanity is the reduction in environmental pollution, especially of the hydrosphere because of rapidly growing industrialization and mining. Pollutants enter our waters, soils, and atmosphere due to the rapid development of agriculture and metallurgical industry and the improper disposal of waste, fertilizers, and pesticides [1]. One of the most common pollutants of the water environment are dyes used in various industries such as textile, food, and cosmetics. Industrial wastewater contains colored compounds that can be dangerous due to toxicity, low biodegradability, etc. Dyes in surface water significantly affect nature/ecosystem, e.g., affect the process of photosynthesis and negatively affect living organisms. Metals such as lead, cadmium, and mercury are particularly dangerous because they are practically not removed from biological objects [2]. In addition, coloring compounds can be harmful to the human body and lead to various diseases [3]. Thus, effective treatment of wastewater containing dyes is required, and chemical oxidation, precipitation and coagulation, biological treatment, membrane methods, and adsorption on activated carbon can be used [4,5]. Reagent treatment, ion exchange, and membrane purification methods are usually used to remove metal ions from water environments. On the contrary, sorption purification methods are simple, less expensive, available, and effective. Carbon sorbents, natural and synthetic sorbents, clay rocks, zeolites, etc., are used as filter materials. However, the ideal materials/technologies—that are cheap and effective—have yet to be found. In addition, porous carbon materials can be used as catalysts/electrode components for energy storage in supercapacitors [6,7], batteries [8], etc.
The main factors when choosing a sorbent for targeted separation are surface area, pore volume, porous structure, and physicochemical properties. In addition, due to environmental friendliness, low cost, and good surface characteristics, biomass-based adsorbents have attracted attention and are widely studied [9]. Carbon sorbent (CS) is a material used to remove both inorganic and organic impurities from the environment. It is characterized by its high specific surface area, well-developed porous structure, and functional groups on the surface of the sorbent. The presence of these groups within the carbon structure, along with stable radicals and fragments with donor–acceptor properties, determines the reaction nature of the carbon interaction with chemical reagents [10]. In addition, CS derived from secondary vegetable raw materials find extensive use across various industries where they are utilized as catalysts and absorbers. These materials can also be employed in hemosorption systems, which perform targeted purification of blood and other physiological fluids from various toxicants, as well as in absorbed preparations such as probiotics. When choosing a raw material for use as a sorbent, attention should also be paid to its availability, cost, and environmental impact. Correspondingly, examples of suitable materials include wood in the form of sawdust, charcoal, peat, fossil coal, peat coke, oil refining products [11], and products of plant origin, such as cellulose, fruit pits, soybean meal, sunflower and coffee husks, nutshells, and empty pods of agricultural crops [12,13]. The preparation of activated carbon from different types of shells was studied for a long time and includes tests with coconut [14], pistachio nut [15], almond [16,17,18,19], and many others. Among the wide range of raw materials for obtaining porous carbon materials (PCM), preference was given to plant-based raw materials, specifically apricot pits, which are essentially waste from the industrial production of large fruit companies. In addition to availability, cost effectiveness, and safety in handling, fruit pits are characterized by natural micro- and mesoporosity. The material obtained by carbonizing apricot pits has a high carbon content (up to 90%). Reported activated carbon obtained from apricot stones with a surface area of 566 m2/g was studied as an adsorbent to remove heavy metal ions Ni(II), Co(II), Cd(II), Cu(II), Pb(II), Cr(III), and Cr(VI) ions from aqueous solutions [20]. Methylene cyanine adsorption by activated carbon with a surface area of 866 m2/g obtained from the apricot shell was tested by Zhu et al. [21]. The activated carbon from apricot stones was used by Abbas as an adsorbent to remove malachite green from the aqueous solution [22]. Abd Ali prepared activated carbon surface areas of 1115 m2/g from apricot stones and evaluated them as an adsorbent for the basic dye (Reactive Blue 222) from aqueous solutions [23].
Currently, chemical activation methods are commonly used to produce sorption materials from natural raw materials, leading to the creation of highly porous activated carbon (AC). The interaction mechanisms between carbon and inorganic substances are determined by the presence of functional groups, radicals, and fragments with catalytic and electrolytic properties in the organic mass. The authors note [24] that the preliminary treatment of carbon with aqueous solutions of acids and alkalis activates the decomposition process of the organic mass, resulting in changes in the mass yield and the composition of liquid and gaseous decomposition products. Therefore, the main goal of the research is to investigate the influence of the chemical activator nature and the reagent/carbon ratio on the yield, structure, and specific surface of carbon, with the aim of obtaining carbon sorption materials.
2. Materials and Methods
2.1. Preparation of Samples
The current study involves three distinct steps: (1) the initial sample obtained by carbonization of raw material of plant origin (RMPO), specifically apricot pits, purchased from the company “T.B. Fruit”, Ukraine (Figure 1); (2) the subsequent activation of derived material with potassium hydroxide (KOH) (purchased from SFERA SIM Ltd., 1 Lviv, Ukraine) [25], resulting in the formation of porous carbon material (PCM); and (3) nitrogen (N)-enriched samples of PCM in concentrated HNO3 (purchased from SFERA SIM Ltd., 1 Lviv, Ukraine) with the following thermal treatment in an argon (Ar) stream [26].

Figure 1.
Raw material of plant origin (apricot pits).
The RMPO was carbonized in a closed furnace in the temperature range of 300–900 °C, and the first digit in the sample number indicates the temperature. The carbonization process aims to remove volatile components from the carbon matrix, maximize the specific carbon content, and obtain a porous material with a high surface area and developed porosity. However, achieving the optimal pore size distribution during carbonization is practically impossible. Therefore, additional processing of the material is required, such as activation. The obtained carbonized carbon was mechanically crushed and mixed with KOH in different mass ratios, stirred for 1–2 h, heated in an Ar atmosphere to 900 °C, and subjected to isothermal exposure. Correspondingly, mineral impurities and ash were chemically washed from the samples using hydrochloric acid (HCl) (purchased from SFERA SIM Ltd., 1 Lviv, Ukraine). Then, the material was rinsed in distilled water until it reached a neutral pH and dried in an oven until a constant mass was achieved. The resulting samples were designated as Cxy, where x = 3, 4, 5, 6, 7, 8, and 9 and corresponds to the used temperature 300, 400, 500, 600, 700, 800, and 900 °C; y = 1, 2, 3, and 4 and corresponds to the ratio of KOH and C as 1:1, 1:2, 1:3, and 1:4. For example, sample C31 was prepared at 300 °C with an equal amount of KOH and C (see Table 1).

Table 1.
List of the prepared activated carbon samples after the activation process by KOH.
Sample C41, prepared at 400 °C and with the ratio KOH and C as 1:1 was used to investigate the formation of surface N-containing functional groups, which enhance reactivity. The proposed process was carried out as follows: 160 mL of 65% HNO3 solution (purchased from SFERA SIM Ltd., 1 Lviv, Ukraine) was added to 12 g of activated PCM (samples C41). The resulting suspension was thoroughly mixed with a magnetic stirrer at room temperature for 3 h, and then washed with distilled water to a neutral pH and dried in air at a temperature of 65 °C for a day. The obtained finely dispersed material exhibited low chemical stability, necessitating additional thermal activation. This activation of the obtained N-containing PCM was carried out in a vertical tubular furnace at temperatures in the range of 250 ÷ 650 °C in an Ar stream for 1 h. The prepared N-enriched samples were labeled as N-C41-t, where t corresponds to the temperature of the heat treatment (from 250 to 650 °C). For example, N-C41-250 represents PCM obtained by chemical activation of C41 material in concentrated HNO3 followed by thermal treatment in Ar at 250 °C.
For a better understanding, all N-enriched carbon samples are organized in Table 2.

Table 2.
List of N-doped carbon materials prepared based on sample C41.
2.2. Characterization of the Samples
The characteristics of the PCM structure including specific surface area (SSA) and total pore volume were determined based on the analysis of nitrogen adsorption/desorption isotherms at its boiling temperature (77 Κ) using a Quantachrome NOVA2200e Analyzer (Quantachrome Instruments, Boynton Beach, FL, USA). Before measurements, the samples were degassed at 180 °C for 18 h. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method (SBET, m2/g) in the region of the isotherm limited by the range of relative pressure P/P0 = 0.050 − 0.035. The total pore volume (Vtotal, cm3/g) was determined based on the amount of nitrogen adsorbed at P/P~1.0. Additionally, the micropore volume (Vmicro, cm3/g), as well as the specific surface areas of micro-(Smicro, m2/g) and mesopores (Smezo, m2/g) were found using the t-method, the Barrett–Joyner–Halenda (BJH) method, and the Density Functional Theory (DFT) [27].
The structural study was conducted using a scanning electron microscope JSM-6700 F (JEOL, Peabody, MA, USA) equipped with an energy system JED-2300.
The infrared (IR) spectra of PCM samples were obtained using a Thermo Nicolet FT-IR spectrometer in reflectance mode. The test sample was mixed with KBr in a ratio of 1:100.
To evaluate the adsorption properties, methylene blue (AMB, mg/g), methyl orange (AMO, mg/g), and iodine (AIodine, mg/g) were used [28]. The optical density of these solutions was measured using a spectrophotometer ULAB 102 UV with a blue light filter and a wavelength of 400 nm in cuvettes with an optical path length of 10 mm.
The carbon adsorption activity by the dye, measured in milligrams per 1 g of the product was calculated as
where C1 is the mass concentration of the initial indicator solution (mg/dm3); C2 is the mass concentration of the solution after contact with activated carbon (AC) (obtained after activating PCM with KOH) (mg/dm3); K is the dilution factor of the solution; 0.025 is the volume of the indicator solution taken for illumination (dm3); and m is the mass of AC (g).
The residual mass concentration of the indicator was determined in comparison with the previously obtained graduation graph [29].
3. Results and Discussion
3.1. Porous Carbon Material (PCM) and Its Activation
In this context, the question of the state of the particle surface becomes important. Functional groups affect the surface hydrophilicity with an electrolyte solution. Since the micropores of carbon materials are not completely wetted due to the presence of graphite crystallites, functional compounds can improve the hydrophilic properties of their surface. They locally change the electrostatic field of the pores (which strengthens the interaction with polarized water molecules [30,31], and thereby increase the carbon sorption activity owing to the improvement in the availability of chemical reagent ions to the pores and, accordingly, the involvement of the additional surface where sorption is possible. In order to confirm the statement about the presence of surface functional groups on the porous carbon material (PCM) surface, the structural analysis of the chemically modified PCM was carried out using IR spectroscopy (Figure 2). The IR spectra of carbon materials are characterized by absorption bands in the entire working range, which is within the so-called fundamental infrared region (500 cm−1–4000 cm−1), mostly without clearly defined absorption peaks. This indicates the presence of various types of surface functionalities. Each type of functional group is characterized by its own set of absorption bands corresponding to the vibrational modes of the chemical bonds of certain atoms. It is worth noting that the absorption band can simultaneously indicate different types of chemical bonds or result from overlapping spectra of various groups [32]. Therefore, IR spectra are analyzed by dividing them into sections that correspond to the vibration modes of certain chemical bonds. The type of surface functional groups is identified by the combination of these bonds.
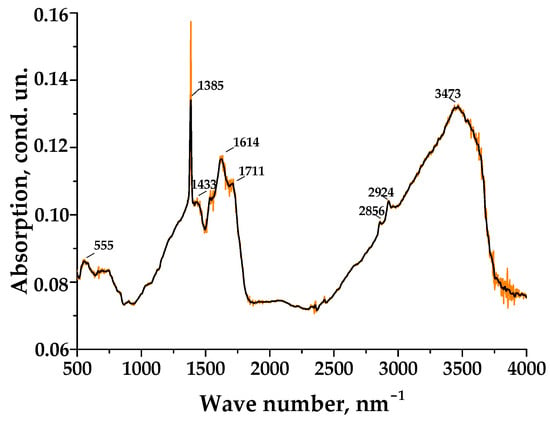
Figure 2.
IR spectrum of surface of activated carbon material C41 prepared at 400 °C and chemically modified by KOH (KOH:C as 1:1).
The IR spectrum of the initial material shows several characteristic modes. Vibrations at 2856 cm−1 and 2924 cm−1 are attributed to asymmetric and symmetric C-H vibrations in CH2 groups. A vibration band at 1385 cm−1 indicates the presence of alcohol compounds on the PCM surface and corresponds to the deformation O-H vibrations. The presence of alkenes is confirmed by the modes at 1433 cm−1 and 1614 cm−1, which characterize the valence vibrations of C-H bonds in CH2/CH3 groups and C=C aromatic rings, respectively. In addition, the starting material spectrum contains absorption bands in the vicinity of 1711 cm−1 and 2924 cm−1, which are attributed to the valence vibrations of C=O and C-H bonds in ketones [33]. Furthermore, there are intense absorption bands with maxima at 1385 and 1614 cm−1 in the spectrum, which may indicate the presence of carboxylic acids in the composition of potassium salts. According to Marsh et al. [34], the activation of carbon material with KOH is accompanied by the flow of gasification reactions, where carbon is oxidized to CO and CO2, and this contributes to the development of a porous structure with the formation of secondary products (K2CO3). It is also worth noting that at high temperatures (550–900 °C), the formation of metallic potassium is possible, which is introduced between graphite layers as a result of the reduction of K2O by carbon. The destruction of the chemically treated sample to a powder state at the carbonization stage and the subsequent porosity formation after washing with water are connected precisely with the emergence of these salts. They do not ensure the consolidation of polymer complexes during the heat treatment stage and are removed by washing with water, thereby revealing the internal structure.
Thus, when exposed to a chemical activator such as KOH, significant structural changes occur in the carbon material with the formation of carboxylic acid salts. This transformation includes the removal of oxygen functional groups from the surface of the carbon material due to chemical modification and heat treatment. These changes affect the chemical composition of the surface of carbon materials, thereby determining their catalytic and sorption properties.
The methods applied to examine the adsorption activity of carbon by the indicator are static and involve determining adsorption isotherms. The adsorption capacity of materials was measured by the interaction of the dye solution (methylene blue (MB)) with the solvent containing a certain amount of adsorbent, which, in our case, is AC (PCM after KOH treatment). After the dye monolayer formation, the solvent was almost completely displaced from the surface of the adsorbent.
Porous carbon samples denoted Cxy, where x = 3, 4, 5, 6, 7, 8, and 9 (corresponds to the used temperature 300, 400, 500, 600, 700, 800, and 900 °C) and y = 2, 3, and 4 (represents to the ratio of KOH and C 1:2, 1:3, and 1:4) were used in the reported measurements. In order to conduct experiments to determine the MB adsorption activity, a calibration graph was previously constructed and is provided in Figure A1 in Appendix A following the main text.
To determine the adsorption capacity, pre-dried and ground PCM weighing 0.1 g was placed in a 50 mL flask, and an aqueous solution of the adsorbent (25 mL of the MB solution with a concentration of 1.5 g/L) was added and stirred for 20 min. Since the solute diffusion rate is much lower compared to gaseous agents, slow adsorption processes were observed without the need for mechanical mixing. Next, the mixed samples were placed in a centrifuge, and after complete precipitation of the adsorbent, the solution was separated. Afterward, it was carefully sampled with a pipette, and, using a spectrophotometer ULAB 102 UV, the value of the optical density was determined according to a previously constructed calibration curve (see Figure A1 in Appendix A). If the optical density exceeded the experimental limits (according to [28], it should not exceed 0.8 optical units), then this solution was diluted by adding 5 mL of it into a 25 mL volumetric flask and topping it up with distilled water to reach the mark.
During the alkaline treatment phase, KOH interacts with oxygen-containing functional groups of carbon, and this leads to the formation of potassium phenolates and carboxylates, as well as the splitting of complex ester groups. These reactions are the driving force for the introduction of K+ and OH− ions, KOH molecules, and their incorporation into the three-dimensional structure of carbon. This can occur in the form of individual ions, molecules, or aggregates of molecules [35,36]. The carbonization of the carbon material activated by KOH contributes to the growth of the specific volume of pores in the structure of the obtained PCM.
To study the influence of the alkali amount on the structural and adsorption properties of the studied samples, the chemical activation with potassium hydroxide was carried out. Figure 3 presents microscopic images of the samples C4y prepared at 400 °C at different KOH:C ratios. These images reveal that the introduction of alkali contributes to the formation of numerous pores and the development of a spongy carbon structure [37]. At lower alkali concentrations, the primary carbonation almost does not destroy the morphology of the raw material. However, an increase in the potassium hydroxide concentration leads to the degradation of cellulose and its migration from the particle volume to the surface. This, in turn, affects the porous structure of the carbon material. While closer examining the inner surface, it becomes evident that the material is permeated with a multitude of pores.
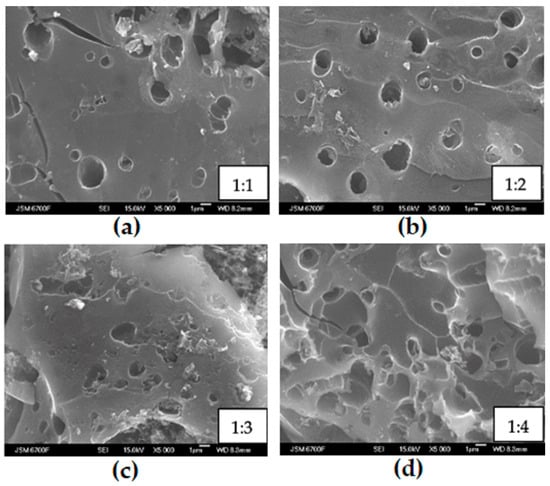
Figure 3.
SEM surface images of the C4y sample surface at different ratios of KOH and C: (a) 1:1 for C41, (b) 1:2 for C42, (c) 1:3 for C44, and (d) 1:4 for C44.
Figure 3 demonstrates that the C4y materials have a more destroyed surface, i.e., with an increase in the KOH concentration, significant changes occur in the surface structure. These findings suggest that the presence of “free” alkali within the framework of carbon material leads to the surface development via reactions with framework fragments: heterolysis of C-O and polarized C-C bonds [38], and the cleavage of O- and S-containing heterocycles [39]. These processes release hydrogen and methane and contribute to the intensive development of the microporous structure thanks to an increase in the number and volume of pores. The use of potassium hydroxide as an activating agent in the process of chemical activation of raw materials of plant origin allows for obtaining a material with a distinctive porous structure and the lace morphology of texture. The sufficient number of pores on the surface serves as an excellent transport system, facilitating the entry of sorbed substances into the micro-or mesopores from either liquid or gaseous phases.
The nature of the change in the adsorption activity depending on the KOH:C ratio is quite complex, and its dependency on the temperature is unclear, although it decreases after 400 °C for all studied samples using MB (Figure 4). The amount of adsorption activity by MB makes it possible to conclude the content of micropores in the adsorbent since the molecule size of MB is quite large (1.97 nm) [28] and their diffusion into micropores (with a diameter of up to 2 nm) is challenging. The analysis of experimental data showed that chemical activation improves the adsorption capacity of the resulting carbons. The most significant increase in activity occurs at a ratio of KOH to C as 1:3, reaching 320 mg/g. The nature of the change in the adsorption activity of the sorbents due to variations in the KOH concentration is similar for most samples. However, for samples C3y, the sorption amount increases with the addition of alkali in the ratio KOH:C = 1:1, but at higher concentrations, the activity decline is 31%. For samples of the C4y series, the maximum capacitance when absorbing methylene blue reaches 280–320 mg/g.
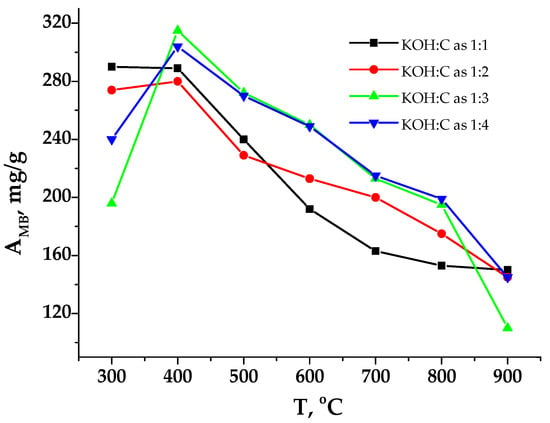
Figure 4.
Dependence of adsorption capacity by MB on carbonization temperature.
The adsorption by methylene blue (AMB) characterizes the ability of AC to sorb large molecules of organic substances from aqueous solutions. It was established that the carbonation temperature and the KOH:C ratio are interconnected and significantly affect the sorption properties of the studied samples [40]. Thus, there is a positive correlation between the capacitance growth and increasing the KOH dosage. Samples C43 and C44 demonstrated the maximum adsorption activity (Figure 3, carbonization temperature for both samples was 400 °C). In the same KOH:C ratio (1:1), the maximum activity by MB (290 mg/g) was observed for sorbents obtained at T = 400 °C. A further increase in temperature leads to a decrease in sorption activity to 150 mg/g. This deterioration is because, at temperatures of 600–900 °C, volatile substances do not form condensation products within carbon pores, which leads to the burnout of the pore walls.
The fact of the matter is that the chemical nature of the PCM surface determines the degree of the adsorption activity by the indicator [41]. When carbon has a nearly perfect porous surface with a high concentration of delocalized electrons, it enables the specific sorption of the dye complex ions due to the interaction of these ions with the central atom of the complex ion. However, as the surface is filled with chemisorbed oxygen, the effective concentration reduces and, accordingly, sorption decreases. Consequently, the adsorption activity declines, and sample C93 exhibits the poorest absorption properties, with an adsorption capacity of only 110 mg/g.
It is worth noting that the presence of certain pore sizes in adsorbents can be inferred from the adsorption activity of the dyes. While the MB adsorption method is the most effective for examining large-porous materials with pore radii exceeding 100 nm, it would be advisable to use another method for studying the adsorption activity of iodine. This method makes it possible to investigate the micropores with effective diameters of 0.5–1.7 nm in the adsorbent.
Likely, the carbonization temperature of the raw material is also an essential factor that affects the sorption properties of carbon. The selection of carbonization and activation temperatures of the raw material is guided by the fact that the main processes of decomposition (pyrolysis) and the initial carbonization of the original solid-phase substance primarily take place in the temperature range of 300–900 °C [42].
Figure 5 presents scanning electron microscopy (SEM) photographs at various magnifications of carbon materials prepared with the same KOH:C ratio as 1:1 but at different carbonized temperatures: C31 at 300 °C (Figure 5a), C51 at 500 °C (Figure 5b), C71 at 700 °C (Figure 5c), which have a meso- and mostly microporous structure, obtained using the method of scanning electron microscopy. The images clearly show surface microcracks and the presence of round or oval micro-sized transport pores in all studied samples. White inclusions associated with ash residues and products of the interaction of potassium hydroxide with carbonaceous material are observed over the entire surface. The structure of the material also contains needle-shaped and fiber inclusions that have the appearance of blurred rings [43].
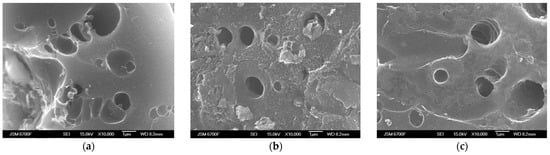
Figure 5.
SEM surface images of porous carbon materials prepared with the same KOH:C as 1:1 but at different carbonized temperatures: (a) C31 at 300 °C, (b) C51 at 500 °C, and (c) C71 at 700 °C.
It can be assumed that the increase in carbonization temperature initiates the process of carbon material graphitization and leads to the formation of graphite-like clusters [44]. In such a case, at temperatures of 700 °C and higher, the formation of the porous structure slows down as the degeneration of narrow micropores occurs via their merging, resulting in a reduction in both pore volume and their SSA.
Figure 6 presents typical nitrogen adsorption/desorption isotherms for the studied carbon materials. They belong to isotherms that are characteristic of multilayer adsorption in micro- and mesopores of organic materials. Notably, a hysteresis loop of the Type H4 according to the IUPAC classification [45] is observed for all samples and is associated with sorption processes within narrow pores.
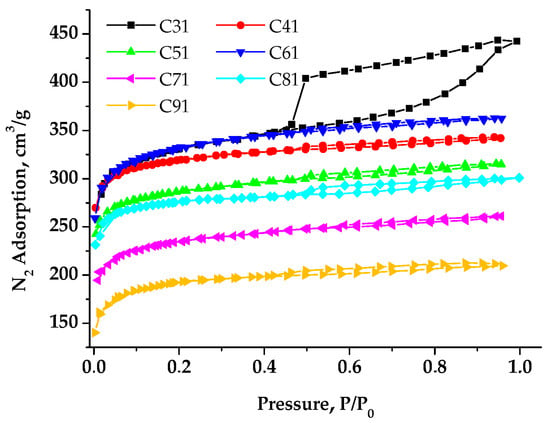
Figure 6.
Nitrogen adsorption isotherms at 77 K of carbon samples.
The sorption isotherms obtained for samples C41–C91 are characteristic of typical microporous materials. This type of isotherm is associated with sorption processes occurring mainly in narrow micropores. A significant hysteresis associated with the process of nitrogen sorption in mesopores is observed for sample C31. The histogram (Figure 7) presents the parameters of the porous structure (SBET, Vtotal) for samples C31–C91 with the contribution of pores of various sizes. The C31 material is characterized by the mesopore area that is three times larger than that of other carbons activated by KOH (with the ratio KOH and C as 1:1).
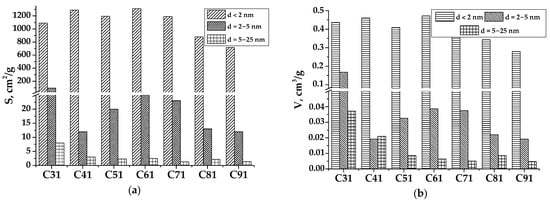
Figure 7.
Dependence of specific surface area (a) and pore volume (b) on pore diameter of PCM prepared at different temperatures with the same ratio between KOH and C (1:1).
The analysis of sorption isotherms made it possible to determine the parameters of the porous surface of carbon materials after chemical modification and temperature treatment (Table 3). Comparing the results obtained by the Brunauer–Emmett–Teller (BET) method, two maxima of the active area growth of the material at temperatures of 300 °C (sample C31) and 600 °C (sample 91) are visible. A further increase in the carbonization temperature leads to a decrease in the specific surface, and for sample C91, it is only 721 m2/g. The nature of the change in the total pore volume (Vtotal) is also different. The largest Vtotal was calculated for sample C31, equal to 0.684 cm3/g, and it was decreased with the heat treatment temperature increased, carbon materials C51 and C71 have almost the same total pore volume. Sample C61 drops out from this group, with the average values of the total pore volume Vtotal~ 0.555 cm3/g, which can be an advantage in microvolume over all other samples.

Table 3.
Main characteristics of porous structure of carbon material.
As already mentioned, the mechanisms of interaction of carbon with inorganic substances are determined by the presence of functional groups, radicals, and fragments with catalytic and electrolytic properties in the organic mass. The preliminary treatment of carbon with aqueous solutions of acids and alkalis activates the process of decomposition of the material, organic mass, leading to changes in the mass yield and composition of liquid and gaseous decomposition products. Therefore, it becomes essential to investigate how these structural changes affect the sorption properties of the samples under study.
The adsorption activity of PCM was determined according to standard methods involving methyl orange (MO) and elemental iodine.
It was observed that the carbonation temperature and the KOH:C ratio are interrelated and have a significant impact on the sorption properties of the studied samples. Specifically, there is a positive trend of capacitance growth with increasing the KOH dosage. Materials carbonized at a temperature of 400 °C have the maximum adsorption activity by methyl orange (MO) (Figure 8a). A characteristic feature of the C4y series is high sorption values across all three methods. When using iodine, the value of Aiodine is the highest for sample C4y (Figure 8b) since it has a large total pore volume Vtotal = 0.521 cm3/g (Table 3), primarily attributed to mesopores with sizes from 2 to 25 nm (Figure 7a).
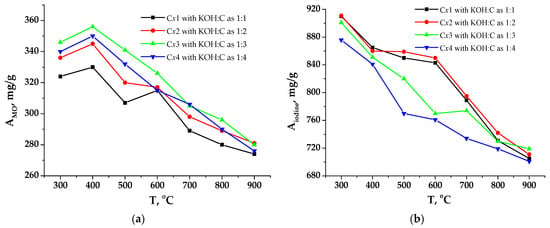
Figure 8.
Dependence of adsorption capacity on carbonization temperature: (a) methyl orange; (b) iodine.
Thus, it could be assumed that as the processing temperature of the carbon material increases, the development of its porous structure slows down, and therefore the sorption properties of these materials also deteriorate. This is due to the formation of predominantly small micropores that are inaccessible to methylene blue and methyl orange. Carbon with a well-developed porous surface (Smeso = 246 m2/g for C31) and a wide pore size distribution (Figure 7b) shows the highest sorption capacitance.
3.2. Nitrogen (N)-Enriched Samples of PCM
The surface of AC is highly heterogeneous both in terms of geometry and electronics. Carbon atoms on the surface are in slightly different electronic states than atoms of the bulk phase, especially in areas with defects in the crystal lattice and on the faces, vertices, and edges of the crystallites. These surface carbon atoms, due to their free valency, readily engage in chemical and sorption interactions, which are more accessible and require less energy. Chemical modification of AC can be achieved using various reagents, and this process involves multiple chemical reactions, including the catalytic decomposition of the reagent, redox interactions with the formation of surface and phase oxides, and partial structural degradation. In this study, nitrogen (N)-enriched samples of PCM obtained at different temperatures were prepared and studied. Considering the previous research results, namely the developed porous structure of the material and high adsorption activity for dyes, C41 was chosen as the starting sample due to the highest adsorption activity by MO (see Figure 8a). The original PCM was obtained via carbonization of RMPO and subsequent activation with KOH at a ratio of KOH and C of 1:1 (sample C41). Subsequently, N-C41-250, N-C41-450, and N-C41-650 were produced by subjecting the C41 material to chemical activation using concentrated HNO3 followed by thermal modification in an argon stream at temperatures of 250, 450, and 650 °C, respectively. It is important to note that thermal modification was not performed for the sample CN-0.
Adsorption/desorption isotherms for nitrogen-containing PCM are depicted in Figure 9. Notably, the isotherm shape remained consistent across all chemically activated materials. However, a slight decrease in the volume of sorbed nitrogen can be observed for sample N-C41-0 compared to porous carbon material that underwent annealing at 400 °C (sample C41 presented in Figure 6). This reduction is likely due to pore blocking caused by the introduction of nitrogen heteroatoms, which probably appeared during the chemical modification of C41 using HNO3. As the temperature rises, an increase in the volume of sorbed nitrogen is evident for materials N-C41-250 and N-C41-250, but at 650 °C, it decreases (sample N-C41-650).
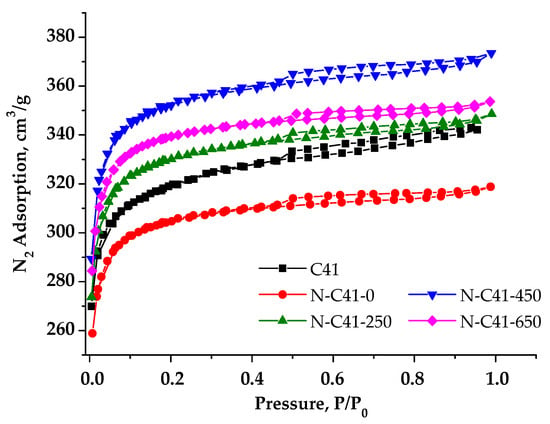
Figure 9.
Nitrogen adsorption/desorption isotherms at 77 K for samples.
The measured isotherms are typical for Type 1 according to the IUPAC classification [27], and they are characterized by loops of the capillary condensation hysteresis of the H4 category at a relative pressure of ~0.5. The analysis of sorption isotherms allowed for the determination of the porous structure parameters of the samples. According to the presented data in Table 4, the nature of the chemical activator significantly affects the change in the porous structure and surface characteristics of PCM. Sample N-C41-450 has the largest SSA with the most developed microporous structure, which makes up 97% of the total surface area.

Table 4.
Structural and adsorption characteristics of PCM before (sample C41) and after modification by nitrogen.
The carbon adsorption activity by the indicator (MO and MB) was calculated in milligrams per 1 g of product (Figure 10) according to standard methods. The final result was determined by averaging two independent calculations, ensuring that the absolute deviation between them did not exceed 10 mg per 1 g of product.
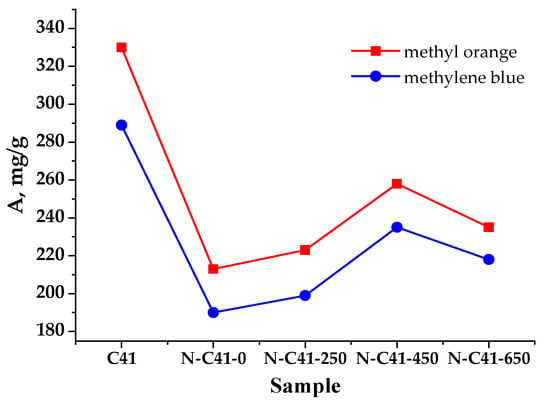
Figure 10.
Sorptive activity of nitrogen-containing carbon materials activated at different temperatures.
Samples N-C41-0 and N-C41-250 are characterized by a microporous structure (Table 4) and have low adsorption activity by MB compared to the C41 material. This is explained by the fact that when the C41 sample is oxidized with nitric acid, the SSA and the pore volume N-C41-0) decrease. Such changes are due to the adsorption of ions and molecules of reactive substances that can occupy a certain volume of pores, as well as an increase in the number of acidic oxygen-containing groups and the formation of new surface heteroatoms that can firmly settle at the entrance and the pore walls available for the N2 adsorption. Heat treatment at T ≤ 450 °C causes an increase in the SSA (N-C41-250), which is thanks to the water desorption, by-products of synthesis, and the removal of functional groups from the material surface and an increase in the adsorption activity by MO and MB, which is associated with an increase in the volume of micropores compared to N-C41-0.
Sample N-C41-450 has the highest specific surface area (1339 m2/g), primarily attributed to its micropores, accounting for 97% of the total surface area. The SSA obtained in this study (1339 m2/g) for KOH-activated carbon prepared at a temperature ≤450 °C is much higher than that reported by Foo et al. (807 m2/g) for KOH-activated carbons prepared by carbonizing oil palm empty fruit bunch at a high temperature of 700 °C and treated in the microwave [46]. Song et al. also reported a slightly lower SSA (1135 m2/g) for activated carbons from coconut shells after carbonization at 500 °C and subsequent heat treatment at 800 °C following KOH-activation [47].
At the same time, Li et al. reported a slightly lower adsorption capacity of 210.97 mg/g for methylene blue obtained using loofah sponge (LSC)-based porous carbons as starting materials by carbonization in an argon atmosphere at 800 °C [48] in contrast to 235 mg/g for N-C41-450 °C obtained in the current work at much lower temperature.
As can be seen in Table 5, the combination of two acids (KOH-HNO3) and two-step heat treatment (400 °C for the carbonization process and 450 °C for annealing), the specific surface area of 1339 m2/g was obtained, is the highest value in comparison with activated carbon materials prepared from similar starting apricot but at different temperatures, time and acids.

Table 5.
Details of the preparation of porous carbon materials in already published papers and results of ongoing work (ordered by processing temperature).
A distinctive feature is the increase in pore volume, which provides high values of the adsorption activity of 235 mg/g for MB and ~260 mg/g for MO. However, for the N-C41-650 sample, carbon material burnout occurs with the participation of oxygen heteroatom material, and as a result, the microporous surface decreases. The sample obtained at temperatures above 650 °C as a sorbent exhibits lower adsorption capacity concerning the absorption of the indicator from the aqueous solution.
4. Conclusions
In conclusion, this research demonstrated a scientific approach to the development of highly effective sorbents based on carbon materials. The methods of scanning electron microscopy and adsorption porosimetry proved that carbon sorbents possess diverse morphologies and porous structures depending on the conditions and modes of production. The study highlights that carbonization of raw materials of plant origin at a temperature of 300 °C, followed by chemical modification with potassium hydroxide, results in PCM with well-developed surfaces and high porosity. Notably, in the case of samples treated at 300 °C (series C3y), mesopores contribute to approximately 20% of the total specific surface area. The analysis of adsorption isotherms showed that by increasing the carbonization temperature of raw material, the pore size distribution in the resulting nanoporous carbon can be controlled. Specifically, at 400–600 °C, micropores dominate, whereas carbonization temperatures exceeding 600 °C lead to a transformation in the carbon matrix, along with the burnout of pore walls and a subsequent reduction in the total pore volume.
It was established that the use of potassium hydroxide (KOH) as a chemical activator influences the activity of surface centers of the sorbent due to the removal of surface functional oxygen compounds from the surface of carbon materials. As the percentage ratio of KOH:C increases, the value of sorption activity for the dyes (methylene blue and methyl orange) also increases. The peak sorption is observed in PCM synthesized by the carbonization of the initial raw material at 400 °C, followed by alkaline activation with a KOH:C ratio of 3:1. In this case, the maximum capacitance was achieved for samples of the C4y series, reaching values of 280–320 mg/g when absorbing methylene blue. However, the highest SSA was found in the KOH-activated carbons prepared at the lowest carbonized temperature and vice versa.
Moreover, the additional chemical modification by HNO3 of the prepared AC showed little increase in the specific surface area. Nevertheless, when the HNO3 and heat treatment were followed by annealing at 450 °C, the SSA increased from 1188 up to 1339 m2/g. This treatment results in a 97% predominance of micropores for sample N-C41-450. The observed decrease in adsorption activity in N-doped carbon materials is likely attributed to the formation of surface nitrogen and oxygen heterostructures, as well as the disordered distribution of pores.
Author Contributions
Conceptualization, resources, formal analysis, V.V.; investigation, V.V. and T.B.; methodology, validation, data curation, V.V., O.O. and T.B.; V.V. writing—original draft preparation, V.V. and O.O.; writing—review and editing, V.V., O.O. and T.B. All authors have read and agreed to the published version of the manuscript.
Funding
V.V.’s work was supported by Project 0121U108649 of the Ministry of Education and Science of Ukraine. O.O. thanks FCT (Fundacao para a Ciencia e a Tecnologia) in the scope of the framework contract foreseen in numbers 4, 5, and 6 of article 23 of the Decree Law 57/2016, of 29 August, UIDB/00481/2020 and UIDP/00481/2020—FCT; and CENTRO-01-0145- FEDER-022083—Centro Portugal Regional Operational Programme (Centro2020) under the PORTUGAL 2020 Partnership Agreement via the European Regional Development Fund.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
In order to conduct experiments for determining the MB adsorption activity, a calibration graph was previously constructed. Accordingly, 0.5; 1.0; 1.5; 2.0; 3.0; 4.0; 5.0; 6.0; 7.0; and 8.0 mL of the MB solution with a concentration of 1500 mg/L were injected into 10 dry volumetric flasks with a capacity of 50 mL each. Respectively, the resulting solutions contain 15; 30; 45; 60; 90; 120; 150; 180; 210; and 240 mg/L of the indicator in 1 L [21]. Then, the optical density for these solutions was determined using a spectrophotometer equipped with a blue light filter and a wavelength of 400 nm in cuvettes with a thickness of the absorbing layer of 10 mm. Distilled water was used as a control solution. Based on the received data, the calibration curve was constructed, showing the dependence of optical density on the concentration of calibration solutions (Figure 2).
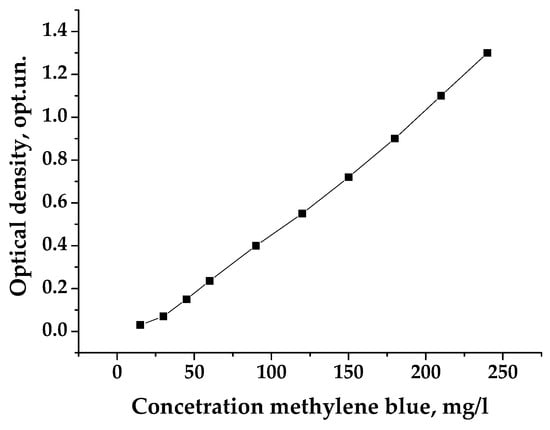
Figure A1.
Calibration curve for measuring concentration by MB.
References
- Pyrzynska, K. Application of carbon sorbents for the concentration and separation of metal ions. Anal. Sci. 2007, 23, 631–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seitkhan, A. Synthesis of carbonized nano mesoporous sorbents based on vegetable raw materials. J. Nanosci. Nanoeng. 2003, 1, 41–44. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, 04691. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-based adsorbents for removal of dyes from wastewater: A review. Front. Environ. Sci. 2021, 9, 558. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Boychuk, T.Y.; Budzulyak, I.M.; Ivanichok, N.Y.; Lisovskiy, R.P.; Rachiy, B.I. Electrochemical properties of hybrid supercapacitors formed from nanosized spinel LiMn1.5Fe0.5O4. J. Nano Electron. Phys. 2015, 7, 1019. [Google Scholar]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 6, 106716. [Google Scholar] [CrossRef]
- Ketabchi, M.R.; Babamohammadi, S.; Davies, W.G.; Gorbounov, M.; Soltani, S.M. Latest advances and challenges in carbon capture using bio-based sorbents: A state-of-the-art review. Carbon. Capture Sci. Technol. 2023, 6, 100087. [Google Scholar] [CrossRef]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Mandzyuk, V.I.; Lisovskyy, R.P. Electrochemical characteristics of capacitor systems formed on chemically modified carbon base. Nanosistemi Nanomater. Nanotehnologii 2008, 6, 1207–1217. [Google Scholar]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Lisovska, S.A.; Ilnytskyy, R.V.; Lisovskyy, R.P.; Ivanichok, N.Y.; Bandura, K.V.; Rachiy, B.I. Structural and sorption properties of nanoporous carbon materials obtained from walnut shells. Phys. Chem. Solid. State 2023, 24, 348–353. [Google Scholar] [CrossRef]
- Sklepova, S.V.; Gasyuk, I.M.; Ivanichok, N.Y.; Kolkovskyi, P.I.; Kotsyubynsky, V.O.; Rachiy, B.I. The porous structure of activated carbon-based on waste coffee grounds. Phys. Chem. Solid State 2022, 23, 484–490. [Google Scholar] [CrossRef]
- Laine, J.; Calafat, A.; Labady, M. Preparation and characterization of activated carbons from coconut shell impregnated with phosphoric acid. Carbon 1989, 27, 191–195. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Linares-Solano, A.; Gonzalez, L.J.d.D.; Sabio, M.M. Active carbons from almond shells as adsorbents in gas and liquid phases. J. Chem. Technol. Biotech. 1980, 30, 65–72. [Google Scholar] [CrossRef]
- Bevla, F.R.; Rico, D.P.; Gomis, A.F.M. Activated carbon from almond shells. Chemical activation. 2. Zinc chloride activation temperature influence. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 269–271. [Google Scholar]
- Balcı, S.; Doğu, T.; Yücel, H. Characterization of activated carbon produced from almond shell and hazelnut shell. J. Chem. Technol. Biotechnol. 1994, 60, 419–426. [Google Scholar] [CrossRef]
- Toles, C.A.; Marshall, W.E.; Johns, M.M.; Wartelle, L.H.; McAloon, A. Acid-activated carbons from almond shells: Physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 71, 87–92. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Zhu, G.; Duan, J.; Zhao, H.; Liu, M.; Li, F. Apricot shell: A potential high-quality raw material for activated carbon. Adv. Mater. Res. 2013, 798, 3–7. [Google Scholar] [CrossRef]
- Abbas, M. Experimental investigation of activated carbon prepared from apricot stones material (ASM) adsorbent for removal of malachite green (MG) from aqueous solution. Adsorpt. Sci. Technol. 2020, 38, 24–45. [Google Scholar] [CrossRef]
- Abd Ali, K.M. Synthesis of activated carbon by chemical activation of apricot stone with adsorption kinetics. J. Mater. Environ. Sci. 2021, 12, 887–898. [Google Scholar]
- Tan, H.; Tall, O.E.; Liu, Z.; Wei, N.; Yapici, T.; Zhan, T.; Han, Y. Selective oxidation of glycerol to glyceric acid in base-free aqueous solution at room temperature catalyzed by platinum supported on carbon activated with potassium hydroxide. ChemCatChem 2016, 8, 1699–1707. [Google Scholar] [CrossRef]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Rachiy, B.I.; Vashchynsky, V.M.; Mandzyuk, V.I.; Lisovsky, R.P.; Shyyko, L.O. Thermochemical activated carbon as an electrode material for supercapacitors. Nanoscale Res. Lett. 2015, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhu, M. New type nitrogen-doped carbon material applied to deep adsorption desulfurization. Energy Fuels 2020, 34, 9320–9327. [Google Scholar] [CrossRef]
- Sing, K.S. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76, 3–11. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Aktaş, Ö.; Çeçen, F. Effect of type of carbon activation on adsorption and its reversibility. J. Chem. Technol. Biotechnol. 2006, 81, 94–101. [Google Scholar] [CrossRef]
- Lang, J.W.; Yan, X.B.; Liu, W.W.; Wang, R.T.; Xue, Q.J. Influence of nitric acid modification of ordered mesoporous carbon materials on their capacitive performances in different aqueous electrolyte. J. Power Sources 2012, 24, 220–229. [Google Scholar] [CrossRef]
- Su, F.; Poh, C.K.; Chen, J.S.; Xu, G.; Wang, D.; Li, Q.; Lin, J.; Lou, X.W. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. [Google Scholar] [CrossRef]
- Zhu, M.; Weber, C.J.; Yang, Y.; Konuta, M.; Starke, U.; Kern, K.; Bittner, A.M. Chemical and electrochemical ageing of carbon materials used in supercapacitor electrodes. Carbon 2008, 46, 1829–1840. [Google Scholar] [CrossRef]
- Huck, C.W. Advances of infrared spectroscopy in natural product research. Phytochem. Lett. 2015, 11, 384–393. [Google Scholar] [CrossRef]
- Marsh, H.; Yan, D.S.; O’Grady, T.M.; Wenneerberg, A. Formation of active carbons from cokes using potassium hydroxide. Carbon 1984, 22, 603–611. [Google Scholar] [CrossRef]
- Kucherenko, V.A.; Tamarkina, Y.V.; Raenko, G.F.; Chernyshova, M.I. Thermolysis of brown coal in the presence of alkali metal hydroxides. Solid Fuel Chem. 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Rachiy, B.I.; Budzulyak, I.M.; Vashchynsky, V.M.; Ivanichok, N.Y.; Nykoliuk, M.O. Electrochemical properties of nanoporous carbon material in aqueous electrolytes. Nanoscale Res. Lett. 2016, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Vashchynskyi, V.M.; Semkiv, I.V.; Kashuba, A.I.; Petrus’, R.Y. Influence of carbonization conditions on porous structure of carbon materials. Khimiya Fiz. Tekhnologiya Poverhni 2022, 13, 349–357. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, T.; Pu, F.; Chen, H.; Wang, Z.; Zhang, H.; Guan, S. Graphene/carbon-coated Si nanoparticle hybrids as high-performance anode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3449–3455. [Google Scholar] [CrossRef]
- Mandzyuk, V.I.; Nagirna, N.I.; Strelchuk, V.V.; Budzulyak, S.I.; Budzulyak, I.M.; Rachiy, B.I. Electrical and optical properties of porous carbon material. Phys. Chem. Solid State 2012, 13, 94–101. [Google Scholar]
- Zubrik, A.; Matik, M.; Hredzák, S.; Lovás, M.; Danková, Z.; Kováčová, M.; Briančin, J. Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J. Clean. Prod. 2017, 143, 643–653. [Google Scholar] [CrossRef]
- Budzulyak, I.M.; Vashchynsky, V.M.; Rachiy, B.I. Adsorption properties of porous carbon materials obtained by chemical activation. JSPE 2015, 13, 84–90. [Google Scholar]
- Şentorun-Shalaby, Ç.; Uçak-Astarlıoglu, M.G.; Artok, L.; Sarıcı, Ç. Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater. 2006, 88, 126–134. [Google Scholar] [CrossRef]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Rachiy, B.I.; Kuzyshyn, M.M.; Vashchynskyi, V.M.; Mykyteichuk, P.M.; Merena, R.I. Adsorption properties of carbon activated with orthophosphoric acid. Khimiya Fiz. Tekhnologiya Poverhni 2014, 5, 204–209. [Google Scholar]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue. Desalination 2011, 275, 302–305. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption studies of coconut shell carbons prepared by KOH activation for removal of lead (II) from aqueous solutions. Sustainability 2014, 6, 86–98. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhai, K.; He, C.; Li, Q.; Guo, P. Methylene blue adsorption from aqueous solution by loofah sponge-based porous carbons. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 28–35. [Google Scholar] [CrossRef]
- Namal, O.O.; Kalipci, E. Adsorption kinetics of methylene blue removal from aqueous solutions using potassium hydroxide (KOH) modified apricot kernel shells. Int. J. Environ. Anal. Chem. 2020, 100, 1549–1565. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).