Assessing the Effect of Intensive Agriculture and Sandy Soil Properties on Groundwater Contamination by Nitrate and Potential Improvement Using Olive Pomace Biomass Slag (OPBS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Presentation of the Study Area
2.2. The R’mel Groundwater
2.3. Sample Collection and Preparation
2.4. Soil Sample Analysis
2.5. Olive Pomace Biomass Slag (OPBS)
2.6. Soil and Olive Pomace Biomass Slag (OPBS) Characterization
2.7. Column Study
2.8. Measurements and Data Analysis
3. Results
3.1. Soil Physicochemical Characterization
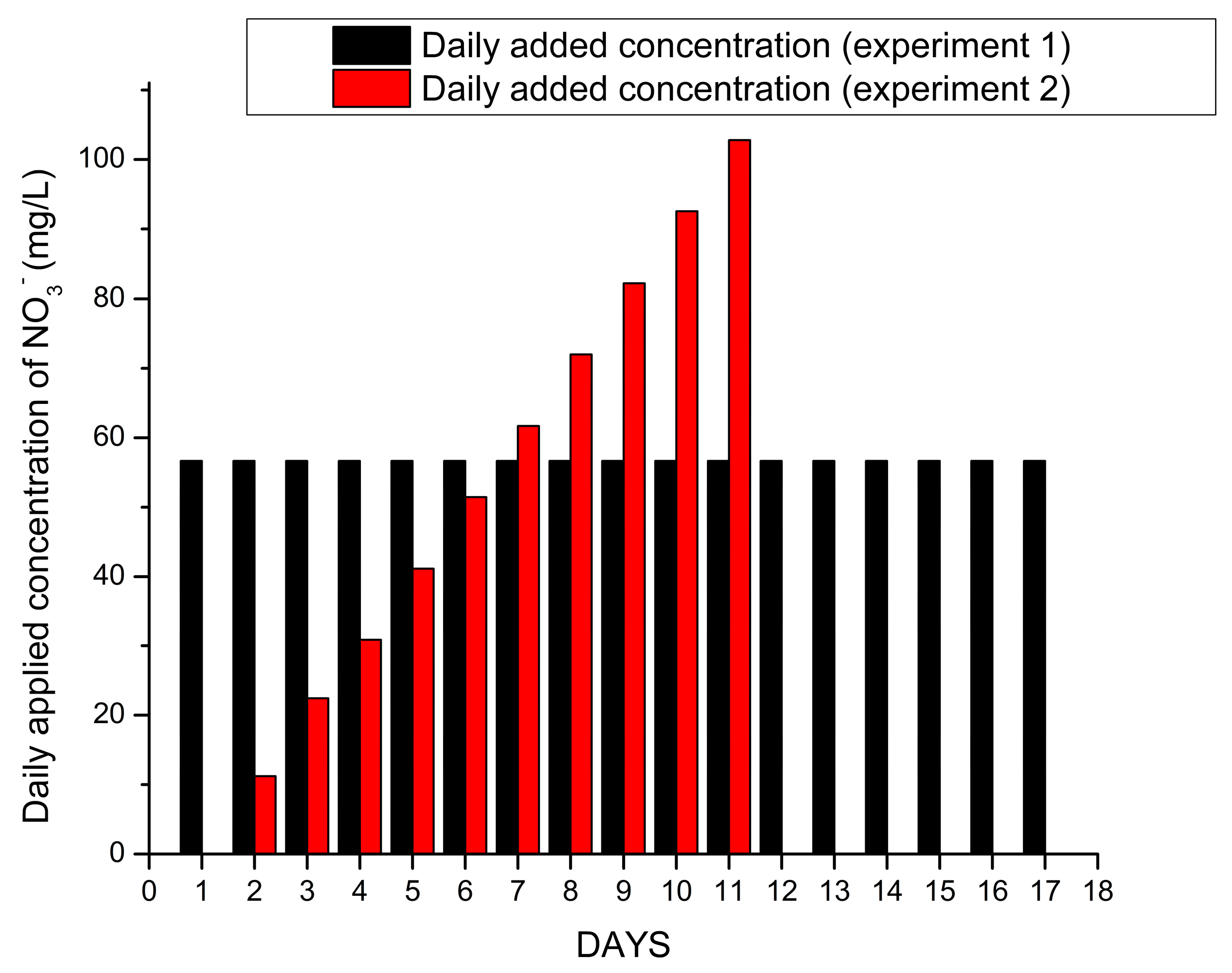
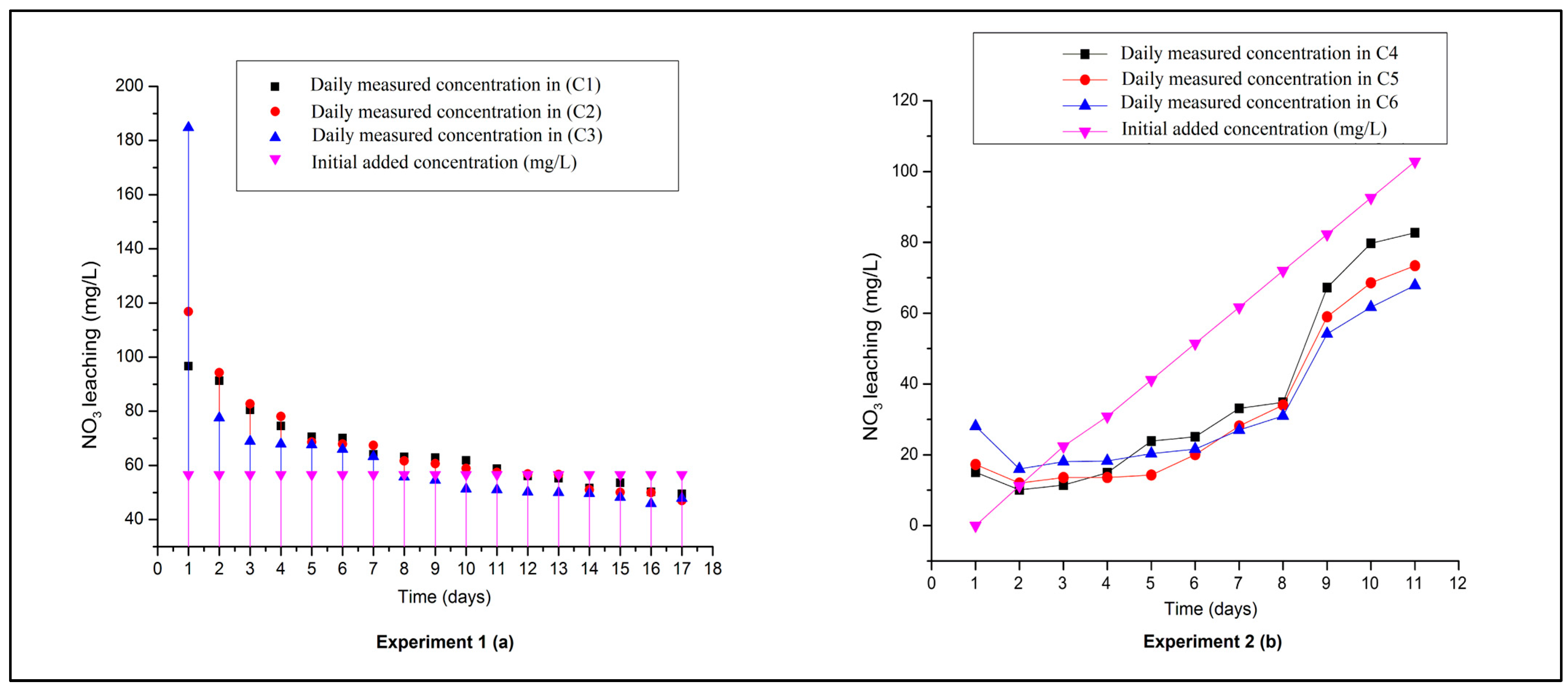
3.2. Column-Leaching Experiments
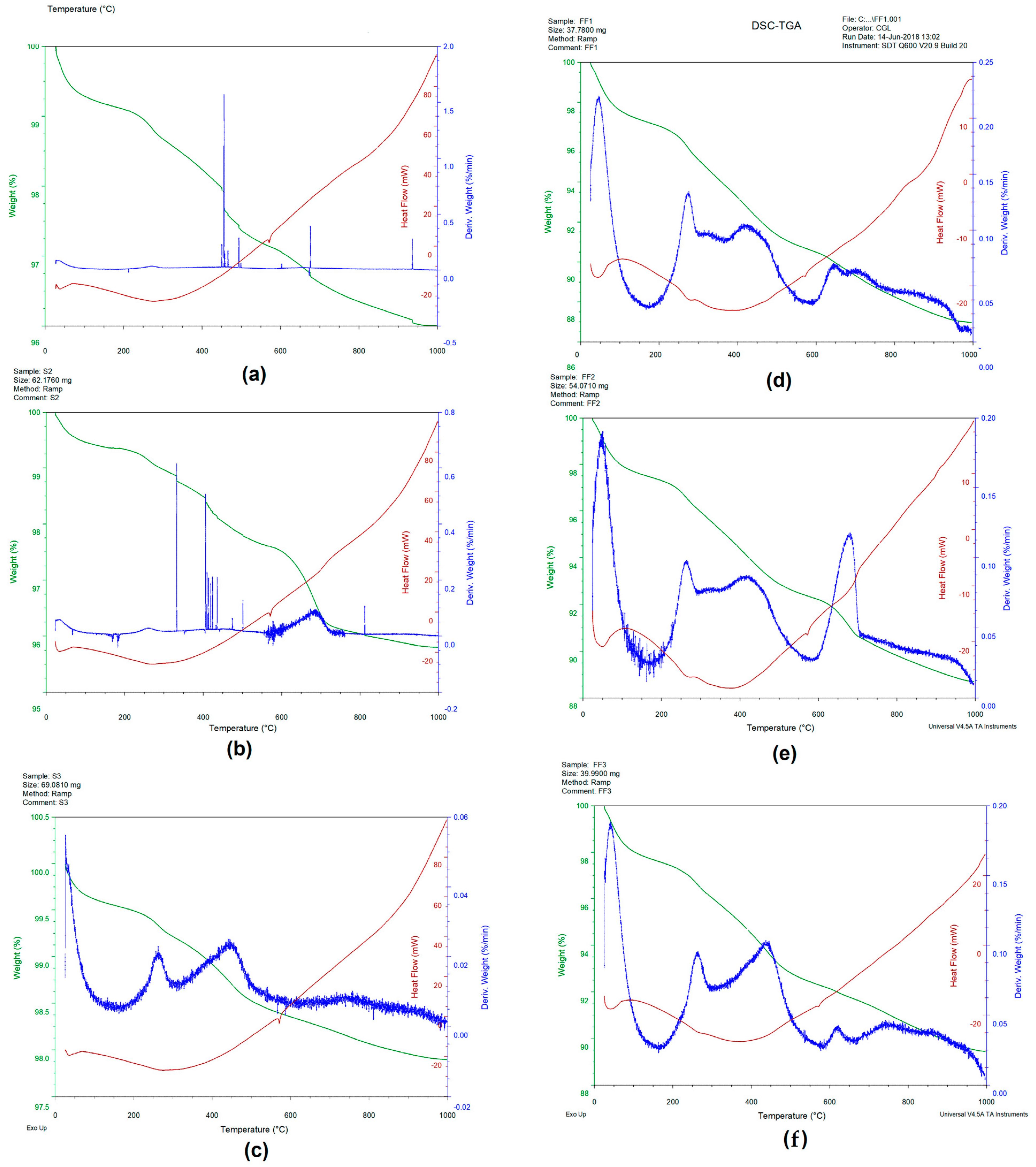
3.3. Soil Thermal Characterization
3.4. Olive Pomace Biomass Slag (OPBS) Analysis
3.5. X-ray Fluorescence Analysis
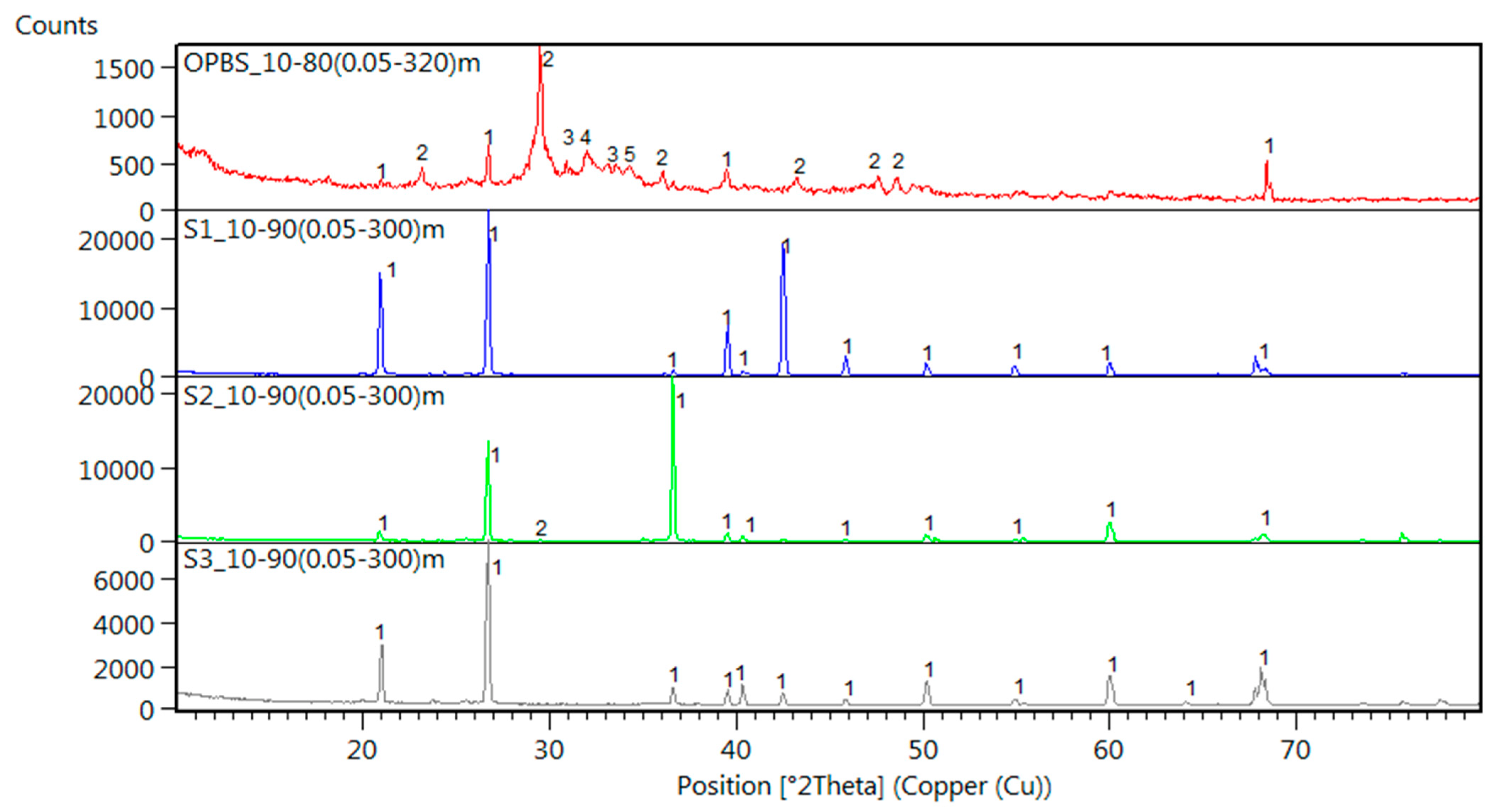
3.6. XRD Analysis
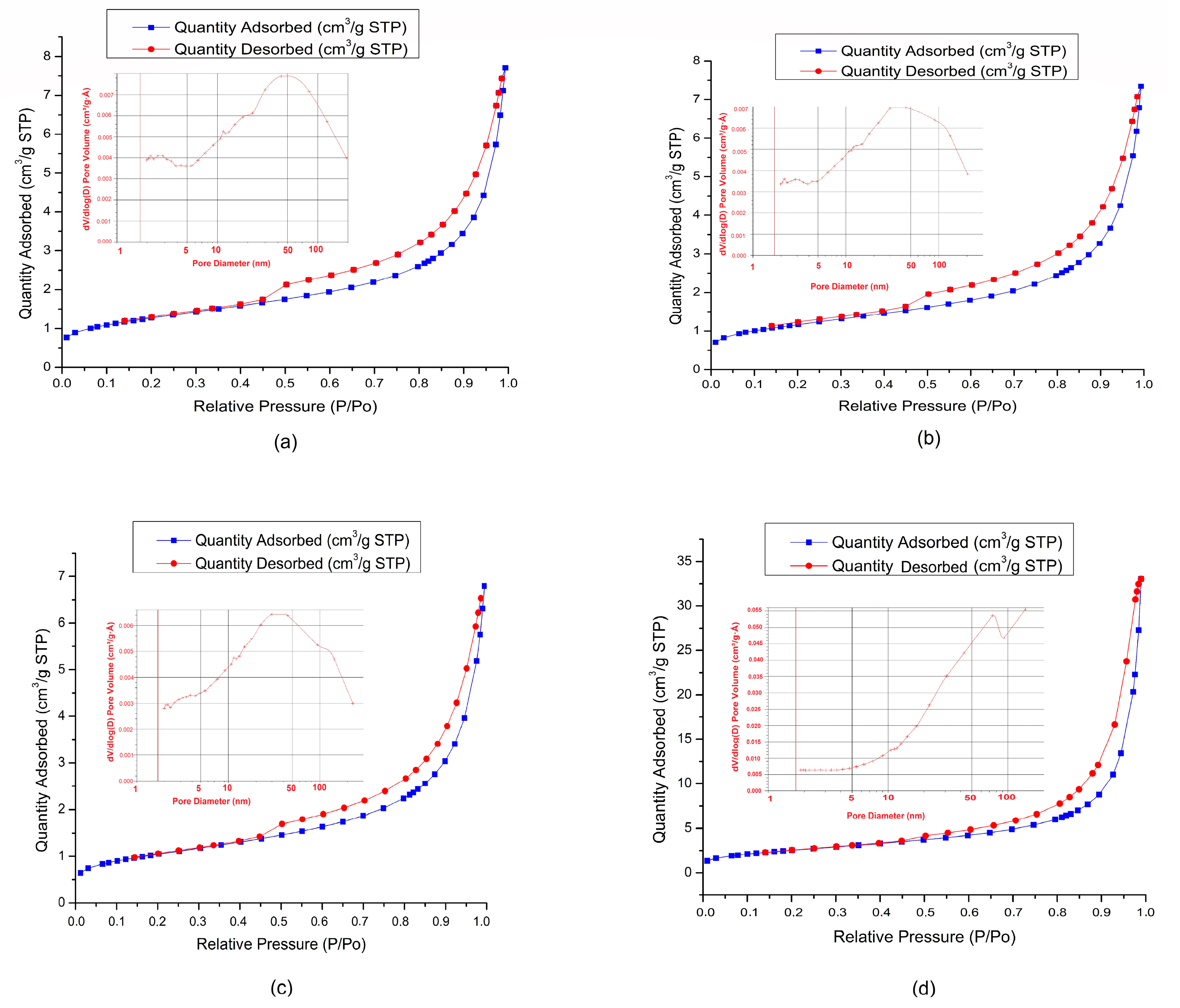
3.7. BET Characterization of the Soil Samples and Biomass Slag
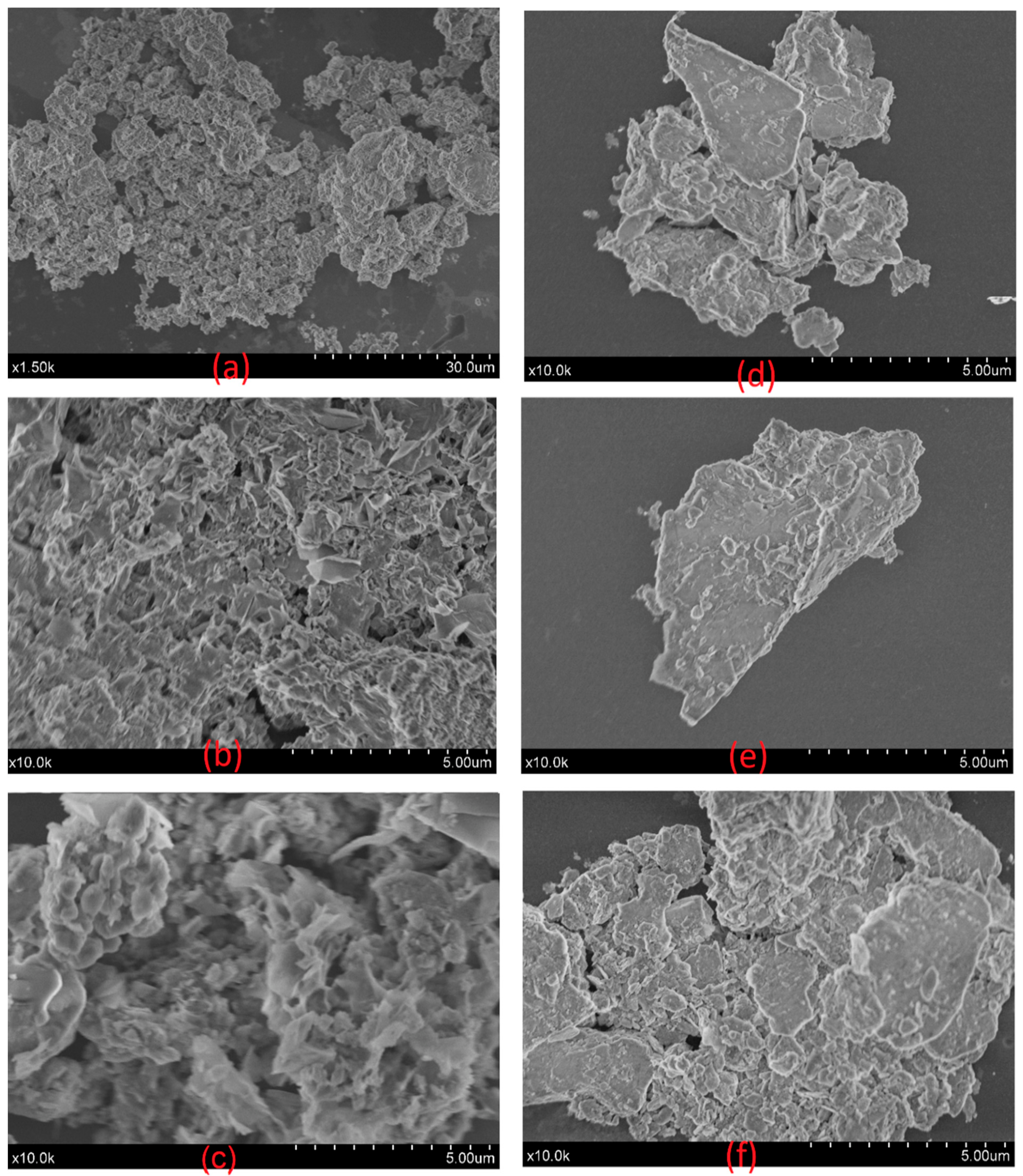
3.8. SEM Characterization
4. Conclusions and Perspectives
- (1)
- The R’mel soils were coarser in texture, with low clay, silt, and OM, low CEC, limited adsorption sites, and poor nutrient availability. In contrast, the clayey fraction (FF) exhibited significant water content, OM, CaCO3, and heavy-metal adsorption capacity despite its low percentage in the soil (<5%).
- (2)
- The column experiments demonstrated that the R’mel soils had a low water- and NO3− retention capacity. Higher leaching rates in percolates were measured, even above the loaded quantities in experiment 1.
- (3)
- The examination of OPBS showed that this residue is non-toxic, has a significant amount of essential plant nutrients such as potassium and calcium, has a moderately porous internal structure, includes organic carbon, and has a high water-retention capacity.
- (4)
- The spreading of OPBS in R’mel soils might be supported by its higher agronomic value as a source of fertilizing elements necessary for plants (Ca, P, K, and C). OPBS can also have a direct/indirect effect on soil properties by improving the physical and chemical characteristics such as water-holding capacity, CEC, and adsorption capacity, and could contribute to the immobilization of trace elements in the soils.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neina, D. The Role of Soil PH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Jones, A. Soil Atlas of Africa; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Keesstra, S.D.; Geissen, V.; Mosse, K.; Piiranen, S.; Scudiero, E.; Leistra, M.; van Schaik, L. Soil as a Filter for Groundwater Quality. Curr. Opin. Environ. Sustain. 2012, 4, 507–516. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Poesen, J.; Ballabio, C.; Lugato, E.; Meusburger, K.; Montanarella, L.; Alewell, C. The New Assessment of Soil Loss by Water Erosion in Europe. Environ. Sci. Policy 2015, 54, 438–447. [Google Scholar] [CrossRef]
- Abd-Elmabod, S.K.; Bakr, N.; Muñoz-Rojas, M.; Pereira, P.; Zhang, Z.; Cerdà, A.; Jordán, A.; Mansour, H.; De la Rosa, D.; Jones, L. Assessment of Soil Suitability for Improvement of Soil Factors and Agricultural Management. Sustainability 2019, 11, 1588. [Google Scholar] [CrossRef]
- Amer, R. Spatial Relationship between Irrigation Water Salinity, Waterlogging, and Cropland Degradation in the Arid and Semi-Arid Environments. Remote Sens. 2021, 13, 1047. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ghosh, B.N.; Mishra, P.K.; Mandal, B.; Rao, C.S.; Sarkar, D.; Das, K.; Anil, K.S.; Lalitha, M.; Hati, K.M. Soil Degradation in India: Challenges and Potential Solutions. Sustainability 2015, 7, 3528–3570. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Hansen, E.M.; Olesen, J.E.; Baral, K.R.; Petersen, S.O. Interactive Effects of Straw Management, Tillage, and a Cover Crop on Nitrous Oxide Emissions and Nitrate Leaching from a Sandy Loam Soil. Sci. Total Environ. 2022, 828, 154316. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Wang, J.; Li, Y.; Lou, Y.; Zhuge, Y.; Dong, Y. Soil Available Nitrogen and Yield Effect under Different Combinations of Urease/Nitrate Inhibitor in Wheat/Maize Rotation System. Agronomy 2022, 12, 1888. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Manzano-Agugliaro, F. Global Trends in Nitrate Leaching Research in the 1960–2017 Period. Sci. Total Environ. 2018, 643, 400–413. [Google Scholar] [CrossRef]
- Lu, B.; Liu, X.; Dong, P.; Tick, G.R.; Zheng, C.; Zhang, Y.; Mahmood-UI-Hassan, M.; Bai, H.; Lamy, E. Quantifying Fate and Transport of Nitrate in Saturated Soil Systems Using Fractional Derivative Model. Appl. Math. Model. 2020, 81, 279–295. [Google Scholar] [CrossRef]
- Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar]
- Stanley, J.; Reading, L. Nitrate Dynamics in Groundwater under Sugarcane in a Wet-Tropics Catchment. Heliyon 2020, 6, e05507. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Kou, C.; Wang, Q. Optimal Fertilizer Application Reduced Nitrogen Leaching and Maintained High Yield in Wheat-Maize Cropping System in North China. Plants 2022, 11, 1963. [Google Scholar] [CrossRef]
- Ingraham, P.A.; Salas, W.A. Assessing Nitrous Oxide and Nitrate Leaching Mitigation Potential in US Corn Crop Systems Using the DNDC Model. Agric. Syst. 2019, 175, 79–87. [Google Scholar] [CrossRef]
- Wongsanit, J.; Teartisup, P.; Kerdsueb, P.; Tharnpoophasiam, P.; Worakhunpiset, S. Contamination of Nitrate in Groundwater and Its Potential Human Health: A Case Study of Lower Mae Klong River Basin, Thailand. Environ. Sci. Pollut. Res. 2015, 22, 11504–11512. [Google Scholar] [CrossRef]
- Huang, J.; Hartemink, A.E. Soil and Environmental Issues in Sandy Soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Karathanasis, A.D.; Harris, W.G. Quantitative Thermal Analysis of Soil Materials. In Quantitative Methods in Soil Mineralogy; Soil Science Society of America: Madison, WI, USA, 1994; pp. 360–411. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Bilecenoglu, M.; Kaya, M.; Eryigit, A. New Data on the Occurrence of Two Alien Fishes, Pisodonophis Semicinctus and Pomadasys Stridens, from the Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2009, 10, 151. [Google Scholar] [CrossRef][Green Version]
- Bakshi, M.; Abhilash, P.C. Nanotechnology for Soil Remediation: Revitalizing the Tarnished Resource. In Nano-Materials as Photocatalysts for Degradation of Environmental Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–370. [Google Scholar]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Sarkar, A.; Sengupta, S.; Sen, S. Nanoparticles for Soil Remediation. In Nanoscience and Biotechnology for Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 249–262. [Google Scholar]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Alessandrino, L.; Colombani, N.; Aschonitis, V.G.; Mastrocicco, M. Nitrate and Dissolved Organic Carbon Release in Sandy Soils at Different Liquid/Solid Ratios Amended with Graphene and Classical Soil Improvers. Appl. Sci. 2022, 12, 6220. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and Modification of Biochar Materials and Their Application in Soil Remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Islam, T.; Li, Y.; Cheng, H. Biochars and Engineered Biochars for Water and Soil Remediation: A Review. Sustainability 2021, 13, 9932. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical Stabilization Remediation for Heavy Metals in Contaminated Soils on the Latest Decade: Available Stabilizing Materials and Associated Evaluation Methods-A Critical Review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Das, S.; Kim, G.W.; Hwang, H.Y.; Verma, P.P.; Kim, P.J. Cropping with Slag to Address Soil, Environment, and Food Security. Front. Microbiol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Liyun, Y.; Ping, X.; Maomao, Y.; Hao, B. The Characteristics of Steel Slag and the Effect of Its Application as a Soil Additive on the Removal of Nitrate from Aqueous Solution. Environ. Sci. Pollut. Res. 2017, 24, 4882–4893. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Hu, Z.; Zhang, L.; Yang, S.; Ruan, R.; Bai, S.; Tan, H. Characteristics of Ash and Slag from Four Biomass-Fired Power Plants: Ash/Slag Ratio, Unburned Carbon, Leaching of Major and Trace Elements. Energy Convers. Manag. 2020, 214, 112897. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Dijkstra, J.J.; Comans, R.N.J. Assessment of Biomass Ash Applications in Soil and Cement Mortars. Chemosphere 2019, 223, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Tanji, A.; Benicha, M.; Mrabet, R. Techniques de Production de l’arachide, Résultats d’enquêtes Au Loukkos. Bull. Transf. Technol. Agric. 2011. [Google Scholar] [CrossRef]
- Mourabit, F.; Ouassini, A.; Azmani, A.; Mueller, R. Nitrate Occurrence in the Groundwater of the Loukkos Perimeter. J. Environ. Monit. 2002, 4, 127–130. [Google Scholar] [CrossRef] [PubMed]
- El Bakouri, H.; Ouassini, A.; Morillo, J.; Usero, J. Pesticides in Ground Water beneath Loukkos Perimeter, Northwest Morocco. J. Hydrol. 2008, 348, 270–278. [Google Scholar] [CrossRef]
- Sarti, O.; Otal, E.; Morillo, J.; Ouassini, A. Integrated Assessment of Groundwater Quality beneath the Rural Area of R’mel, Northwest of Morocco. Groundw. Sustain. Dev. 2021, 14, 100620. [Google Scholar] [CrossRef]
- Hmamou, M.; Bounakaya, B. The role of artificial impoundments in improving agricultural production in the semi-arid regions of northern morocco. Geogr. Environ. Sustain. 2020, 13, 32–42. [Google Scholar] [CrossRef]
- Metson, A.J. Methods of Chemical Analysis for Soil Survey Samples; New Zealand Department of Scientific and Industrial Research: Wellington, New Zealand, 1961. [Google Scholar]
- Alam, M.T.; Dai, B.; Wu, X.; Hoadley, A.; Zhang, L. A Critical Review of Ash Slagging Mechanisms and Viscosity Measurement for Low-Rank Coal and Bio-Slags. Front. Energy 2021, 15, 46–67. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.M.; Han, X.X.; Wang, H. Fusion Characteristic Study on Seaweed Biomass Ash. Energy Fuels 2008, 22, 2229–2235. [Google Scholar] [CrossRef]
- Zarabi, M.; Jalali, M. Leaching of Nitrogen and Base Cations from Calcareous Soil Amended with Organic Residues. Environ. Technol. 2012, 33, 1577–1588. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Phosphorus. In Principles of Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001; pp. 453–479. [Google Scholar]
- Jackson, R.S. 5—Site Selection and Climate. In Wine Science, 4th ed.; Jackson, R.S., Ed.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2014; pp. 307–346. ISBN 978-0-12-381468-5. [Google Scholar]
- Fujii, K.; Hayakawa, C.; Panitkasate, T.; Maskhao, I.; Funakawa, S.; Kosaki, T.; Nawata, E. Acidification and Buffering Mechanisms of Tropical Sandy Soil in Northeast Thailand. Soil Tillage Res. 2017, 165, 80–87. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Remon, E. Tolérance et Accumulation Des Métaux Lourds Par La Végétation Spontanée Des Friches Métallurgiques: Vers de Nouvelles Méthodes de Bio-Dépollution. Ph.D. Thesis, Université Jean Monnet, Saint-Étienne, France, 2006. [Google Scholar]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil Organic Matter Prevails over Heavy Metal Pollution and Vegetation as a Factor Shaping Soil Microbial Communities at Historical Zn–Pb Mining Sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef] [PubMed]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of Fertilizer Application on Soil Heavy Metal Concentration. Environ. Monit. Assess. 2010, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kelepertzis, E. Accumulation of Heavy Metals in Agricultural Soils of Mediterranean: Insights from Argolida Basin, Peloponnese, Greece. Geoderma 2014, 221–222, 82–90. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Wang, Y.; Sun, L.; Yu, H. Multivariate and Geostatistical Analyses of the Spatial Distribution and Sources of Heavy Metals in Agricultural Soil in Dehui, Northeast China. Chemosphere 2013, 92, 517–523. [Google Scholar] [CrossRef]
- Mertens, J.; Smolders, E. Zinc. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin, Germanry, 2013; pp. 465–493. [Google Scholar]
- Batra, V.S.; Urbonaite, S.; Svensson, G. Characterization of Unburned Carbon in Bagasse Fly Ash. Fuel 2008, 87, 2972–2976. [Google Scholar] [CrossRef]
- Motesharezadeh, B.; Bell, R.W.; Ma, Q. Lime Application Reduces Potassium and Nitrate Leaching on Sandy Soils (Die Kalkanwendung Reduziert Das Auswaschen von Kalium Und Nitrat Auf Sandigen Böden). J. Kult. 2021, 73, 83–93. [Google Scholar]
- Wang, L.; Skjevrak, G.; Hustad, J.E.; Skreiberg, Ø. Investigation of Biomass Ash Sintering Characteristics and the Effect of Additives. Energy Fuels 2014, 28, 208–218. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Mäkelä, M.; Pöykiö, R.; Manskinen, K.; Dahl, O. Comparison of the Forest Fertilizer Properties of Ash Fractions from Two Power Plants of Pulp and Paper Mills Incinerating Biomass-Based Fuels. Fuel Process. Technol. 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Mandal, J.K.; Mukherjee, S.; Saha, N.; Halder, N.; Biswas, T.; Chakraborty, S.; Hassan, S.; Hassan, M.M.; Abo-Shosha, A.A.; Hossain, A. Assessing the Capability of Chemical Ameliorants to Reduce the Bioavailability of Heavy Metals in Bulk Fly Ash Contaminated Soil. Molecules 2021, 26, 7019. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Moreno, N.; Alvarez-Ayuso, E.; García-Sánchez, A.; Cama, J.; Ayora, C.; Simón, M. Immobilization of Heavy Metals in Polluted Soils by the Addition of Zeolitic Material Synthesized from Coal Fly Ash. Chemosphere 2006, 62, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Dermatas, D.; Meng, X. Utilization of Fly Ash for Stabilization/Solidification of Heavy Metal Contaminated Soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Ciccu, R.; Ghiani, M.; Peretti, R.; Serci, A.; Zucca, A. Heavy Metal Immobilization Using Fly Ash in Soils Contaminated by Mine Activity. In Proceedings of the 2001 International Ash Utilization Symposium, Lexington, KY, USA, 22–24 October 2001. [Google Scholar]
- Hu, X.; Huang, X.; Zhao, H.; Liu, F.; Wang, L.; Zhao, X.; Gao, P.; Li, X.; Ji, P. Possibility of Using Modified Fly Ash and Organic Fertilizers for Remediation of Heavy-Metal-Contaminated Soils. J. Clean. Prod. 2021, 284, 124713. [Google Scholar] [CrossRef]
- Ciccu, R.; Ghiani, M.; Serci, A.; Fadda, S.; Peretti, R.; Zucca, A. Heavy Metal Immobilization in the Mining-Contaminated Soils Using Various Industrial Wastes. Miner. Eng. 2003, 16, 187–192. [Google Scholar] [CrossRef]
- Ou, Y.; Ma, S.; Zhou, X.; Wang, X.; Shi, J.; Zhang, Y. The Effect of a Fly Ash-Based Soil Conditioner on Corn and Wheat Yield and Risk Analysis of Heavy Metal Contamination. Sustainability 2020, 12, 7281. [Google Scholar] [CrossRef]
- Chong, I.Q.; Azman, E.A.; Ng, J.F.; Ismail, R.; Awang, A.; Hasbullah, N.A.; Murdad, R.; Ahmed, O.H.; Musah, A.A.; Alam, M.A. Improving Selected Chemical Properties of a Paddy Soil in Sabah Amended with Calcium Silicate: A Laboratory Incubation Study. Sustainability 2022, 14, 13214. [Google Scholar] [CrossRef]
- Bruand, A.; Hartmann, C.; Lesturgez, G. Physical Properties of Tropical Sandy Soils: A Large Range of Behaviours. In Management of Tropical Sandy Soils for Sustainable Agriculture; A Holistic Approach for Sustainable Development of Problem Soils in the Tropics; FAO: Rome, Italy, 2005. [Google Scholar]
- Matichenkov, V.V.; Bocharnikova, E.A.; Calvert, D.V.; Snyder, G.H. Comparison Study of Soil Silicon Status in Sandy Soils of South Florida. In Proceedings of the Fifty-Ninth Annual Meeting of the Soil and Crop Science Society of Florida, Sarasota, FL, USA, 22–24 September 1999; Volume 59, pp. 132–137. [Google Scholar]


| Parameter | S01 | S02 | S03 | Mean |
|---|---|---|---|---|
| pH | 7.27 | 8.75 | 6.33 | 7.55 |
| EC (mS/Cm) | 0.26 | 0.23 | 0.32 | 0.27 |
| OM % CF | 2.81 | 2.36 | 1.57 | 2.25 |
| OM % FF | 9.11 | 7.5 | 7.65 | 8.09 |
| CEC (meq/100 g) | 9.28 | 9.11 | 8.18 | 8.86 |
| Bulk density | 1.28 | 1.34 | 1.32 | 1.31 |
| Sand % | 95.84 | 95.87 | 95.42 | 95.71 |
| Silt % | 1.18 | 2.47 | 2.33 | 1.99 |
| Clay % | 2.98 | 2.36 | 1.66 | 2.33 |
| PO34− (g·Kg−1) * | 1.26 | 2.04 | 0.81 | 1.37 |
| Ca (g·Kg−1) * | 7.5 | 13.37 | 5.24 | 8.7 |
| Fe (g·Kg−1) * | 43.76 | 47.41 | 46.19 | 45.79 |
| K (g·Kg−1) * | 3.48 | 3.158 | 2.05 | 2.9 |
| Mg (g·Kg−1) * | 4.58 | 4.86 | 3.42 | 4.29 |
| Mn (g·Kg−1) * | 1.62 | 1.35 | 1.53 | 1.5 |
| Na (g·Kg−1) * | 0.33 | 0.27 | 0.18 | 0.26 |
| As (mg·Kg−1) * | 62.3 | 53.3 | 58 | 57.9 |
| Cd (mg·Kg−1) * | 6.8 | 2.64 | 1.48 | 3.6 |
| Co (mg·Kg−1) * | 22.1 | 20.3 | 32.6 | 25 |
| Cr (mg·Kg−1) * | 123.5 | 125.4 | 89.6 | 112.8 |
| Cu (mg·Kg−1) * | 108.1 | 38.9 | 17.1 | 54.7 |
| Mo (mg·Kg−1) * | 2.84 | 0.82 | 0.97 | 1.5 |
| Ni (mg·Kg−1) * | 52.4 | 36.6 | 48.6 | 45.9 |
| Pb (mg·Kg−1) * | 37.3 | 23.1 | 27.6 | 29.4 |
| Zn (mg·Kg−1) * | 224.7 | 162.14 | 114.25 | 167 |
| Parameter | pH | Moisture % | Unburned Carbon % | Ca % | Fe % | K% | Mg% | Na% |
|---|---|---|---|---|---|---|---|---|
| OPBS | 12.1 | 7.18 | 19.97 | 10.59 | 0.95 | 8.24 | 1.56 | 0.15 |
| Trace Element in (mg. Kg−1) | As | Cd | Co | Cr | Cu | Mn | Mo | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| OPBS | 2.12 | 0.25 | 2.49 | 48.43 | 48.30 | 396.33 | 1.72 | 42.64 | 1.93 | 47.34 |
| Sample | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | %Mineral Fraction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.F 1 | 55.17 | 10.86 | 9.9 | 0.3 | 1.57 | 1.73 | 0.78 | 1.42 | 0.75 | 0.61 | 0.21 | 83.3 |
| C.F 1 | 71.43 | 7.19 | 5.94 | 0.16 | 0.55 | 1.03 | 0.76 | 0.94 | 0.55 | 0.34 | 0.15 | 89.04 |
| F.F 2 | 58.79 | 11.09 | 9.76 | 0.23 | 1.59 | 2.56 | 0.76 | 1.38 | 0.71 | 0.53 | 0.17 | 87.57 |
| C.F 2 | 72.45 | 7.45 | 5.00 | 0.16 | 0.53 | 1.21 | 0.72 | 1.11 | 0.45 | 0.38 | 0.14 | 89.6 |
| F.F 3 | 60.64 | 12.51 | 10.74 | 0.3 | 1.3 | 1.01 | 0.51 | 1.3 | 0.81 | 0.37 | 0.13 | 89.62 |
| C.F 3 | 75.70 | 7.23 | 5.52 | 0.19 | 0.42 | 0.54 | 0.63 | 1.01 | 0.48 | 0.32 | 0.09 | 92.13 |
| OPBS | 26.29 | 3.26 | 1.07 | 0.05 | 3.52 | 22.38 | 0.56 | 17.63 | 0.12 | 2.75 | 1.41 | 79.04 |
| Sample | S1 | S2 | S3 | Biomass Slag |
|---|---|---|---|---|
| BET surface area (m2/g) | 4.56 | 4.18 | 3.74 | 9.37 |
| Langmuir surface area (m2/g) | 6.30 | 5.79 | 5.20 | 13.20 |
| Total pore volume (cm3/g) | 0.008868 | 0.008574 | 0.008034 | 0.031480 |
| Pore diameter (nm) | 11.9869 | 12.3592 | 12.5881 | 20.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarti, O.; El Mansouri, F.; Otal, E.; Morillo, J.; Ouassini, A.; Brigui, J.; Saidi, M. Assessing the Effect of Intensive Agriculture and Sandy Soil Properties on Groundwater Contamination by Nitrate and Potential Improvement Using Olive Pomace Biomass Slag (OPBS). C 2023, 9, 1. https://doi.org/10.3390/c9010001
Sarti O, El Mansouri F, Otal E, Morillo J, Ouassini A, Brigui J, Saidi M. Assessing the Effect of Intensive Agriculture and Sandy Soil Properties on Groundwater Contamination by Nitrate and Potential Improvement Using Olive Pomace Biomass Slag (OPBS). C. 2023; 9(1):1. https://doi.org/10.3390/c9010001
Chicago/Turabian StyleSarti, Otmane, Fouad El Mansouri, Emilia Otal, José Morillo, Abdelhamid Ouassini, Jamal Brigui, and Mohamed Saidi. 2023. "Assessing the Effect of Intensive Agriculture and Sandy Soil Properties on Groundwater Contamination by Nitrate and Potential Improvement Using Olive Pomace Biomass Slag (OPBS)" C 9, no. 1: 1. https://doi.org/10.3390/c9010001
APA StyleSarti, O., El Mansouri, F., Otal, E., Morillo, J., Ouassini, A., Brigui, J., & Saidi, M. (2023). Assessing the Effect of Intensive Agriculture and Sandy Soil Properties on Groundwater Contamination by Nitrate and Potential Improvement Using Olive Pomace Biomass Slag (OPBS). C, 9(1), 1. https://doi.org/10.3390/c9010001











