Ferromagnetic Biochar Prepared from Hydrothermally Modified Calcined Mango Seeds for Fenton-like Degradation of Indigo Carmine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Non-Modified Biochar (BNM) and Ferromagnetic Biochar (BNMF)
2.3. Material Characterization
2.4. Experimental Procedure for the Degradation of Indigo Carmine in Aqueous Solution
3. Results
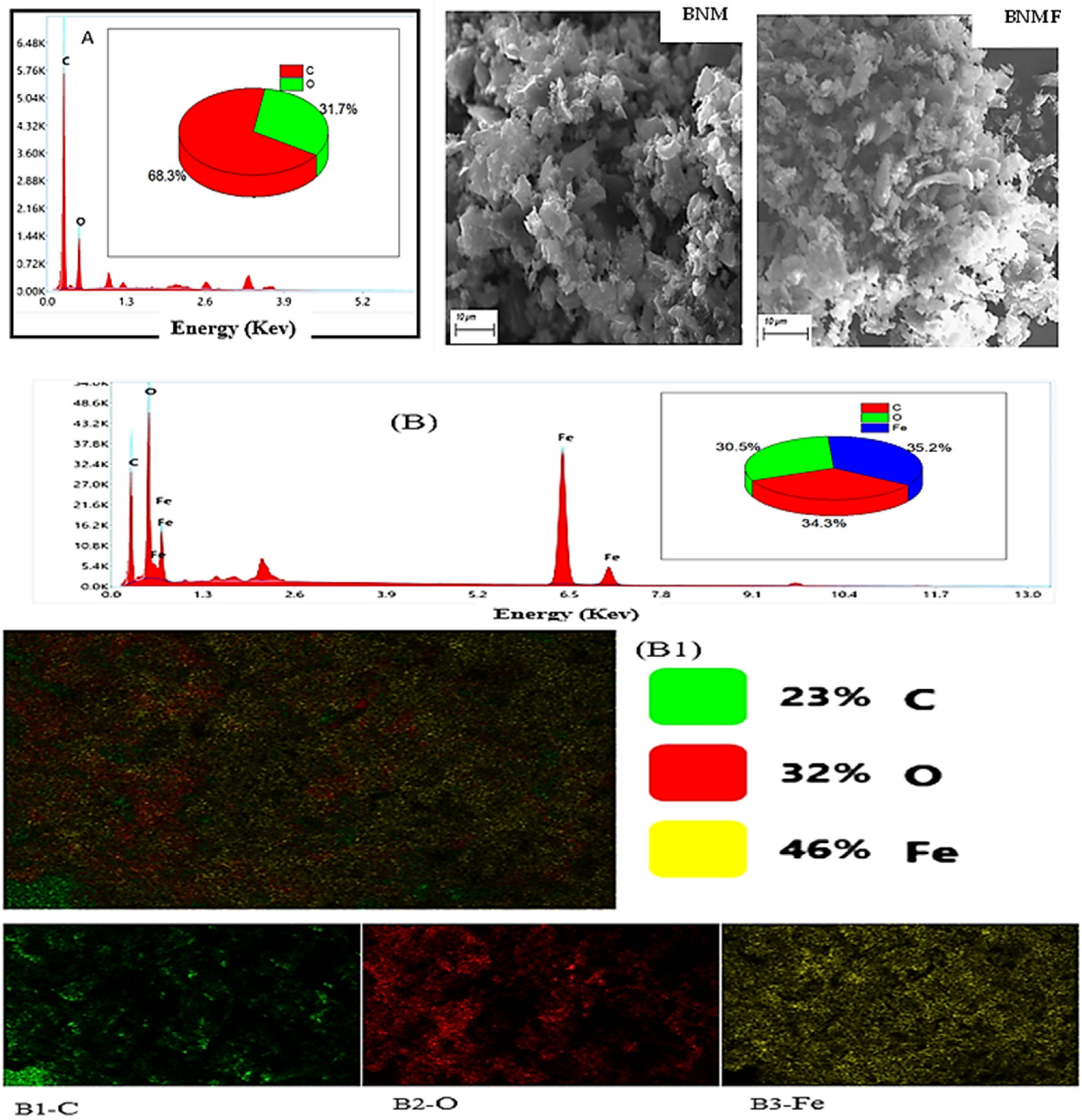
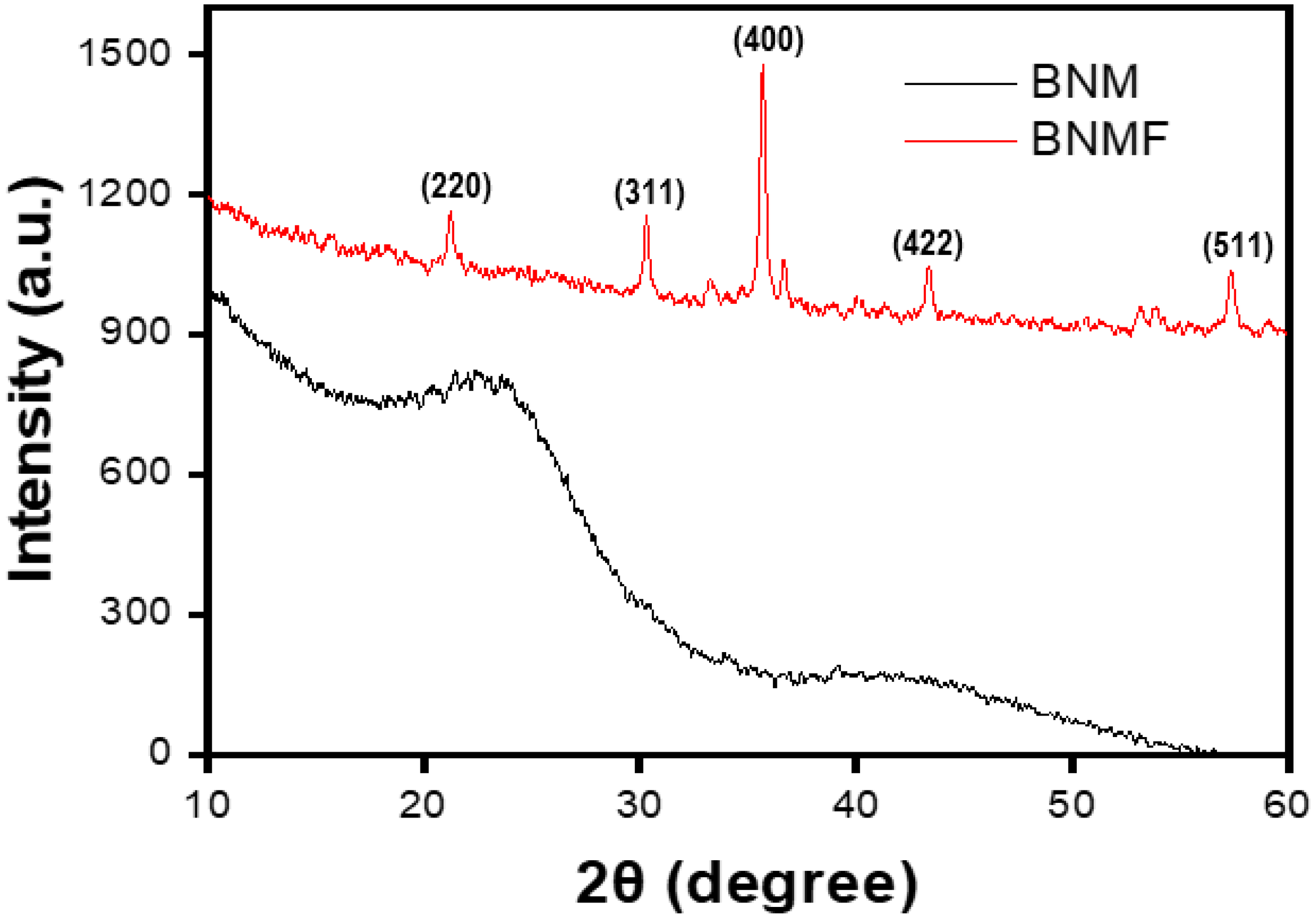
3.1. Characterization of Materials
3.2. Degradation of Indigo Carmine in Aqueous Solution
3.2.1. Influence of the Type of Catalyst on the Degradation Process
3.2.2. Influence of pH on the Degradation Process
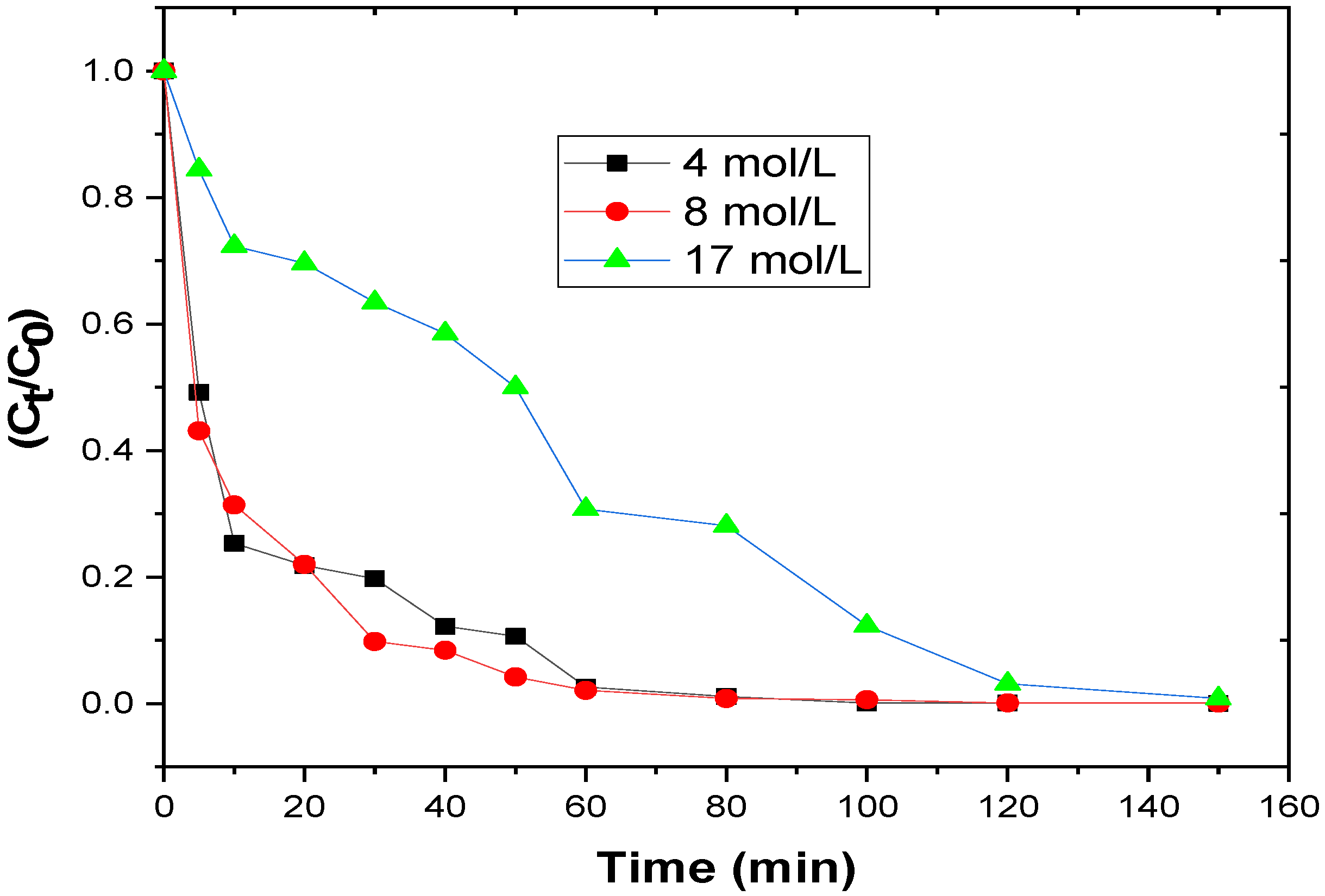
3.2.3. Influence of H2O2 Concentration on the Degradation Process
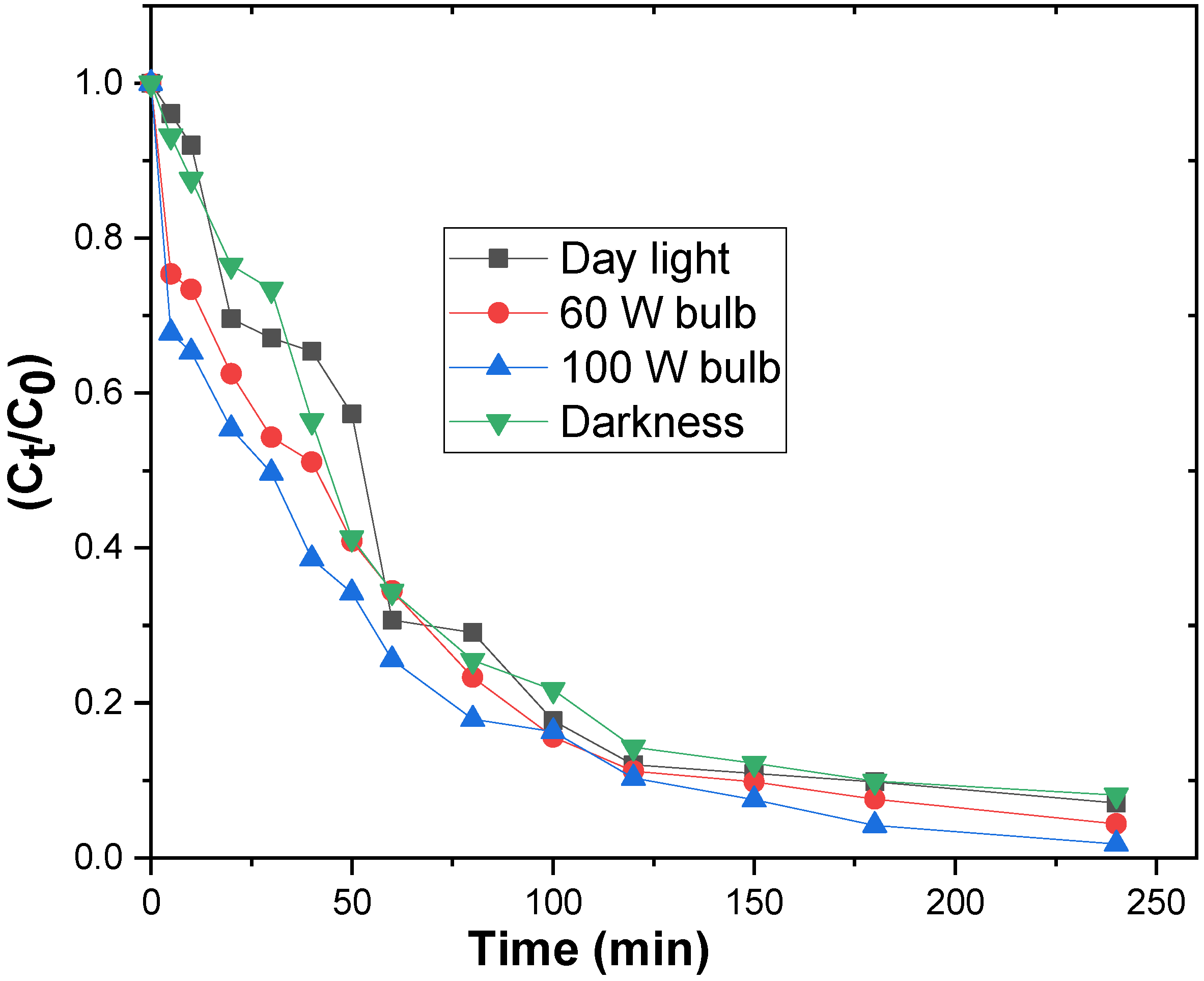
3.2.4. Influence of Light Intensity on the Degradation Process
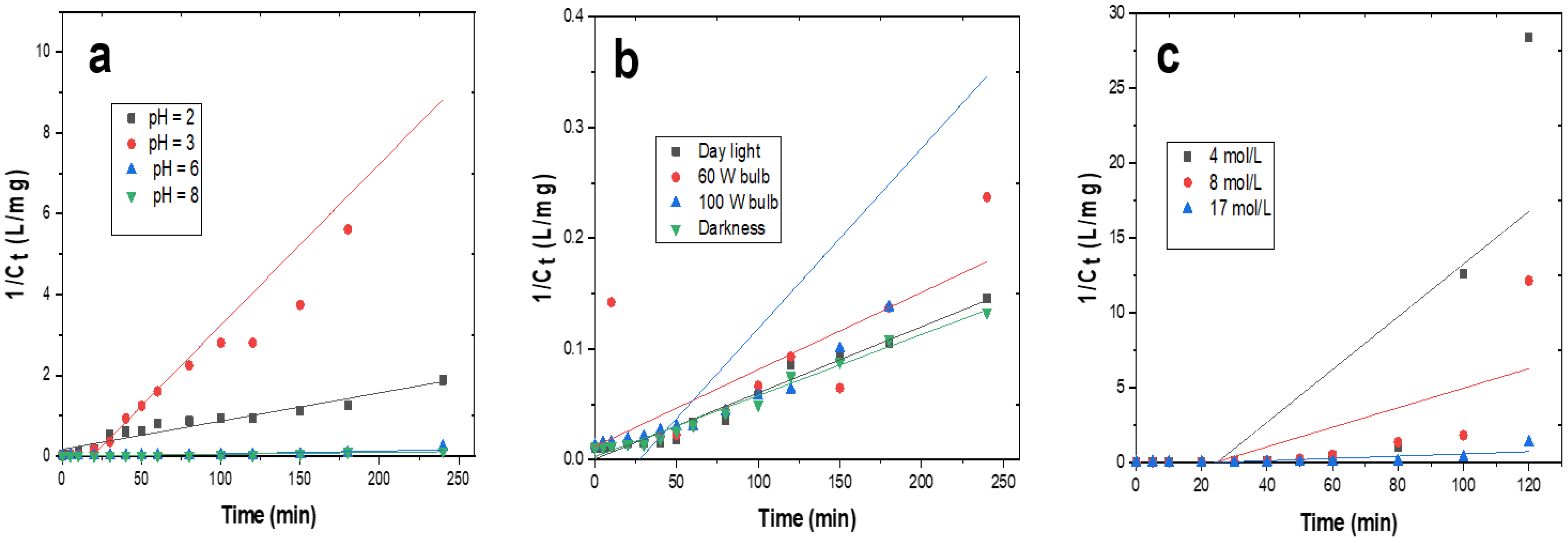
3.3. Kinetic Studies of the Degradation of Indigo Carmine
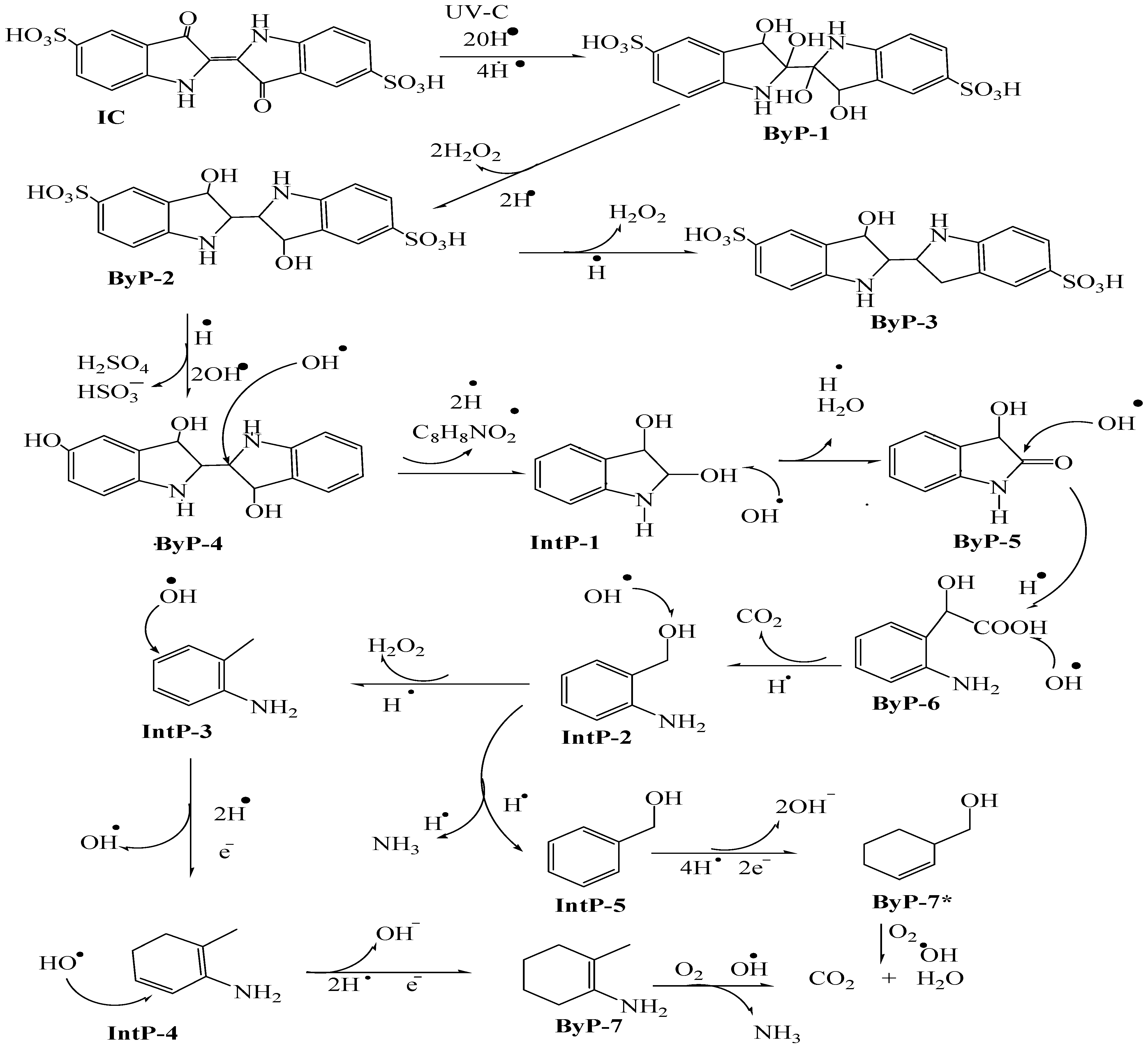
3.4. Plausible Mechanism of Indigo Carmine Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuete, I.-H.T.; Tchuifon, D.R.T.; Ndifor-Angwafor, G.N.; Kamdem, A.T.; Anagho, S.G. Kinetic, Isotherm and Thermodynamic Studies of the Adsorption of Thymol Blue onto Powdered Activated Carbons from Garcinia Cola Nut Shells Impregnated with H3PO4 and KOH: Non-Linear Regression Analysis. JEAS 2020, 10, 1–27. [Google Scholar] [CrossRef]
- Ngaha, M.C.D.; Njanja, E.; Doungmo, G.; Tamo Kamdem, A.; Tonle, I.K. Indigo Carmine and 2,6-Dichlorophenolindophenol Removal Using Cetyltrimethylammonium Bromide-Modified Palm Oil Fiber: Adsorption Isotherms and Mass Transfer Kinetics. Int. J. Biomater. 2019, 2019, 6862825. [Google Scholar] [CrossRef] [PubMed]
- Alsukaibi, A.K.D. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Ngouoko, J.J.K.; Tajeu, K.Y.; Temgoua, R.C.T.; Doungmo, G.; Doench, I.; Tamo, A.K.; Kamgaing, T.; Osorio-Madrazo, A.; Tonle, I.K. Hydroxyapatite/L-Lysine Composite Coating as Glassy Carbon Electrode Modifier for the Analysis and Detection of Nile Blue A. Materials 2022, 15, 4262. [Google Scholar] [CrossRef]
- Ngouoko, J.J.K.; Tajeu, K.Y.; Fotsop, C.G.; Tamo, A.K.; Doungmo, G.; Temgoua, R.C.T.; Kamgaing, T.; Tonle, I.K. Calcium Carbonate Originating from Snail Shells for Synthesis of Hydroxyapatite/L-Lysine Composite: Characterization and Application to the Electroanalysis of Toluidine Blue. Crystals 2022, 12, 1189. [Google Scholar] [CrossRef]
- Nindjio, G.F.K.; Tagne, R.F.T.; Jiokeng, S.L.Z.; Fotsop, C.G.; Bopda, A.; Doungmo, G.; Temgoua, R.C.T.; Doench, I.; Njoyim, E.T.; Tamo, A.K.; et al. Lignocellulosic-Based Materials from Bean and Pistachio Pod Wastes for Dye-Contaminated Water Treatment: Optimization and Modeling of Indigo Carmine Sorption. Polymers 2022, 14, 3776. [Google Scholar] [CrossRef]
- Donlifack Atemkeng, C.; Tamo, A.K.; Doungmo, G.; Amola, L.A.; Kouanang Ngouoko, J.J.; Kamgaing, T. Thermodynamic, Nonlinear Kinetic, and Isotherm Studies of Bisphenol a Uptake onto Chemically Activated Carbons Derived from Safou (Dacryodes Edulis) Seeds. J. Chem. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Bhat, R.A.; Qadri, H. (Eds.) Bioremediation and Biotechnology; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-35690-3. [Google Scholar]
- Genázio Pereira, P.C.; Reimão, R.V.; Pavesi, T.; Saggioro, E.M.; Moreira, J.C.; Veríssimo Correia, F. Lethal and Sub-Lethal Evaluation of Indigo Carmine Dye and Byproducts after TiO2 Photocatalysis in the Immune System of Eisenia Andrei Earthworms. Ecotoxicol. Environ. Saf. 2017, 143, 275–282. [Google Scholar] [CrossRef]
- Debina, B.; Eric, S.N.; Fotio, D.; Arnaud, K.T.; Lemankreo, D.-Y.; Rahman, A.N. Adsorption of Indigo Carmine Dye by Composite Activated Carbons Prepared from Plastic Waste (PET) and Banana Pseudo Stem. MSCE 2020, 8, 39–55. [Google Scholar] [CrossRef]
- Ortiz, E.; Gómez-Chávez, V.; Cortés-Romero, C.M.; Solís, H.; Ruiz-Ramos, R.; Loera-Serna, S. Degradation of Indigo Carmine Using Advanced Oxidation Processes: Synergy Effects and Toxicological Study. JEP 2016, 7, 1693–1706. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Soomro, U.; Muqeet, M.; Ahmed, Z. Adsorption of Indigo Carmine Dye onto the Surface-Modified Adsorbent Prepared from Municipal Waste and Simulation Using Deep Neural Network. J. Hazard. Mater. 2021, 408, 124433. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent Developments in Hazardous Pollutants Removal from Wastewater and Water Reuse within a Circular Economy. npj Clean Water 2022, 5, 109. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Liu, D.; Yi, M.; Chang, F.; Li, H.; Du, Y. A Review of Sulfate Radical-Based and Singlet Oxygen-Based Advanced Oxidation Technologies: Recent Advances and Prospects. Catalysts 2022, 12, 1092. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C. (Ed.) Advanced Oxidation Processes—Applications, Trends, and Prospects; IntechOpen: London, UK, 2020; ISBN 978-1-78984-890-8. [Google Scholar]
- Xu, M.; Wu, C.; Zhou, Y. Advancements in the Fenton Process for Wastewater Treatment. In Advanced Oxidation Processes—Applications, Trends, and Prospects; Bustillo-Lecompte, C., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-890-8. [Google Scholar]
- Krupińska, I. Impact of the Oxidant Type on the Efficiency of the Oxidation and Removal of Iron Compounds from Groundwater Containing Humic Substances. Molecules 2020, 25, 3380. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Zhang, L.; He, G.; He, H.; Hu, C. Selective H2O2Conversion to Hydroxyl Radicals in the Electron-Rich Area of Hydroxylated C-g-C3N4/CuCo–Al2O3. J. Mater. Chem. A 2017, 5, 7153–7164. [Google Scholar] [CrossRef]
- Gasim, M.F.; Choong, Z.-Y.; Koo, P.-L.; Low, S.-C.; Abdurahman, M.-H.; Ho, Y.-C.; Mohamad, M.; Suryawan, I.W.K.; Lim, J.-W.; Oh, W.-D. Application of Biochar as Functional Material for Remediation of Organic Pollutants in Water: An Overview. Catalysts 2022, 12, 210. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, Z.; Xu, P.; Lin, H.; Wu, X.; Yang, L.; Huang, J. Characterization and Application of Magnetic Biochars from Corn Stalk by Pyrolysis and Hydrothermal Treatment. BioResources 2016, 12, 1077–1089. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.-P.; Qin, S.-N.; Guo, J.-R.; Zhang, Y.-Q.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; Jin, Y. Study on the Hg0 Removal Characteristics and Synergistic Mechanism of Iron-Based Modified Biochar Doped with Multiple Metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef]
- Ozel, F.; Kockar, H. Growth and Characterizations of Magnetic Nanoparticles under Hydrothermal Conditions: Reaction Time and Temperature. J. Magn. Magn. Mater. 2015, 373, 213–216. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, L. Pollution Caused by Agricultural Waste Burning and Possible Alternate Uses of Crop Stubble: A Case Study of Punjab. In Knowledge Systems of Societies for Adaptation and Mitigation of Impacts of Climate Change; Nautiyal, S., Rao, K.S., Kaechele, H., Raju, K.V., Schaldach, R., Eds.; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 367–385. ISBN 978-3-642-36142-5. [Google Scholar]
- Usmani, M.; Kondal, A.; Wang, J.; Jutla, A. Environmental Association of Burning Agricultural Biomass in the Indus River Basin. GeoHealth 2020, 4, e2020GH000281. [Google Scholar] [CrossRef]
- Gupta, S. Agriculture Crop Residue Burning and Its Consequences on Respiration Health of School-Going Children. Glob. Pediatr. Health 2019, 6, 2333794X1987467. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Lall, A.; Kamdem Tamo, A.; Doench, I.; David, L.; Nunes de Oliveira, P.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan-Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Deussi Ngaha, M.C.; Kougoum Tchieda, V.; Kamdem Tamo, A.; Doungmo, G.; Njanja, E.; Kenfack Tonle, I. Aminoalcohol-functionalization of Alkali Palm Oil Fiber and Application as Electrochemical Sensor for 2-nitrophenol Determination. Electroanalysis 2022, 9, 5222. [Google Scholar] [CrossRef]
- Marquez-Bravo, S.; Doench, I.; Molina, P.; Bentley, F.E.; Tamo, A.K.; Passieux, R.; Lossada, F.; David, L.; Osorio-Madrazo, A. Functional Bionanocomposite Fibers of Chitosan Filled with Cellulose Nanofibers Obtained by Gel Spinning. Polymers 2021, 13, 1563. [Google Scholar] [CrossRef]
- Kamdem Tamo, A.; Doench, I.; Morales Helguera, A.; Hoenders, D.; Walther, A.; Madrazo, A.O. Biodegradation of Crystalline Cellulose Nanofibers by Means of Enzyme Immobilized-Alginate Beads and Microparticles. Polymers 2020, 12, 1522. [Google Scholar] [CrossRef]
- Djouonkep, L.D.W.; Tamo, A.K.; Doench, I.; Selabi, N.B.S.; Ilunga, E.M.; Lenwoue, A.R.K.; Gauthier, M.; Cheng, Z.; Osorio-Madrazo, A. Synthesis of High Performance Thiophene-Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies. Molecules 2022, 27, 325. [Google Scholar] [CrossRef]
- Ngankam, E.S.; Dai-Yang, L.; Debina, B.; Baçaoui, A.; Yaacoubi, A.; Rahman, A.N. Preparation and Characterization of Magnetic Banana Peels Biochar for Fenton Degradation of Methylene Blue. MSA 2020, 11, 382–400. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, Z.; Chen, X.; Zhou, X.; Gao, Z.F.; Deng, S.; Wan, S.; Lv, X.; Shi, Y.; Han, W. Fabrication of Nano Iron Oxide-Modified Biochar from Co-Hydrothermal Carbonization of Microalgae and Fe(II) Salt for Efficient Removal of Rhodamine B. Nanomaterials 2022, 12, 2271. [Google Scholar] [CrossRef]
- Zhou, W.; Rajic, L.; Chen, L.; Kou, K.; Ding, Y.; Meng, X.; Wang, Y.; Mulaw, B.; Gao, J.; Qin, Y.; et al. Activated Carbon as Effective Cathode Material in Iron-Free Electro-Fenton Process: Integrated H2O2 Electrogeneration, Activation, and Pollutants Adsorption. Electrochim. Acta 2019, 296, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Armynah, B.; Atika; Djafar, Z.; Piarah, W.H.; Tahir, D. Analysis of Chemical and Physical Properties of Biochar from Rice Husk Biomass. J. Phys. Conf. Ser. 2018, 979, 012038. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Ridwan; Marsi; Setiabudidaya, D. Removal of Procion Red MX-5B from Songket’s Industrial Wastewater in South Sumatra Indonesia Using Activated Carbon-Fe3O4 Composite. Sustain. Environ. Res. 2018, 28, 158–164. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, M.; Zhang, Z.; Angst, U.; Elsener, B. The Kinetic Competition between Transport and Oxidation of Ferrous Ions Governs Precipitation of Corrosion Products in Carbonated Concrete. RILEM Tech. Lett. 2018, 3, 8–16. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of PH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Yang, Y.; Sun, P. Fenton-Like Oxidation of Antibiotic Ornidazole Using Biochar-Supported Nanoscale Zero-Valent Iron as Heterogeneous Hydrogen Peroxide Activator. Int. J. Environ. Res. Public Health 2020, 17, 1324. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Nnadozie, E.C. Synthesis and Structural Studies of Manganese Ferrite and Zinc Ferrite Nanocomposites and Their Use as Photoadsorbents for Indigo Carmine and Methylene Blue Dyes. ACS Omega 2020, 5, 32386–32394. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Lee, W. Degradation of Diclofenac by Pyrite Catalyzed Fenton Oxidation. Appl. Catal. B Environ. 2013, 134–135, 93–102. [Google Scholar] [CrossRef]
- Dias, F.F.; Oliveira, A.A.S.; Arcanjo, A.P.; Moura, F.C.C.; Pacheco, J.G.A. Residue-Based Iron Catalyst for the Degradation of Textile Dye via Heterogeneous Photo-Fenton. Appl. Catal. B Environ. 2016, 186, 136–142. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical Oxidation of Methylene Blue Using a Fenton-like Reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Karale, R.S.; Manu, B.; Shrihari, S. Fenton and Photo-Fenton Oxidation Processes for Degradation of 3-Aminopyridine from Water. APCBEE Procedia 2014, 9, 25–29. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Qin, Q.; Peng, C. Sustainable and Feasible Reagent-Free Electro-Fenton via Sequential Dual-Cathode Electrocatalysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2108573118. [Google Scholar] [CrossRef]
- Kousar, T.; Bokhari, T.H.; Altaf, A.; ul Haq, A.; Muneer, M.; Farhat, L.B.; Alwadai, N.; Alfryyan, N.; Jilani, M.I.; Iqbal, M.; et al. SnO2/UV/H2O2 and TiO2/UV/H2O2 Efficiency for the Degradation of Reactive Yellow 160A: By-Product Distribution, Cytotoxicity and Mutagenicity Evaluation. Catalysts 2022, 12, 553. [Google Scholar] [CrossRef]
- Giwa, A.-R.A.; Bello, I.A.; Olabintan, A.B.; Bello, O.S.; Saleh, T.A. Kinetic and Thermodynamic Studies of Fenton Oxidative Decolorization of Methylene Blue. Heliyon 2020, 6, e04454. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like Processes with in-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Degradation of Emerging Contaminants: Advances and Prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Ramos, R.O.; Albuquerque, M.V.C.; Lopes, W.S.; Sousa, J.T.; Leite, V.D. Degradation of Indigo Carmine by Photo-Fenton, Fenton, H2O2/UV-C and Direct UV-C: Comparison of Pathways, Products and Kinetics. J. Water Process Eng. 2020, 37, 101535. [Google Scholar] [CrossRef]
- Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Coupling of Advanced Oxidation Technologies and Biochar for the Removal of Dyes in Water. Water 2022, 14, 2531. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, Y.; Liu, J.; Ye, Z.; Zhang, H.; Ma, J.; Jia, Y.; Gao, W.; Li, Y. Preparation and Characterization of Magnetic Porous Carbon Microspheres for Removal of Methylene Blue by a Heterogeneous Fenton Reaction. ACS Appl. Mater. Interfaces 2014, 6, 7275–7285. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton Catalysts: A Review of Recent Advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Maezono, T.; Tokumura, M.; Sekine, M.; Kawase, Y. Hydroxyl Radical Concentration Profile in Photo-Fenton Oxidation Process: Generation and Consumption of Hydroxyl Radicals during the Discoloration of Azo-Dye Orange II. Chemosphere 2011, 82, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.F. Water. In Sustainable Design and Build; Elsevier: Amsterdam, The Netherlands, 2019; pp. 301–418. ISBN 978-0-12-816722-9. [Google Scholar]
- Torres-Blancas, T.; Roa-Morales, G.; Barrera-Díaz, C.; Ureña-Nuñez, F.; Cruz-Olivares, J.; Balderas-Hernandez, P.; Natividad, R. Ozonation of Indigo Carmine Enhanced by Fe/ Pimenta Dioica L. Merrill Particles. Int. J. Photoenergy 2015, 2015, 608412. [Google Scholar] [CrossRef]
- Bernal, M.; Romero, R.; Roa, G.; Barrera-Díaz, C.; Torres-Blancas, T.; Natividad, R. Ozonation of Indigo Carmine Catalyzed with Fe-Pillared Clay. Int. J. Photoenergy 2013, 2013, 918025. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Silva-Agredo, J.; González, I.; Torres-Palma, R.A. Comparative Degradation of Indigo Carmine by Electrochemical Oxidation and Advanced Oxidation Processes. Electrochim. Acta 2014, 140, 427–433. [Google Scholar] [CrossRef]
- Terres, J.; Battisti, R.; Andreaus, J.; de Jesus, P.C. Decolorization and Degradation of Indigo Carmine Dye from Aqueous Solution Catalyzed by Horseradish Peroxidase. Biocatal. Biotransform. 2014, 32, 64–73. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Xiang, L.; Huang, Q.; Qiu, J.; Zhang, H.; Sivaiah, M.V.; Baron, F.; Barrault, J.; Petit, S.; et al. Heterogeneous Photo-Fenton Decolorization of Orange II over Al-Pillared Fe-Smectite: Response Surface Approach, Degradation Pathway, and Toxicity Evaluation. J. Hazard. Mater. 2015, 287, 32–41. [Google Scholar] [CrossRef]
- Luan, J.; Chen, M.; Hu, W. Synthesis, Characterization and Photocatalytic Activity of New Photocatalyst ZnBiSbO4 under Visible Light Irradiation. Int. J. Mol. Sci. 2014, 15, 9459–9480. [Google Scholar] [CrossRef]




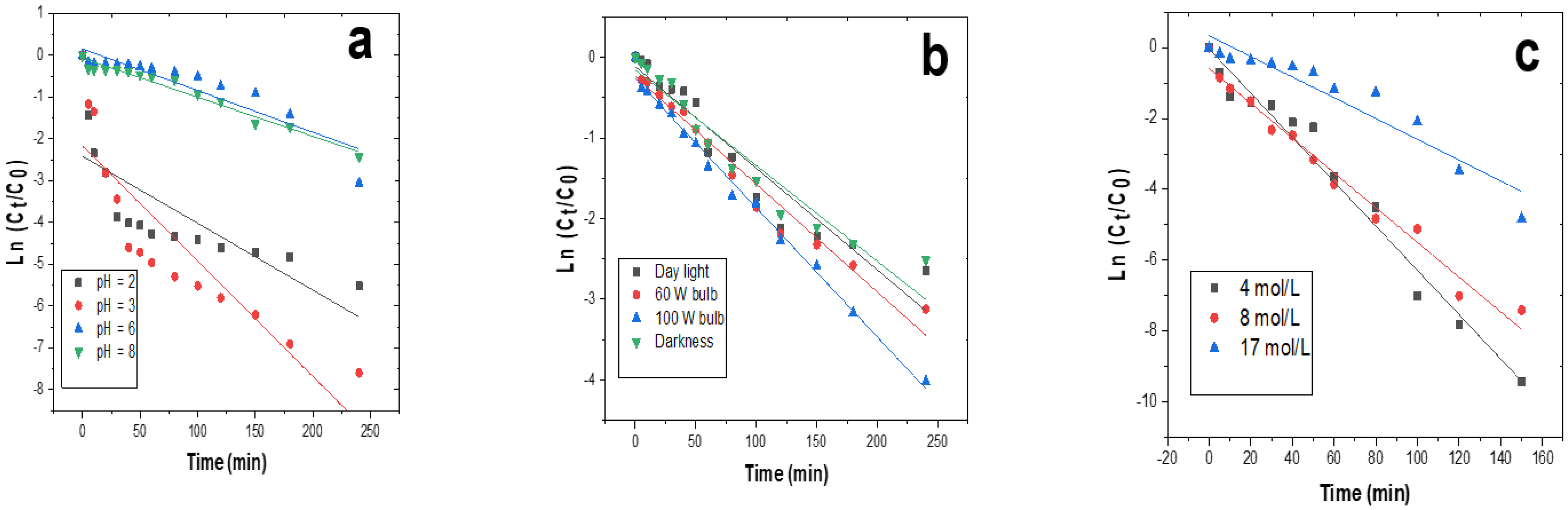
| Parameters | Variables | R2 | k (min−1) | t1/2 (min) |
|---|---|---|---|---|
| pH | 2 | 0.63 | 0.0162 | 42.59 |
| 3 | 0.78 | 0.0271 | 25.46 | |
| 6 | 0.93 | 0.0115 | 60.00 | |
| 8 | 0.93 | 0.0114 | 60.52 | |
| Concentration of H2O2 (mol/L) | 4 | 0.97 | 0.0615 | 11.21 |
| 8 | 0.98 | 0.0478 | 14.43 | |
| 17 | 0.90 | 0.0295 | 23.38 | |
| Intensity of the light | Day light | 0.93 | 0.0119 | 58.98 |
| 60 W bulb | 0.97 | 0.0132 | 52.27 | |
| 100 W bulb | 0.99 | 0.0158 | 43.67 | |
| Darkness | 0.93 | 0.0119 | 57.88 |
| Parameters | Variables | R2 | k (dm3/mol·min) | t1/2 (min) |
|---|---|---|---|---|
| pH | 2 | 0.930 | 0.011 | 1.075 |
| 3 | 0.921 | 0.088 | 0.129 | |
| 6 | 0.880 | 0.001 | 18.796 | |
| 8 | 0.880 | 0.001 | 17.820 | |
| Concentration H2O2 (mol/L) | 4 | 0.538 | 0.622 | 0.022 |
| 8 | 0.696 | 0.103 | 0.106 | |
| 17 | 0.554 | 0.006 | 1.891 | |
| Intensity of the light | Day light | 0.968 | 0.001 | 18.331 |
| 60 W bulb | 0.938 | 0.001 | 14.930 | |
| 100 W bulb | 0.785 | 0.002 | 5.739 | |
| Darkness | 0.981 | 0.001 | 18.911 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bopda, A.; Mafo, S.G.M.; Ndongmo, J.N.; Kenda, G.T.; Fotsop, C.G.; Kuete, I.-H.T.; Ngakou, C.S.; Tchuifon, D.R.T.; Tamo, A.K.; Nche, G.N.-A.; et al. Ferromagnetic Biochar Prepared from Hydrothermally Modified Calcined Mango Seeds for Fenton-like Degradation of Indigo Carmine. C 2022, 8, 81. https://doi.org/10.3390/c8040081
Bopda A, Mafo SGM, Ndongmo JN, Kenda GT, Fotsop CG, Kuete I-HT, Ngakou CS, Tchuifon DRT, Tamo AK, Nche GN-A, et al. Ferromagnetic Biochar Prepared from Hydrothermally Modified Calcined Mango Seeds for Fenton-like Degradation of Indigo Carmine. C. 2022; 8(4):81. https://doi.org/10.3390/c8040081
Chicago/Turabian StyleBopda, Aurelien, Sandrale Grace Mokue Mafo, Josiane Nguimatsia Ndongmo, Georges Teikam Kenda, Cyrille Ghislain Fotsop, Idris-Hermann Tiotsop Kuete, Christian Sadeu Ngakou, Donald Raoul Tchuifon Tchuifon, Arnaud Kamdem Tamo, George Ndifor-Angwafor Nche, and et al. 2022. "Ferromagnetic Biochar Prepared from Hydrothermally Modified Calcined Mango Seeds for Fenton-like Degradation of Indigo Carmine" C 8, no. 4: 81. https://doi.org/10.3390/c8040081
APA StyleBopda, A., Mafo, S. G. M., Ndongmo, J. N., Kenda, G. T., Fotsop, C. G., Kuete, I.-H. T., Ngakou, C. S., Tchuifon, D. R. T., Tamo, A. K., Nche, G. N.-A., & Anagho, S. G. (2022). Ferromagnetic Biochar Prepared from Hydrothermally Modified Calcined Mango Seeds for Fenton-like Degradation of Indigo Carmine. C, 8(4), 81. https://doi.org/10.3390/c8040081







