Carbon Dots versus Nano-Carbon/Organic Hybrids—Divergence between Optical Properties and Photoinduced Antimicrobial Activities
Abstract
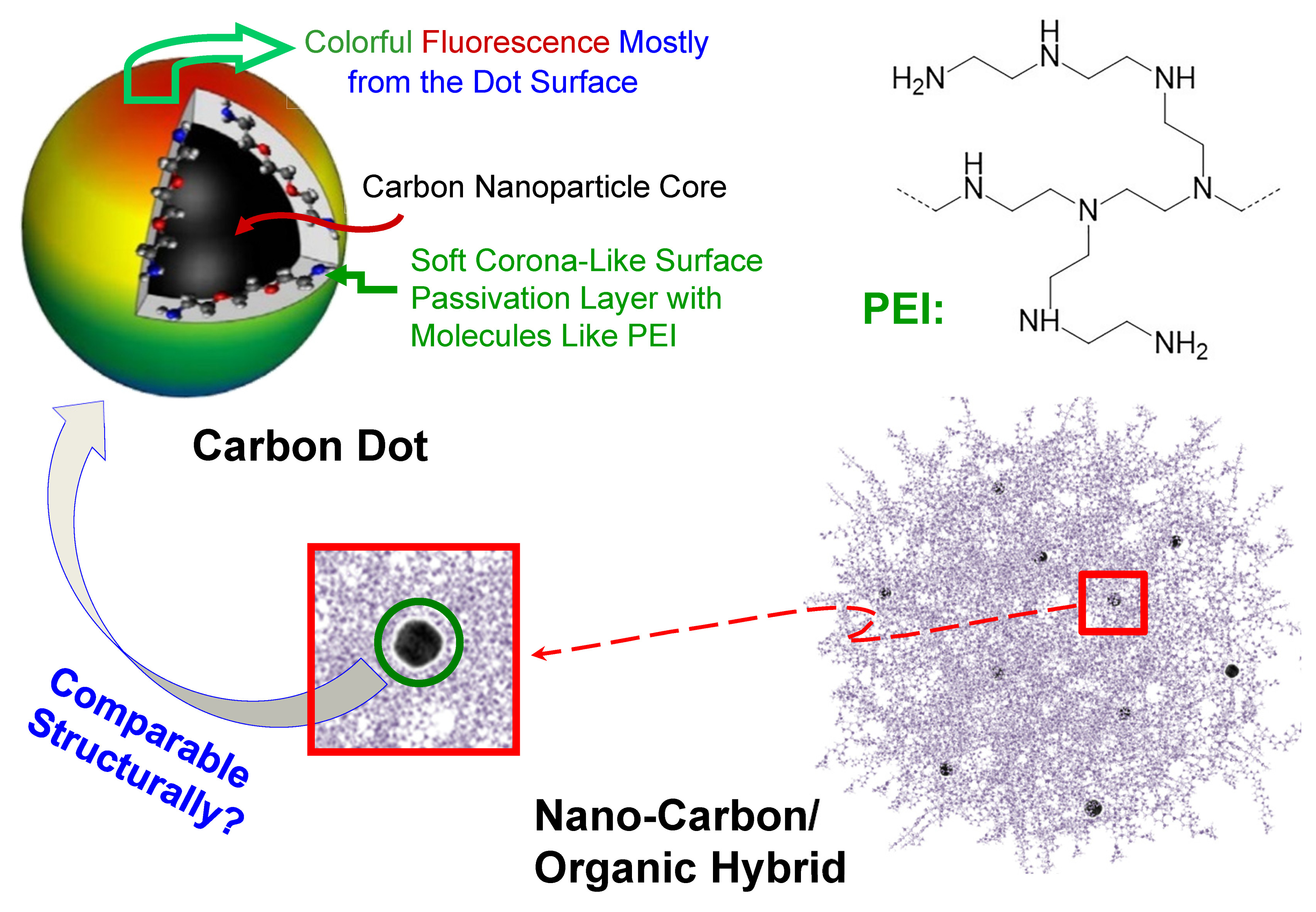
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurement
2.3. CS200 Sample
2.4. CS330 Sample
2.5. CSMT Sample
2.6. PEI-CDots
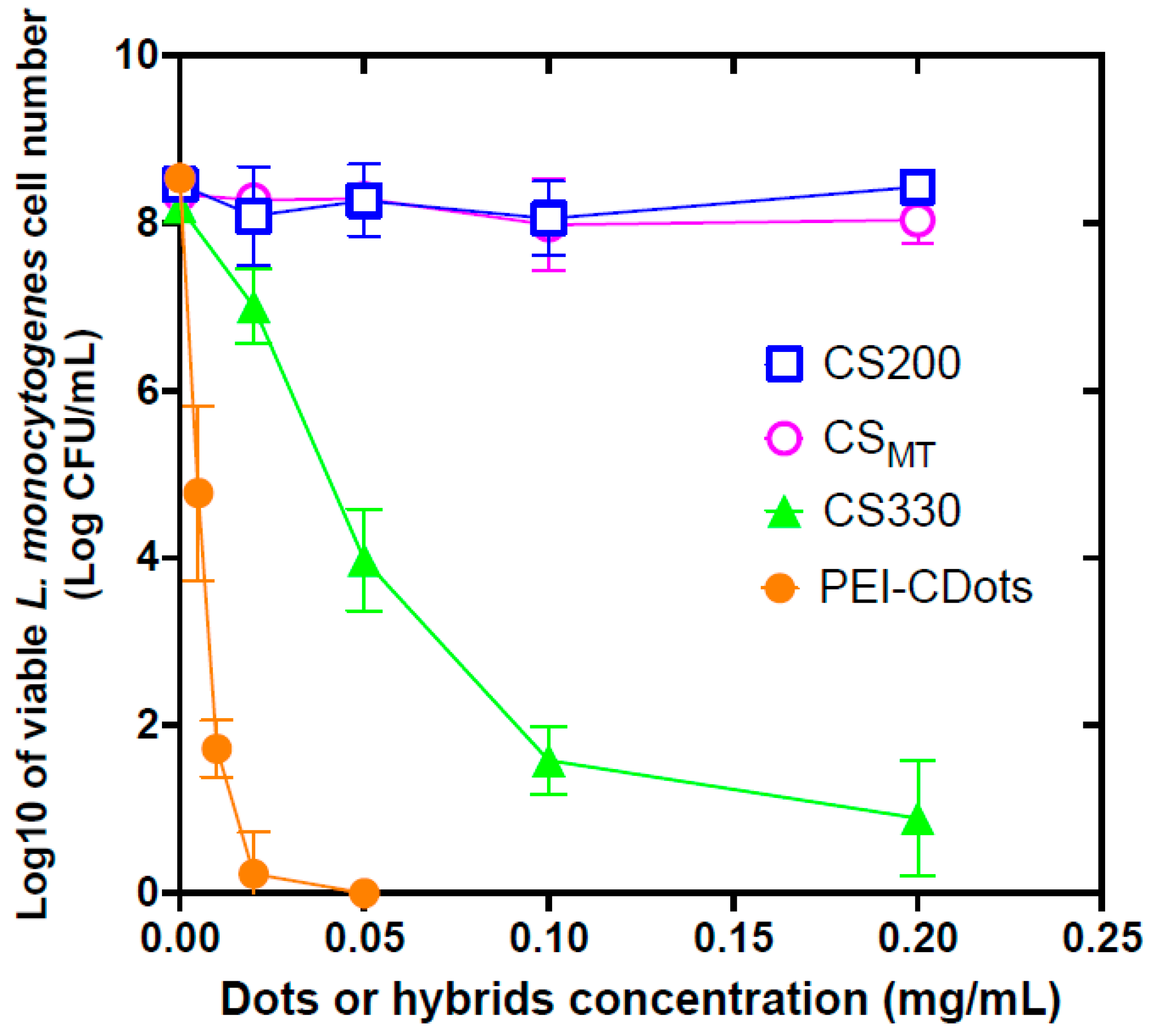
2.7. Antibacterial Evaluations
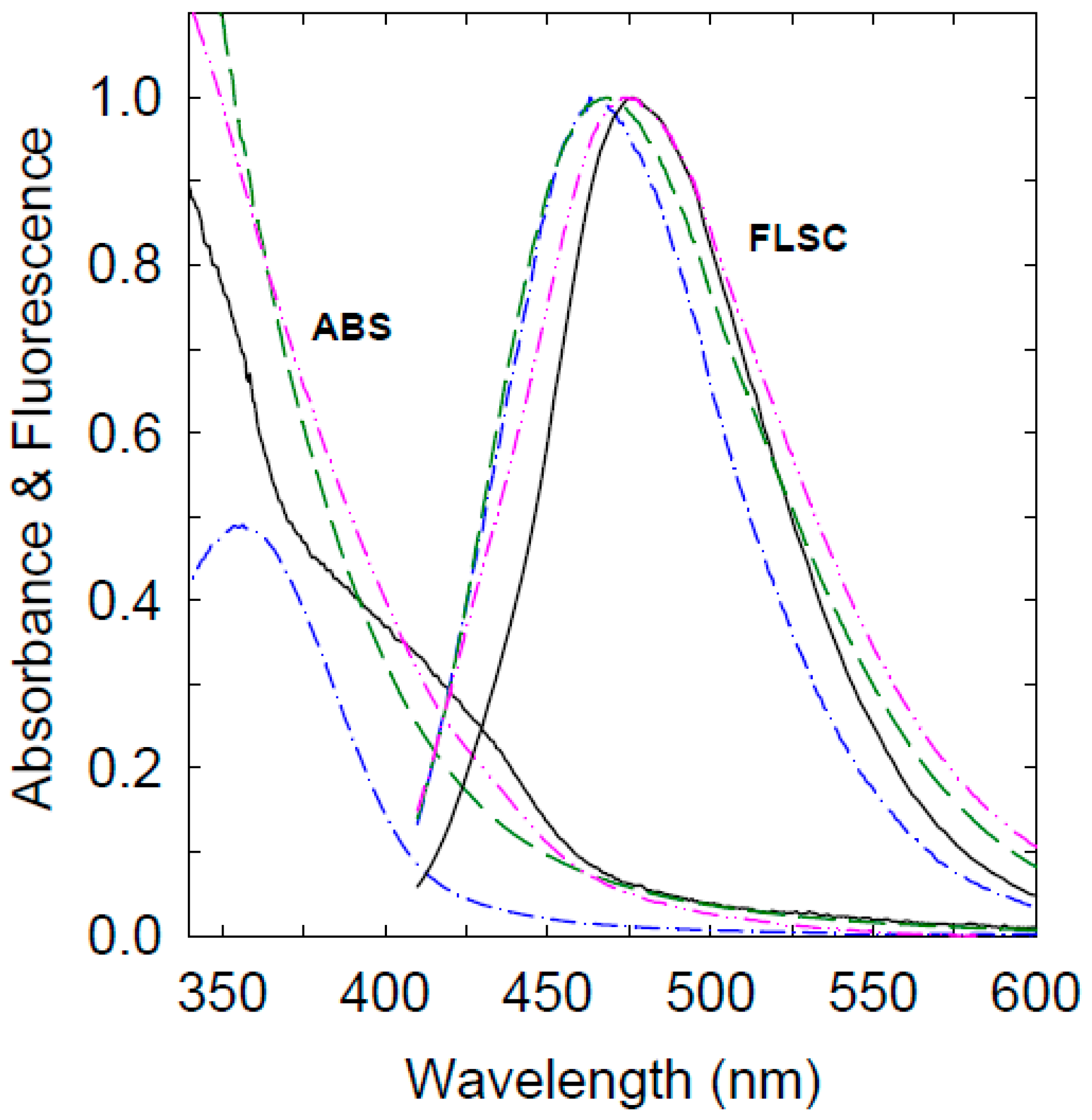
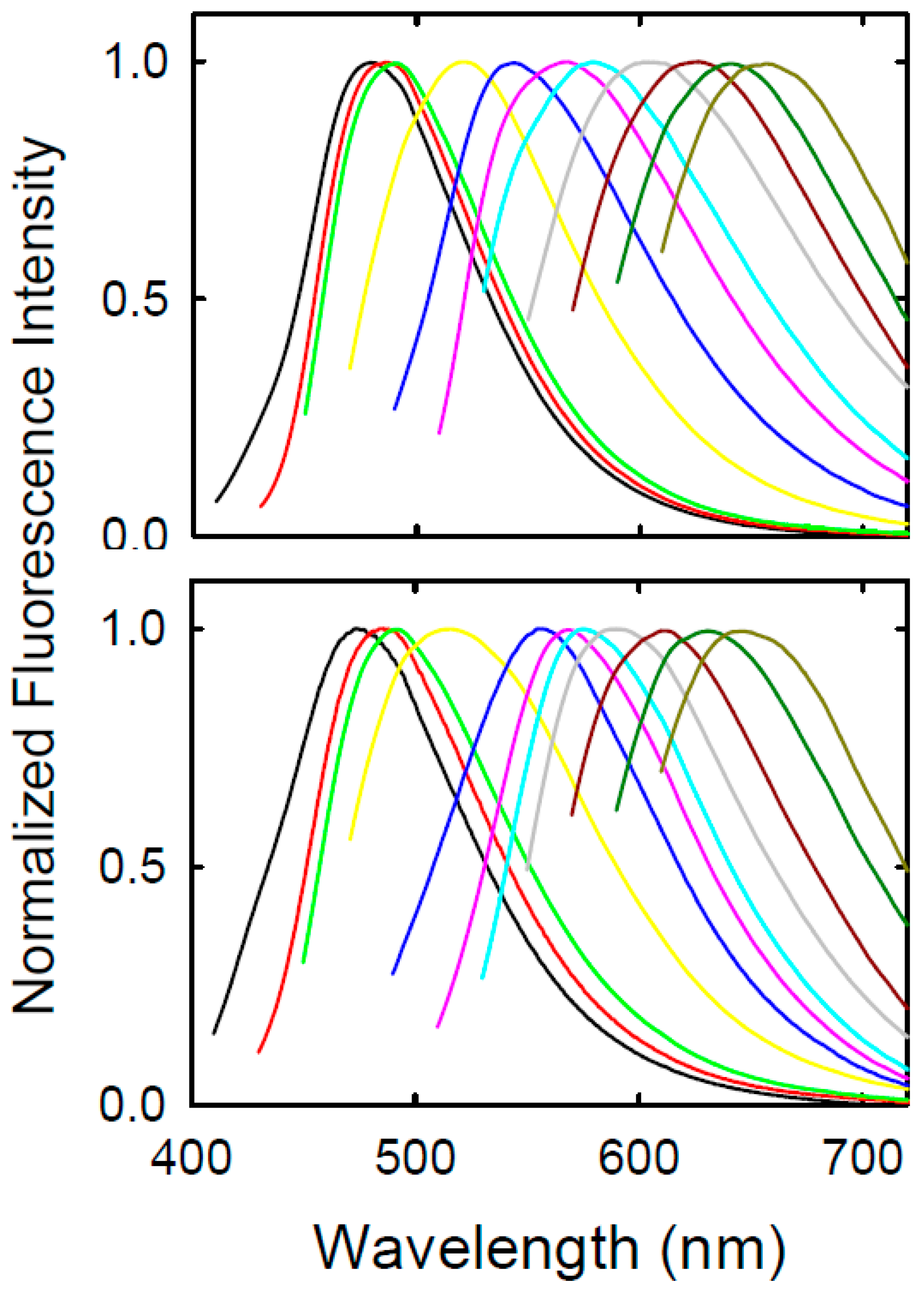
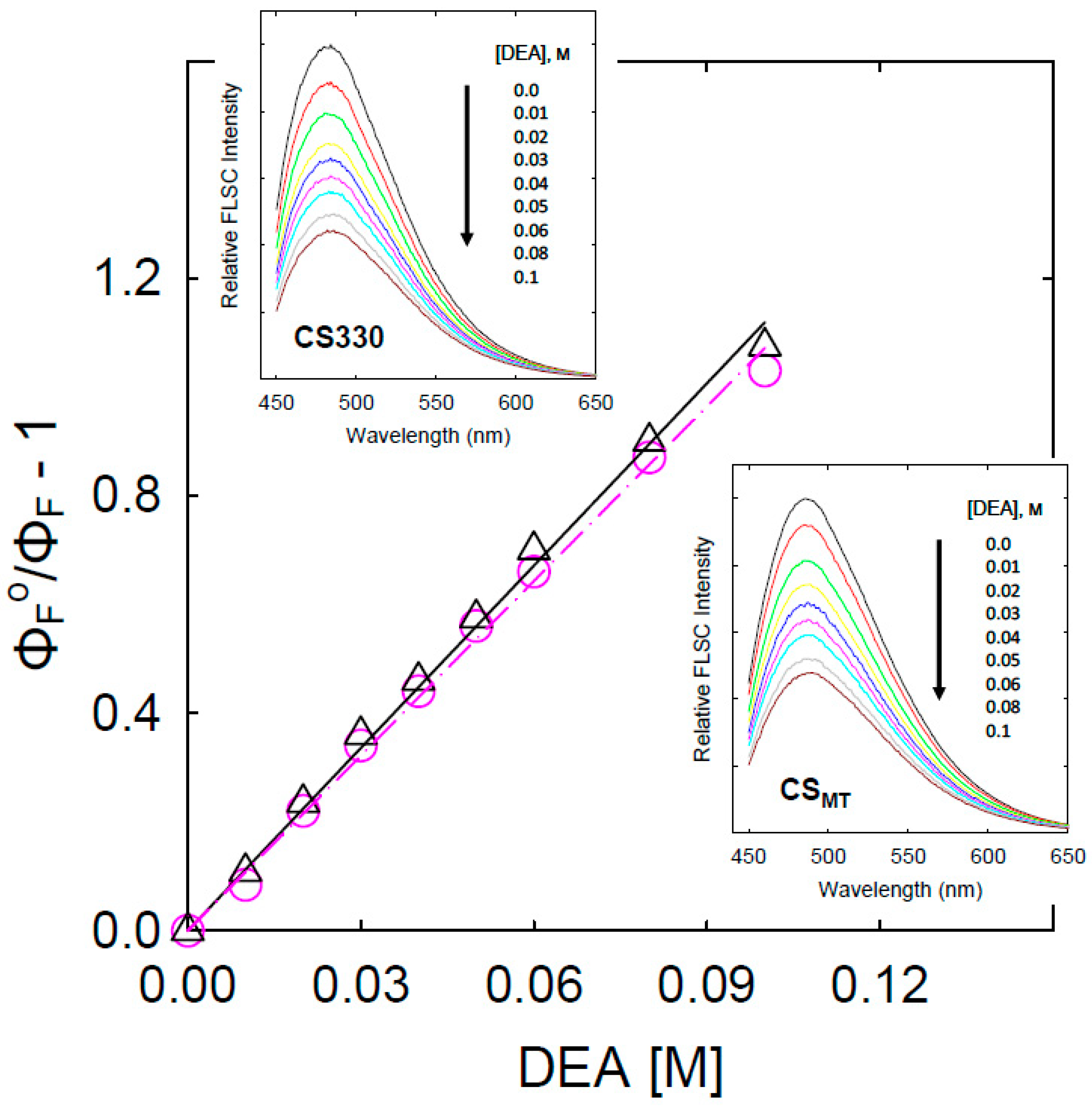
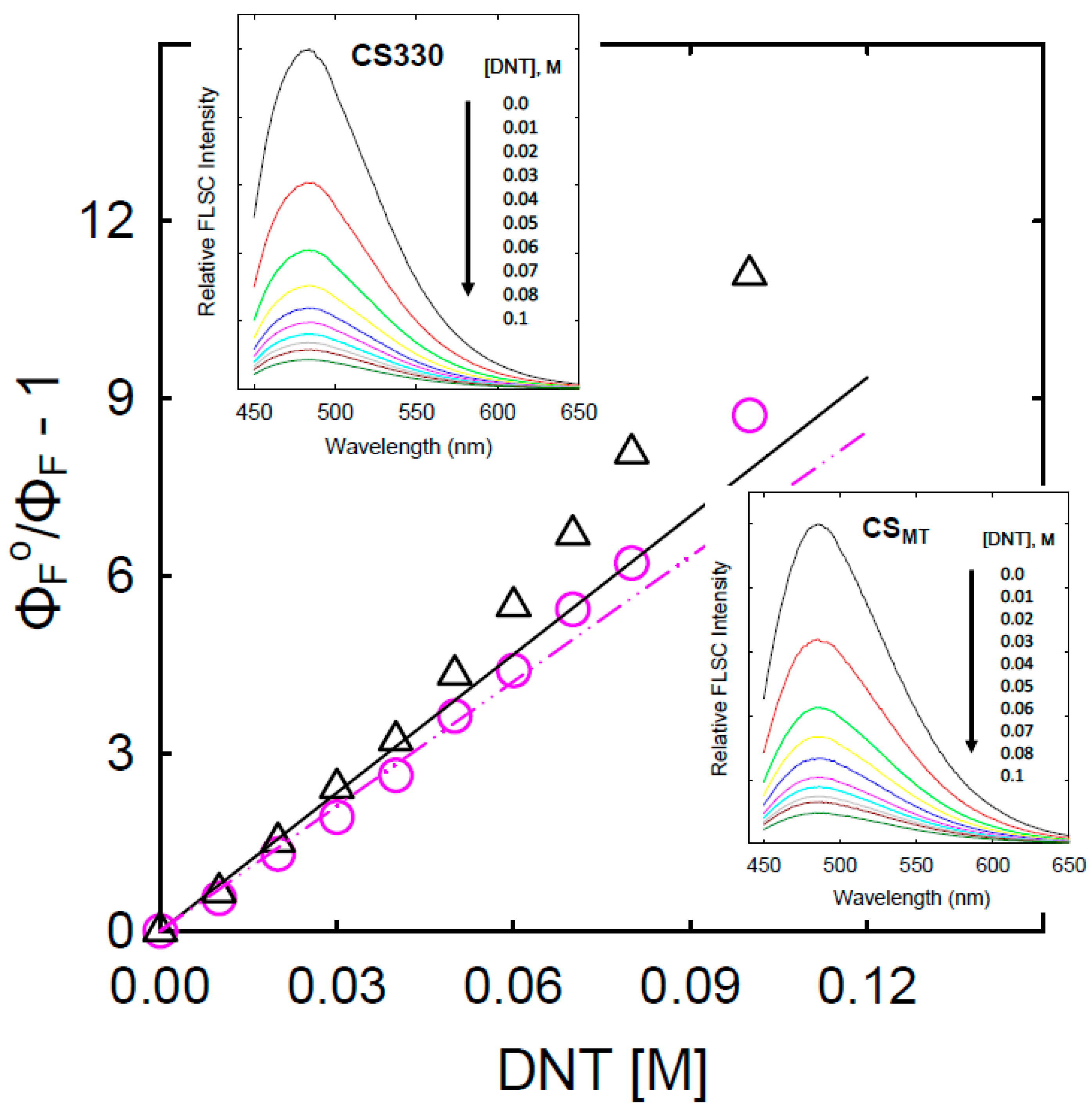
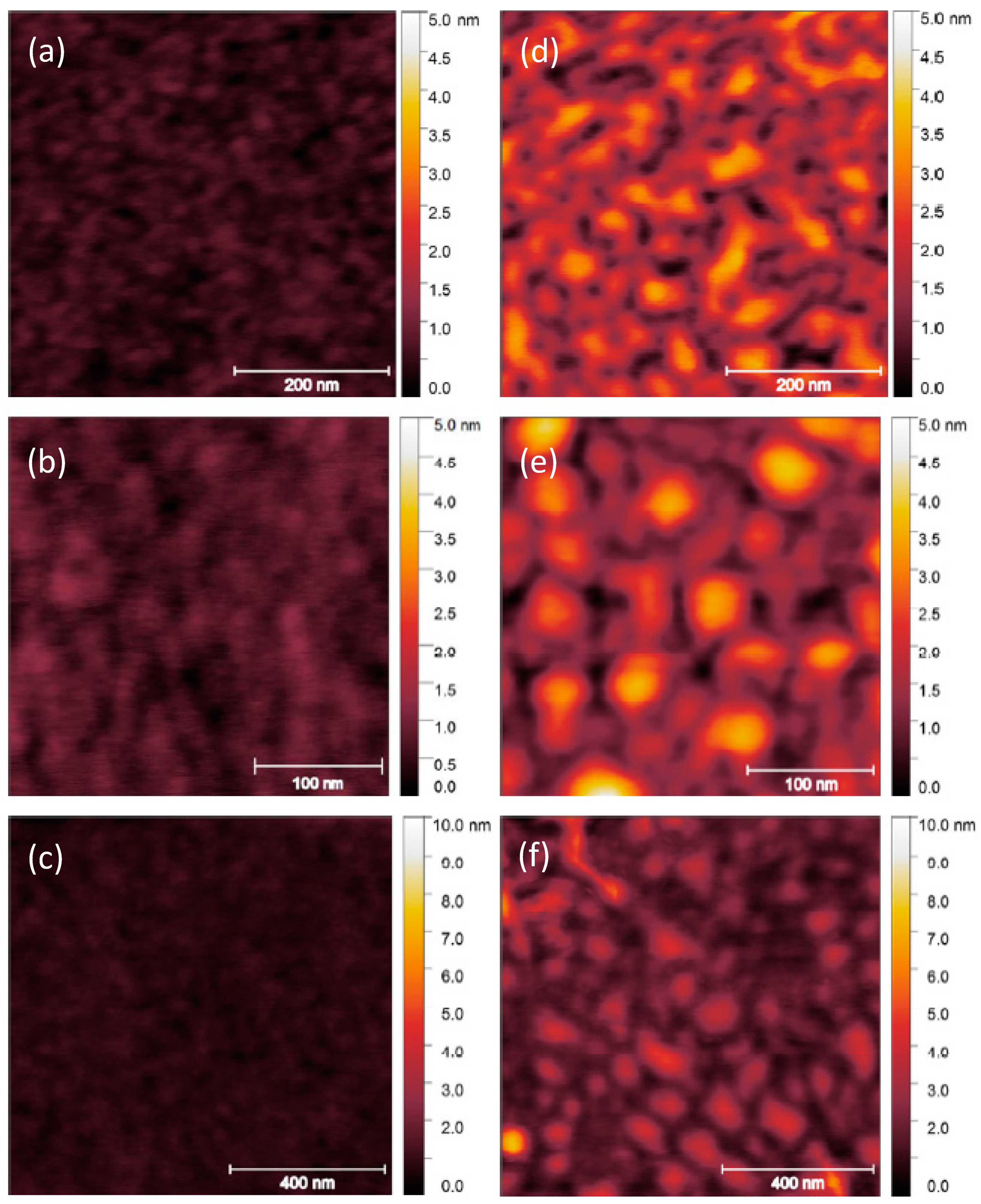
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.-P. Carbon Dots—Exploring Carbon at Zero-Dimension; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P. Fluorescent Carbon Nanoparticles. U.S. Patent 7,829,772, 9 November 2010. [Google Scholar]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.-P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2013, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.G.; Sahu, S.; Yang, S.-T.; Sonkar, S.K.; Wang, J.; Wang, H.; LeCroy, G.E.; Cao, L.; Sun, Y.-P. Carbon “Quantum” Dots for Optical Bioimaging. J. Mater. Chem. B 2013, 1, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, A.; Tian, Y. Functional Surface Engineering of C-Dots for Fluorescent Biosensing and in Vivo Bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-Based Quantum Dots for Fluorescence Imaging of Cells and Tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon Quantum Dots and Applications in Photocatalytic Energy Conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Yang, S.-T.; Yang, F.; Liu, Y.; Fernando, K.A.S.; Bunker, C.E.; Hu, Y.; Luo, P.G.; Sun, Y.-P. Functionalized Carbon Nanoparticles: Syntheses and Applications in Optical Bioimaging and Energy Conversion. Coord. Chem. Rev. 2016, 320, 66–81. [Google Scholar] [CrossRef]
- Peng, Z.; Han, X.; Li, S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon Dots: Biomacromolecule Interaction, Bioimaging and Nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Hutton, G.A.M.; Martindale, B.C.M.; Reisner, E. Carbon Dots as Photosensitisers for Solar-Driven Catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Q.; Chang, H.-T. Recent Advances and Sensing Applications of Carbon Dots. Small Methods 2020, 4, 1900387. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon Dots for In Vivo Bioimaging and Theranostics. Small 2019, 15, 1805087. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. A Review on the Effects of Carbon Dots in Plant Systems. Mater. Chem. Front. 2020, 4, 437–448. [Google Scholar] [CrossRef]
- Indriyati; Primadona, I.; Permatasari, F.A.A.; Irham, M.A.; Nasir, D.E.M.; Iskandar, F. Recent Advances and Rational Design Strategies of Carbon Dots towards Highly Efficient Solar Evaporation. Nanoscale 2021, 13, 7523–7532. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; Cacioppo, M.; Prato, M.A. Multifunctional Chemical Toolbox to Engineer Carbon Dots for Biomedical and Energy Applications. Nat. Nanotech. 2022, 17, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Wang, P.; Yang, L.; Quimby, J.L.; Sun, Y.-P. Carbon “Quantum” Dots for Bioapplications. Exp. Bio. Med. 2022, 247, 300–309. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.; Chi, Y.; Lin, X.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots for Chemical Sensing. Carbon 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Dong, Y.; Chi, Y.; Chen, G. Carbon Quantum Dot-Functionalized Aerogels for NO2 Gas Sensing. Anal. Chem. 2013, 85, 8065–8069. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Tian, W.; Chi, Y.; Chen, G. “Turn-on” Fluorescent Detection of Cyanide Based on Polyamine-Functionalized Carbon Quantum Dots. RSC Adv. 2014, 4, 3685–3689. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Luo, H.; Gao, Y. One-Step Preparation of Nitrogen-Doped and Surface-Passivated Carbon Quantum Dots with High Quantum Yield and Excellent Optical Properties. RSC Adv. 2014, 4, 7648. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Zhang, C. Polyethyleneimine-Functionalized Fluorescent Carbon Dots: Water Stability, PH Sensing, and Cellular Imaging. ChemNanoMat 2015, 1, 122–127. [Google Scholar] [CrossRef]
- Pierrat, P.; Wang, R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichler, A.; Pons, F.; Lebeau, L. Efficient in Vitro and in Vivo Pulmonary Delivery of Nucleic Acid by Carbon Dot-Based Nanocarriers. Biomaterials 2015, 51, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hu, Y.; Wang, P.; Yang, L.; Al Awak, M.M.; Tang, Y.; Twara, F.K.; Qian, H.; Sun, Y.-P. Modified Facile Synthesis for Quantitatively Fluorescent Carbon Dots. Carbon 2017, 122, 389–394. [Google Scholar] [CrossRef]
- Wang, P.; Meziani, M.J.; Fu, Y.; Bunker, C.E.; Hou, X.; Yang, L.; Msellek, H.; Zaharias, M.; Darby, J.P.; Sun, Y.-P. Carbon Dots versus Nano-Carbon/Organic Hybrids—Dramatically Different Behaviors in Fluorescence Sensing of Metal Cations with Structural and Mechanistic Implications. Nanoscale Adv. 2021, 3, 2316–2324. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, A.; Ghoshal, S.; Jain, S.; Hazra, M.K.; Nandi, C.K. Small Molecular Organic Nanocrystals Resemble Carbon Nanodots in Terms of Their Properties. Chem. Sci. 2018, 9, 175–180. [Google Scholar] [CrossRef]
- Hinterberger, V.; Damm, C.; Haines, P.; Guldi, D.M.; Peukert, W. Purification and Structural Elucidation of Carbon Dots by Column Chromatography. Nanoscale 2019, 11, 8464–8474. [Google Scholar] [CrossRef]
- Liang, W.; Ge, L.; Hou, X.; Ren, X.; Yang, L.; Bunker, C.E.; Overton, C.M.; Wang, P.; Sun, Y.-P. Evaluation of Commercial “Carbon Quantum Dots” Sample on Origins of Red Absorption and Emission Features. C J. Carbon Res. 2019, 5, 70. [Google Scholar] [CrossRef]
- Liang, W.; Wang, P.; Meziani, M.J.; Ge, L.; Yang, L.; Patel, A.K.; Morgan, S.O.; Sun, Y.-P. On the Myth of “Red/Near-IR Carbon Quantum Dots” from Thermal Processing of Specific Colorless Organic Precursors. Nanoscale Adv. 2021, 3, 4186–4195. [Google Scholar] [CrossRef]
- Liang, W.; Wang, P.; Yang, L.; Overton, C.M.; Hewitt, B.; Sun, Y.-P. Chemical Reactions in Thermal Carbonization Processing of Citric Acid—Urea Mixtures. Gen. Chem. 2021, 7, 210011–210017. [Google Scholar]
- Bartolomei, B.; Bogo, A.; Amato, F.; Ragazzon, G.; Prato, M. Nuclear Magnetic Resonance Reveals Molecular Species in Carbon Nanodot Samples Disclosing Flaws. Angew. Chem. Int. Ed. 2022, 61, e202200038. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Al Awak, M.M.; Yang, F.; Yan, S.; Xiong, Q.; Wang, P.; Tang, Y.; Yang, L.; LeCroy, G.E.; Bunker, C.E.; et al. Photoexcited State Properties of Carbon Dots from Thermally Induced Functionalization of Carbon Nanoparticles. J. Mater. Chem. C. 2016, 4, 10554–10561. [Google Scholar] [CrossRef]
- Ge, L.; Pan, N.; Jin, J.; Wang, P.; LeCroy, G.E.; Liang, W.; Yang, L.; Teisl, L.R.; Tang, Y.; Sun, Y.-P. Systematic Comparison of Carbon Dots from Different Preparations—Consistent Optical Properties and Photoinduced Redox Characteristics in Visible Spectrum, and Structural and Mechanistic Implications. J. Phys. Chem. C 2018, 122, 21667–21676. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; LeCroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L.; et al. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Abu Rabe, D.I.; Mohammed, O.O.; Dong, X.; Patel, A.K.; Overton, C.M.; Tang, Y.; Kathariou, S.; Sun, Y.-P.; Yang, L. Carbon Dots for Highly Effective Photodynamic Inactivation of Multidrug-Resistant Bacteria. Mater. Adv. 2020, 1, 321–325. [Google Scholar] [CrossRef]
- Dong, X.; Ge, L.; Abu Rabe, D.I.; Mohammed, O.O.; Wang, P.; Tang, Y.; Kathariou, S.; Yang, L.; Sun, Y.-P. Photoexcited State Properties and Antibacterial Activities of Carbon Dots Relevant to Mechanistic Features and Implications. Carbon 2020, 170, 137–145. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Messina, F.; Sciortino, A.; Bunker, C.E.; Wang, P.; Fernando, K.A.S.; Sun, Y.-P. Characteristic Excitation Wavelength Dependence of Fluorescence Emissions in Carbon “Quantum” Dots. J. Phys. Chem. C 2017, 121, 28180–28186. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Fernando, K.A.S.; Bunker, C.E.; Wang, P.; Tomlinson, N.; Sun, Y.-P. Steady-State and Time-Resolved Fluorescence Studies on Interactions of Carbon “Quantum” Dots with Nitrotoluenes. Inorg. Chim. Acta 2017, 468, 300–307. [Google Scholar] [CrossRef]
- Kortan, A.R.; Hull, R.; Opila, R.L.; Bawendi, M.G.; Steigerwald, M.L.; Carroll, P.J.; Brus, L.E. Nucleation and Growth of CdSe on ZnS Quantum Crystallite Seeds, and Vice Versa, in Inverse Micelle Media. J. Am. Chem. Soc. 1990, 112, 1327–1332. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = sulfur, selenium, tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Cao, L.; Anilkumar, P.; Wang, X.; Liu, J.-H.; Sahu, S.; Meziani, M.J.; Myers, E.; Sun, Y.-P. Reverse Stern-Volmer Behavior for Luminescence Quenching in Carbon Nanoparticles. Can. J. Chem. 2011, 89, 104–109. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited Quantum Dots for Killing Multidrug-Resistant Bacteria. Nat. Mater. 2016, 15, 529–534. [Google Scholar] [CrossRef]
- Hara, K.; Holland, S.; Woo, J. Effects of Exogenous Reactive Oxygen Species Scavengers on the Survival of Escherichia coli B23 during Exposure to UV-A Radiation. J. Exp. Microbiol. Immunol. 2004, 12, 62–66. [Google Scholar]
- Ishiyama, K.; Nakamura, K.; Ikai, H.; Kanno, T.; Kohno, M.; Sasaki, K.; Niwano, Y. Bactericidal Action of Photogenerated Singlet Oxygen from Photosensitizers Used in Plaque Disclosing Agents. PLoS ONE 2012, 7, e37871. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adcock, A.F.; Wang, P.; Cao, E.Y.; Ge, L.; Tang, Y.; Ferguson, I.S.; Abu Sweilem, F.S.; Petta, L.; Cannon, W.; Yang, L.; et al. Carbon Dots versus Nano-Carbon/Organic Hybrids—Divergence between Optical Properties and Photoinduced Antimicrobial Activities. C 2022, 8, 54. https://doi.org/10.3390/c8040054
Adcock AF, Wang P, Cao EY, Ge L, Tang Y, Ferguson IS, Abu Sweilem FS, Petta L, Cannon W, Yang L, et al. Carbon Dots versus Nano-Carbon/Organic Hybrids—Divergence between Optical Properties and Photoinduced Antimicrobial Activities. C. 2022; 8(4):54. https://doi.org/10.3390/c8040054
Chicago/Turabian StyleAdcock, Audrey F., Ping Wang, Elton Y. Cao, Lin Ge, Yongan Tang, Isaiah S. Ferguson, Fares S. Abu Sweilem, Lauren Petta, William Cannon, Liju Yang, and et al. 2022. "Carbon Dots versus Nano-Carbon/Organic Hybrids—Divergence between Optical Properties and Photoinduced Antimicrobial Activities" C 8, no. 4: 54. https://doi.org/10.3390/c8040054
APA StyleAdcock, A. F., Wang, P., Cao, E. Y., Ge, L., Tang, Y., Ferguson, I. S., Abu Sweilem, F. S., Petta, L., Cannon, W., Yang, L., Bunker, C. E., & Sun, Y.-P. (2022). Carbon Dots versus Nano-Carbon/Organic Hybrids—Divergence between Optical Properties and Photoinduced Antimicrobial Activities. C, 8(4), 54. https://doi.org/10.3390/c8040054








