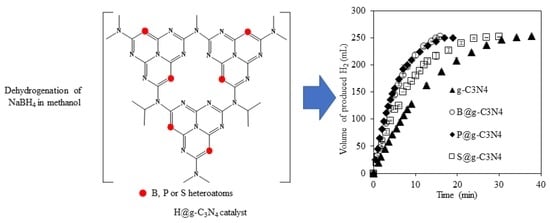
Use of Heteroatom-Doped g-C3N4 Particles as Catalysts for Dehydrogenation of Sodium Borohydride in Methanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
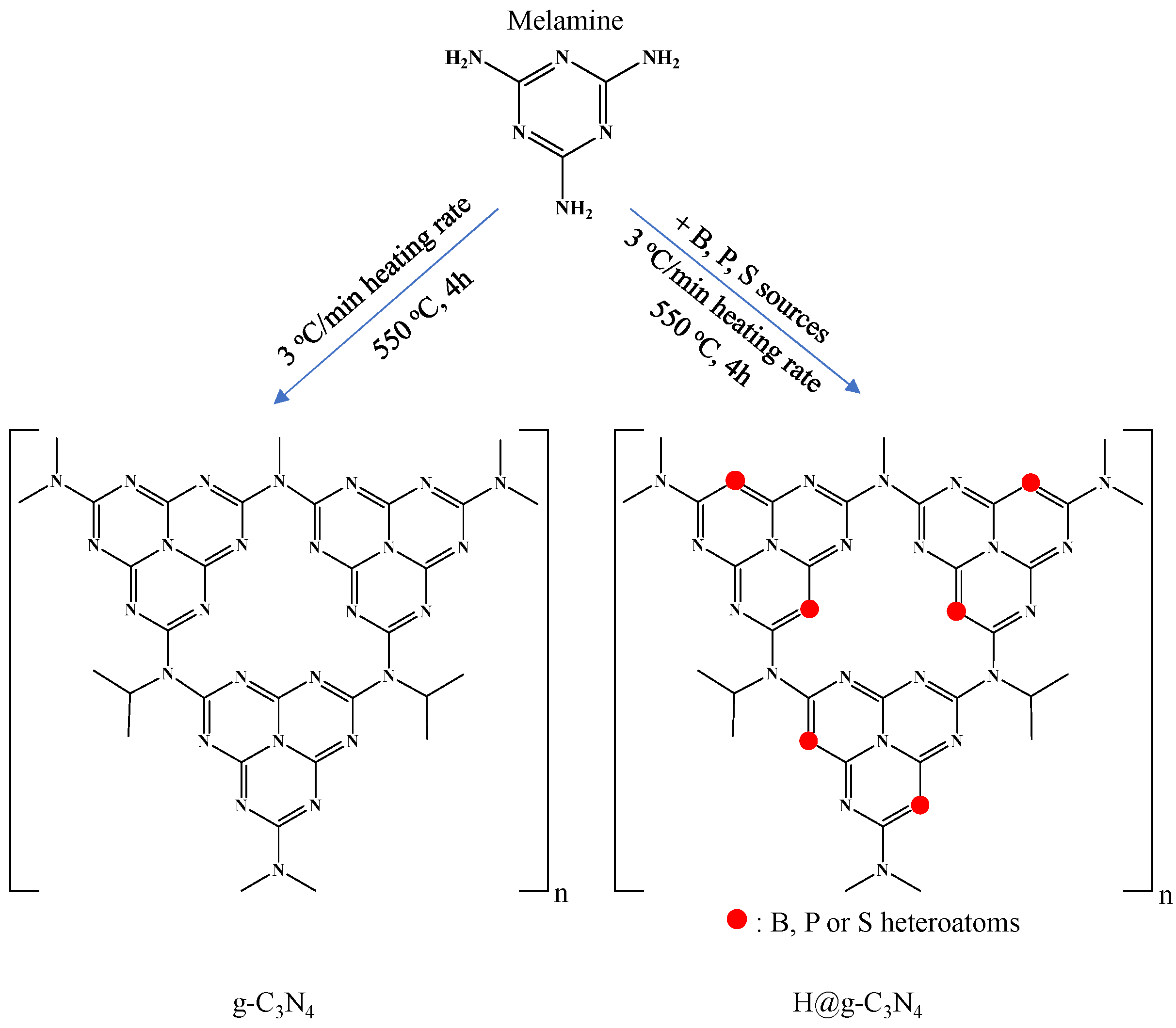
2.2. Synthesis of g-C3N4 and Heteroatom-Doped g-C3N4 (H@g-C3N4)
2.3. Instruments
2.4. Catalytic Activity of H@g-C3N4 on Dehydrogenation of NaBH4 in Methanol
2.5. Calculation of Activation Parameters
2.6. Reusability of g-C3N4-Based Structures on Dehydrogenation of NaBH4 in Methanol
3. Results and Discussion
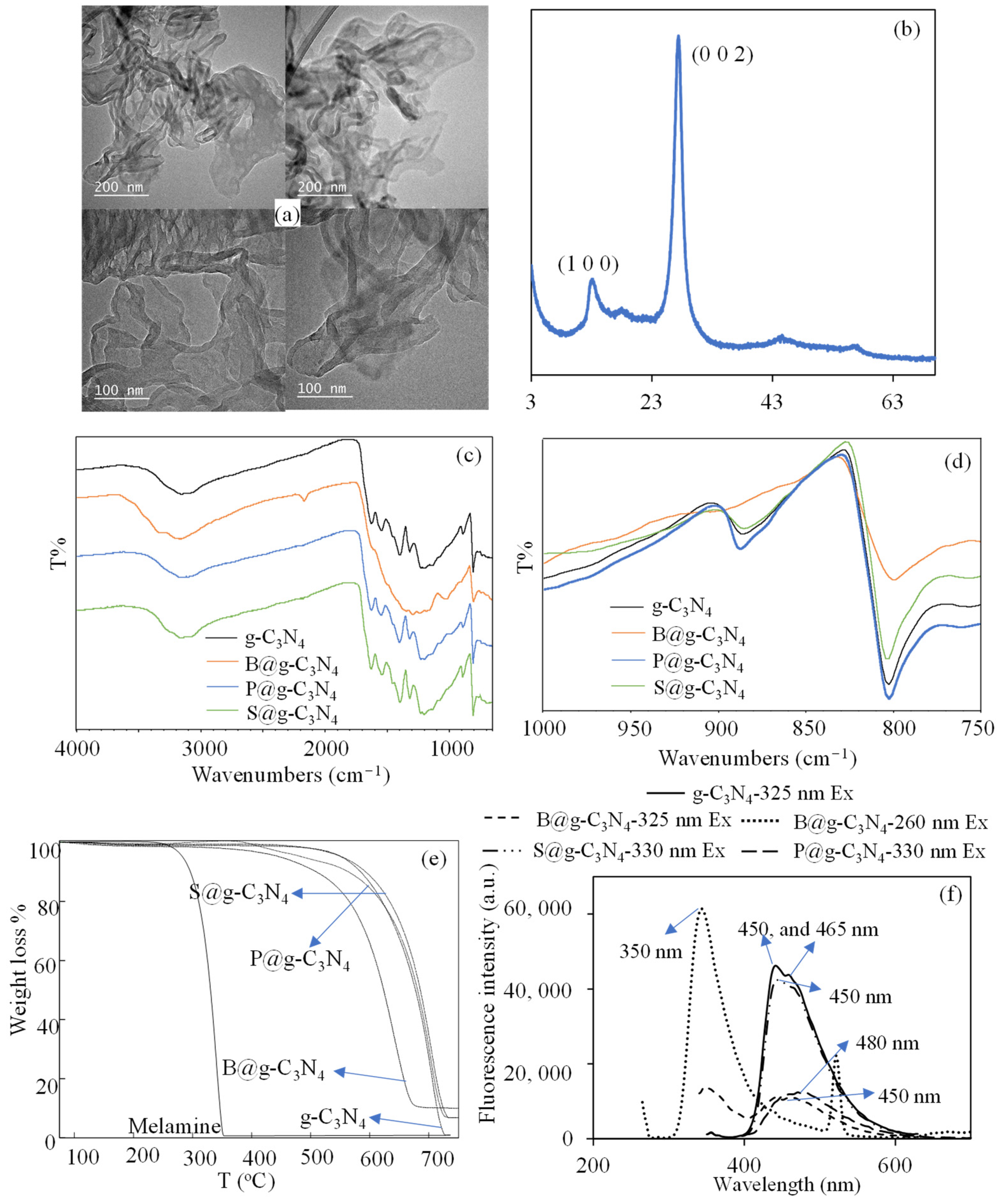
3.1. Synthesis and Characterization of H@g-C3N4
3.2. The Usage of g-C3N4-Based Structures as a Catalyst on Dehydrogenation of NaBH4 in Methanol
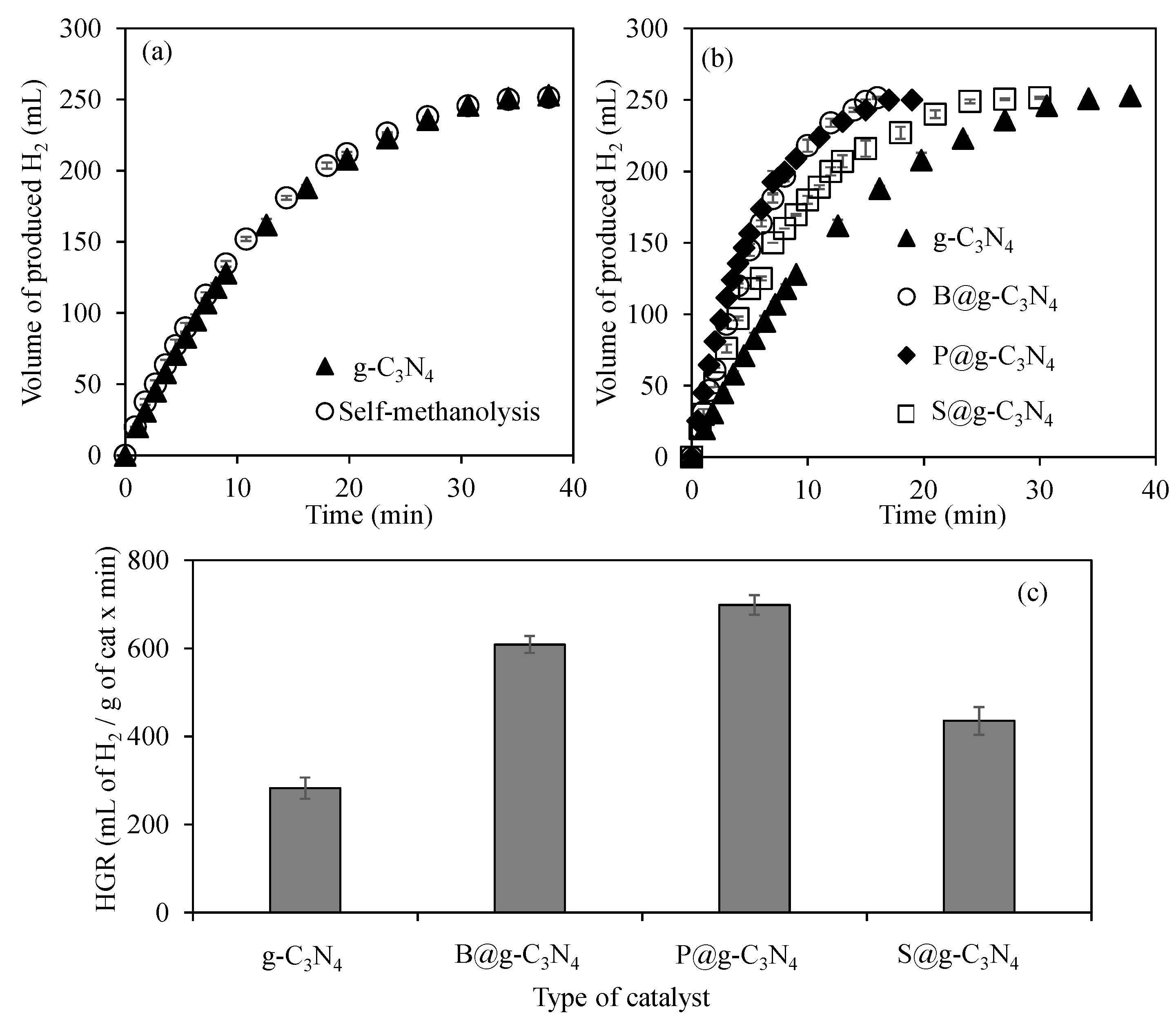
3.2.1. The Effect of Heteroatom Doping on the Catalytic Activity of g-C3N4
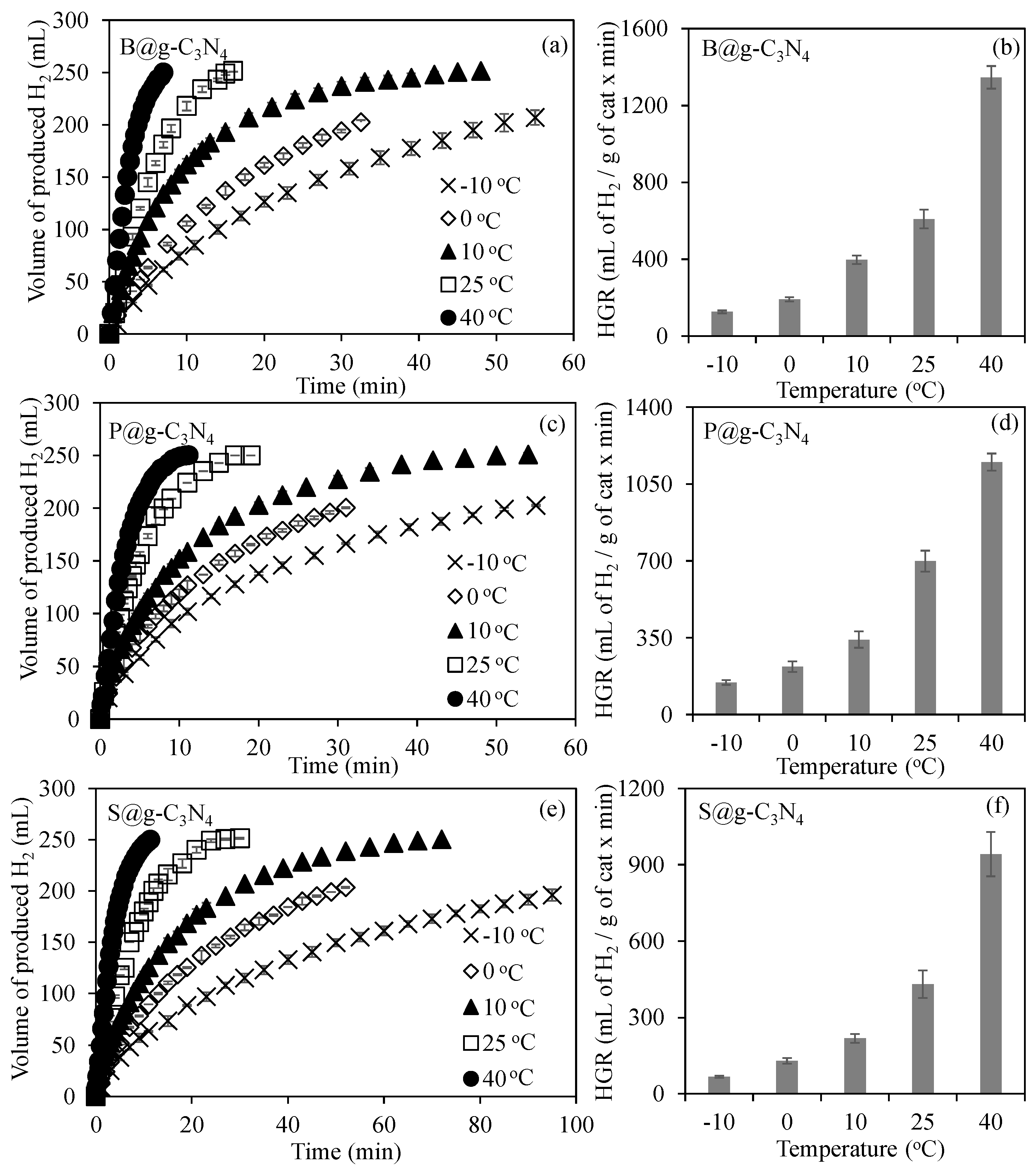
3.2.2. The Effect of Temperature on g-C3N4-Based-Structures-Catalyzed Dehydrogenation of NaBH4 in Methanol
3.3. Comparison of Activation Parameters for g-C3N4-Based-Structures-Catalyzed Reaction
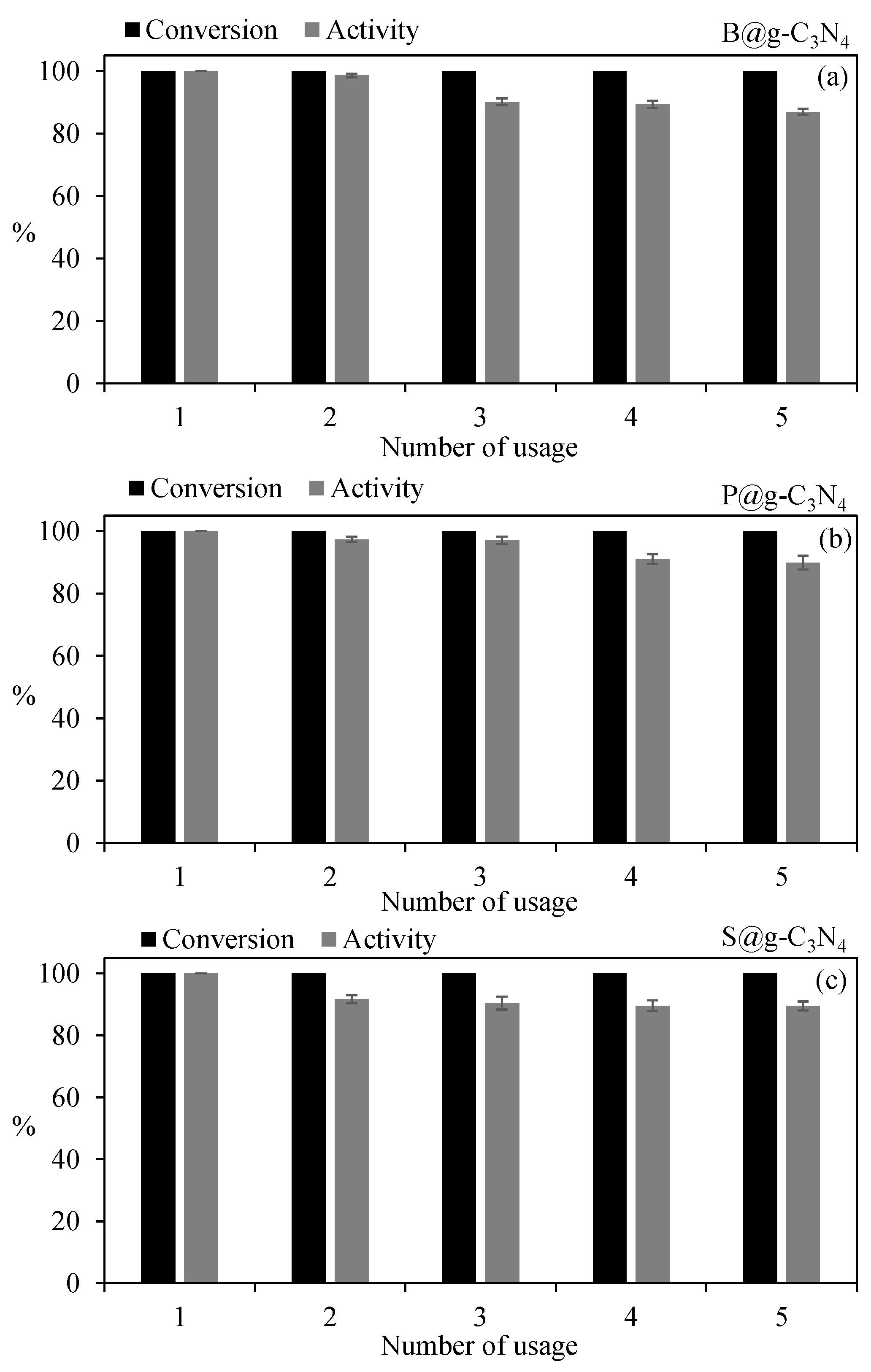
3.4. Reusabilities of g-C3N4-Based Catalysts on Dehydrogenation of NaBH4 in Methanol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid State Mater. Sci. 2021, 46, 189–217. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.; Nie, R. Synthesis and biomedical applications of graphitic carbon nitride quantum dots. J. Mater. Chem. B 2019, 7, 5432–5448. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Sun, Q.; Jena, P. Functionalized Graphitic Carbon Nitride for Efficient Energy Storage. J. Phys. Chem. C 2013, 117, 6055–6059. [Google Scholar] [CrossRef]
- Luo, Y.; Yan, Y.; Zheng, S.; Xue, H.; Pang, H. Graphitic carbon nitride based materials for electrochemical energy storage. J. Mater. Chem. A 2019, 7, 901–924. [Google Scholar] [CrossRef]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, D.; Wang, F.; Chen, M. Element-doped graphitic carbon nitride: Confirmation of doped elements and applications. Nanoscale Adv. 2021, 3, 4370–4387. [Google Scholar] [CrossRef] [PubMed]
- Starukh, H.; Praus, P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts 2020, 10, 1119. [Google Scholar] [CrossRef]
- Saka, C. Surface modification with oxygen doping of g-C3N4 nanoparticles by carbon vacancy for efficient dehydrogenation of sodium borohydride in methanol. Fuel 2022, 310, 122444. [Google Scholar] [CrossRef]
- Saka, C. Facile fabrication of P-doped g-C3N4 particles with nitrogen vacancies for efficient dehydrogenation of sodium borohydride methanolysis. Fuel 2022, 313, 122688. [Google Scholar] [CrossRef]
- Ramya, K.; Dhathathreyan, K.; Sreenivas, J.; Kumar, S.; Narasimhan, S. Hydrogen production by alcoholysis of sodium borohydride. Int. J. Energy Res. 2013, 37, 1889–1895. [Google Scholar] [CrossRef]
- Lo, C.-T.F.; Karan, K.; Davis, B.R. Kinetic Studies of Reaction between Sodium Borohydride and Methanol, Water, and Their Mixtures. Ind. Eng. Chem. Res. 2007, 46, 5478–5484. [Google Scholar] [CrossRef]
- Filiz, B.C.; Figen, A.K. Insight into the role of solvents in enhancing hydrogen production: Ru-Co nanoparticles catalyzed sodium borohydride dehydrogenation. Int. J. Hydrogn Energy 2019, 44, 28471–28482. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Guo, Q. Research progress on catalysts for hydrogen generation through sodium borohydride alcoholysis. Int. J. Hydrogn Energy 2022, 47, 5929–5946. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, T.; Zhang, H.; Zhao, Y. Advances in catalysts for hydrogen production by methanolysis of sodium borohydride. Int. J. Hydrogn Energy 2022, 47, 14589–14610. [Google Scholar] [CrossRef]
- Hannauer, J.; Demirci, U.B.; Pastor, G.; Geantet, C.; Herrmann, J.M.; Miele, P. Hydrogen release through catalyzed methanolysis of solid sodium borohydride. Energy Environ. Sci. 2010, 3, 1796. [Google Scholar] [CrossRef]
- Ocon, J.D.; Tuan, T.N.; Yi, Y.; de Leon, R.L.; Lee, J.K.; Lee, J. Ultrafast and stable hydrogen generation from sodium borohydride in methanol and water over Fe–B nanoparticles. J. Power Sources 2013, 243, 444–450. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Wang, Y.; Zhu, H. The preparation and performance of a novel spherical spider web-like structure Ru–Ni / Ni foam catalyst for NaBH4 methanolysis. Int. J. Hydrogn Energy 2019, 44, 13185–13194. [Google Scholar] [CrossRef]
- Sahiner, N. Carbon spheres from lactose as green catalyst for fast hydrogen production via methanolysis. Int. J. Hydrogn Energy 2018, 43, 9687–9695. [Google Scholar] [CrossRef]
- Demirci, S.; Yildiz, M.; Inger, E.; Sahiner, N. Porous carbon particles as metal-free superior catalyst for hydrogen release from methanolysis of sodium borohydride. Renew. Energy 2020, 147, 69–76. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Asiri, A.M. Chitosan coated cellulose cotton fibers as catalyst for the H2 production from NaBH4 methanolysis. Int. J. Hydrogn Energy 2019, 44, 4143–4155. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Visible-Light-Induced Hydrogen and Oxygen Formation over Pt/Au/WO3 Photocatalyst Utilizing Two Types of Photoabsorption Due to Surface Plasmon Resonance and Band-Gap Excitation. J. Am. Chem. Soc. 2014, 136, 586–589. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Wu, M.; Yan, J.-M.; Zhang, X.-W.; Zhao, M. Synthesis of g-C3N4 with heating acetic acid treated melamine and its photocatalytic activity for hydrogen evolution. Appl. Surf. Sci. 2015, 354, 196–200. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, X.; Wang, X.; Cai, H.; He, P.; Ma, J.; Li, L.; Ding, Y.; Fan, X. Large-Scale Preparation of g-C3N4 Porous Nanotubes with Enhanced Photocatalytic Activity by Using Salicylic Acid and Melamine. Ind. Eng. Chem. Res. 2020, 59, 1065–1072. [Google Scholar] [CrossRef]
- Demirci, S.; Ari, B.; Şengel, S.B.; Inger, E.; Sahiner, N. Boric acid versus boron trioxide as catalysts for green energy source H2 production from sodium borohydride methanolysis. MANAS J. Eng. 2021, 9, 142–152. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A 2009, 94, 387–392. [Google Scholar] [CrossRef]
- Li, G.; Yang, N.; Wang, W.; Zhang, W.F. Synthesis, Photophysical and Photocatalytic Properties of N-Doped Sodium Niobate Sensitized by Carbon Nitride. J. Phys. Chem. C 2009, 113, 14829–14833. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, L.; Dai, P.; Ji, S. Hydrogen generation from methanolysis of sodium borohydride over Co/Al2O3 catalyst. J. Nat. Gas Chem. 2012, 21, 488–494. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Wang, Y.; Luo, Y.; Chen, Y.; Zhu, H. Co-P nanoparticles supported on dandelion-like CNTs-Ni foam composite carrier as a novel catalyst for hydrogen generation from NaBH4 methanolysis. Int. J. Hydrogn Energy 2018, 43, 8805–8814. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, Y.; Luo, Y.; Zhu, H. Highly dispersed RuCo bimetallic nanoparticles supported on carbon black: Enhanced catalytic activity for hydrogen generation from NaBH4 methanolysis. J. Mater. Sci. 2018, 53, 6831–6841. [Google Scholar] [CrossRef]
- Karakaş, D.E. A novel cost-effective catalyst from orange peel waste protonated with phosphoric acid for hydrogen generation from methanolysis of NaBH4. Int. J. Hydrogn Energy 2022, 47, 12231–12239. [Google Scholar] [CrossRef]
- Demirci, S.; Suner, S.S.; Yildiz, M.; Sahiner, N. Polymeric ionic liquid forms of PEI microgels as catalysts for hydrogen production via sodium borohydride methanolysis. J. Mol. Liq. 2022, 360, 119562. [Google Scholar] [CrossRef]
- Liu, S.; Xu, M.; Pang, C.; Lester, E.; Wu, T. Theoretical insights of catalytic oxidation of Hg0 on g-C3N4-supported Fe/Co/Ni-based bi-metallic catalysts using O2 in coal-fired flue gas as the oxidant. Fuel 2021, 306, 121593. [Google Scholar] [CrossRef]
- Koo, H.M.; Wang, X.; Kim, A.R.; Shin, C.-H.; Bae, J.W. Effects of self-reduction of Co nanoparticles on mesoporous graphitic carbon-nitride to CO hydrogenation activity to hydrocarbons. Fuel 2021, 287, 119437. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Li, O.L.; Kang, J. Novel synthesis of highly phosphorus-doped carbon as an ultrahigh-rate anode for sodium ion batteries. Carbon 2020, 168, 448–457. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresource Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef] [PubMed]
| Materials | Activation Parameters | Ref. | ||
|---|---|---|---|---|
| Ea (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol.K) | ||
| Self methanolysis | 52.9 | - | - | [36] |
| 62.9 | [18] | |||
| B@g-C3N4 | 31.2 | 28.2 | −189.1 | This study |
| P@g-C3N4 | 26.9 | 24.0 | −191.6 | |
| S@g-C3N4 | 31.2 | 28.2 | −191.5 | |
| O doped g-C3N4 | 36.1 | - | - | [15] |
| P doped g-C3N4 | 30.3 | - | - | [16] |
| Co-P/CNTs-Ni foam | 49.9 | - | - | [37] |
| Ru–Co/C | 36.8 | - | - | [38] |
| CS from lactose | 23.8 | 21.4 | −173 | [25] |
| Metal-free OP-H3PO4-Cat | 12.5 | - | - | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, S.; Sahiner, N. Use of Heteroatom-Doped g-C3N4 Particles as Catalysts for Dehydrogenation of Sodium Borohydride in Methanol. C 2022, 8, 53. https://doi.org/10.3390/c8040053
Demirci S, Sahiner N. Use of Heteroatom-Doped g-C3N4 Particles as Catalysts for Dehydrogenation of Sodium Borohydride in Methanol. C. 2022; 8(4):53. https://doi.org/10.3390/c8040053
Chicago/Turabian StyleDemirci, Sahin, and Nurettin Sahiner. 2022. "Use of Heteroatom-Doped g-C3N4 Particles as Catalysts for Dehydrogenation of Sodium Borohydride in Methanol" C 8, no. 4: 53. https://doi.org/10.3390/c8040053
APA StyleDemirci, S., & Sahiner, N. (2022). Use of Heteroatom-Doped g-C3N4 Particles as Catalysts for Dehydrogenation of Sodium Borohydride in Methanol. C, 8(4), 53. https://doi.org/10.3390/c8040053