Easy and Low-Cost Method for Synthesis of Carbon–Silica Composite from Vinasse and Study of Ibuprofen Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbon–silica Composite Synthesis
2.3. Materials Characterization
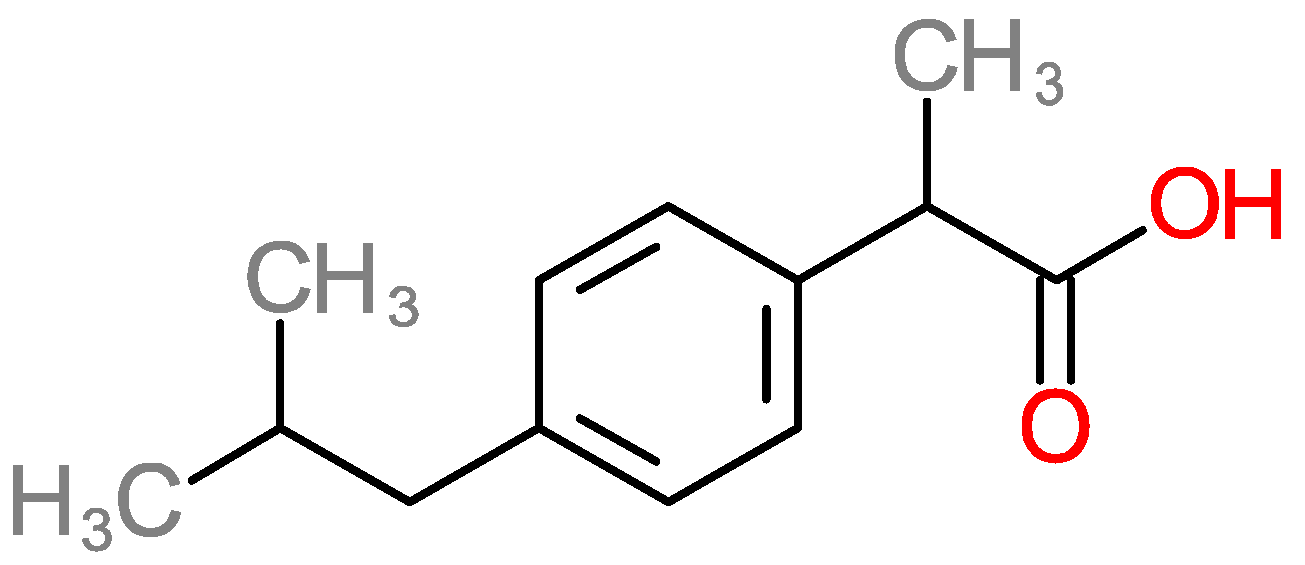
2.4. Adsorption of Ibuprofen
3. Results
3.1. Characterization of Carbon–silica Composite
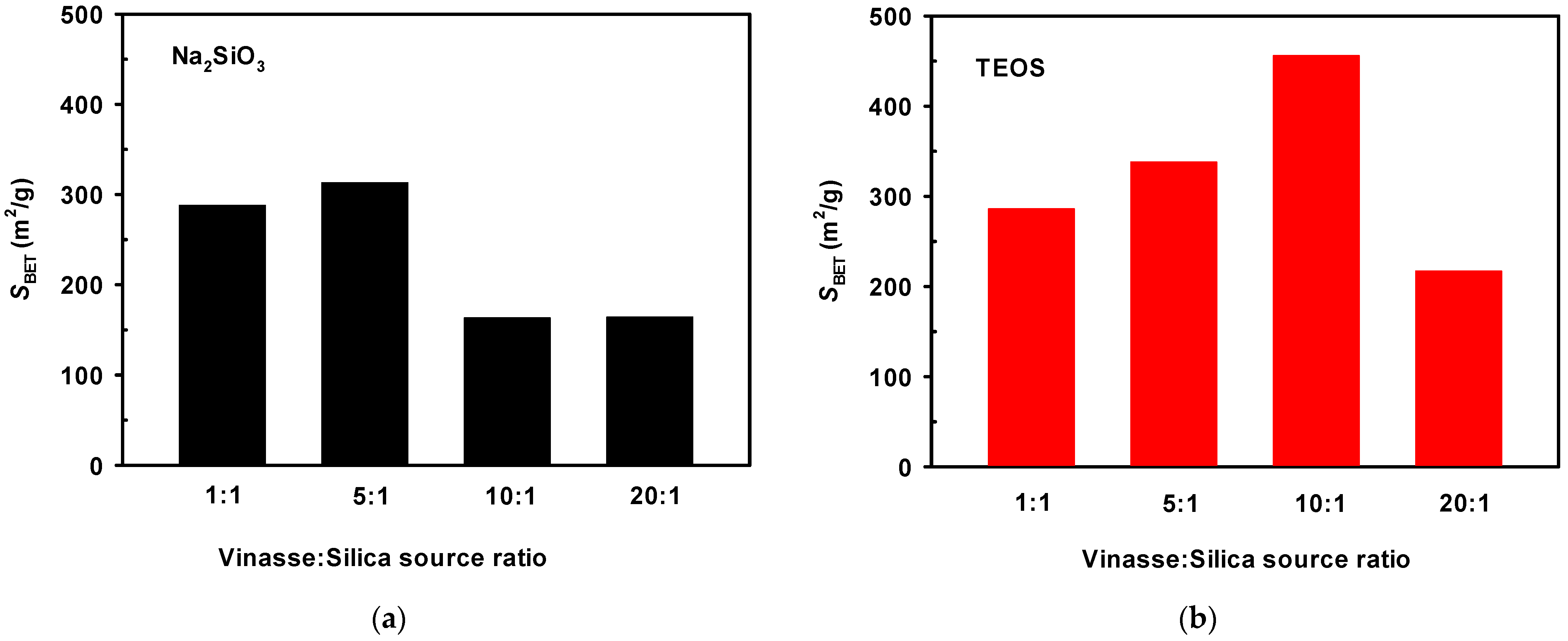
3.1.1. Specific Surface Area of Composites under Different Ratio
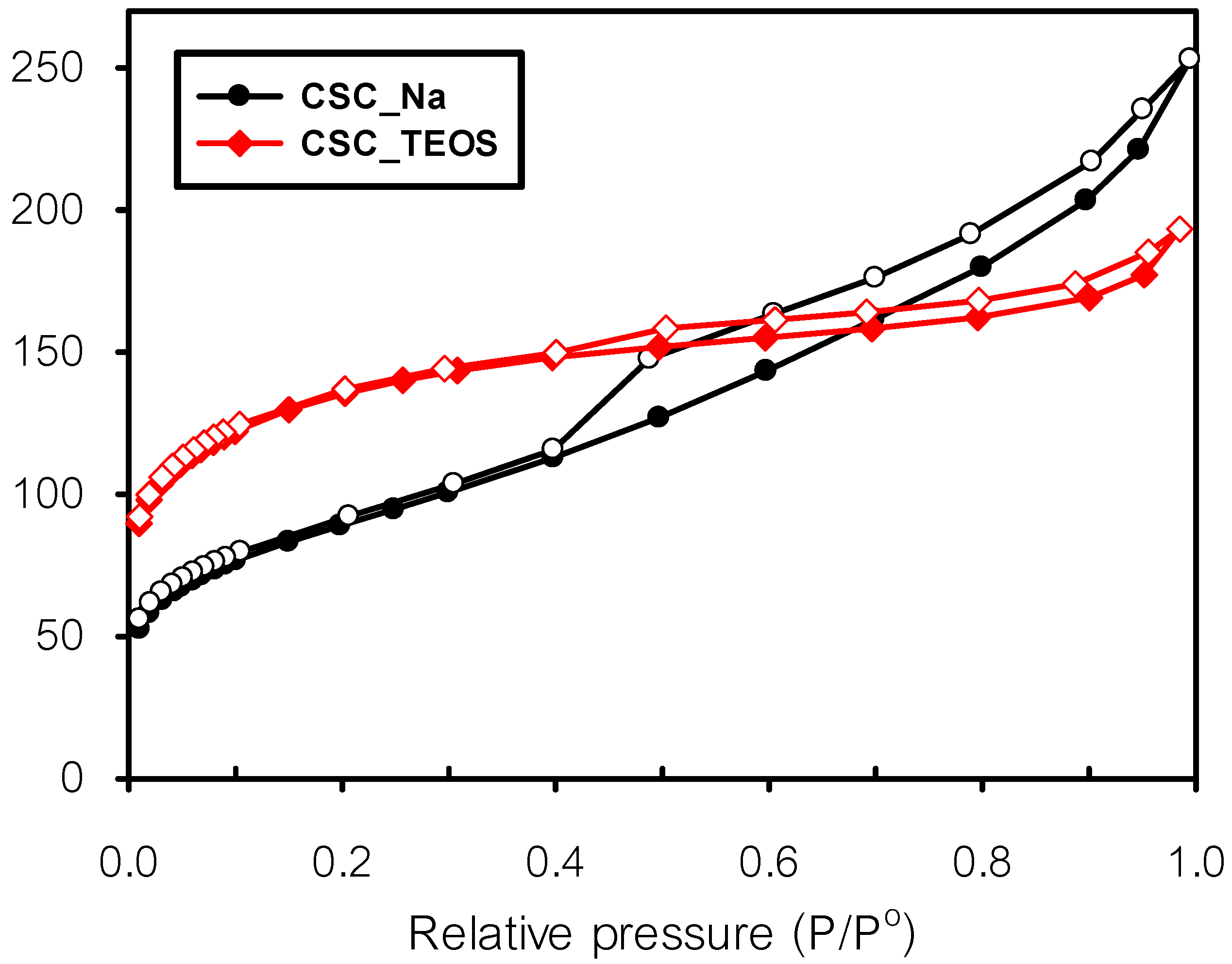
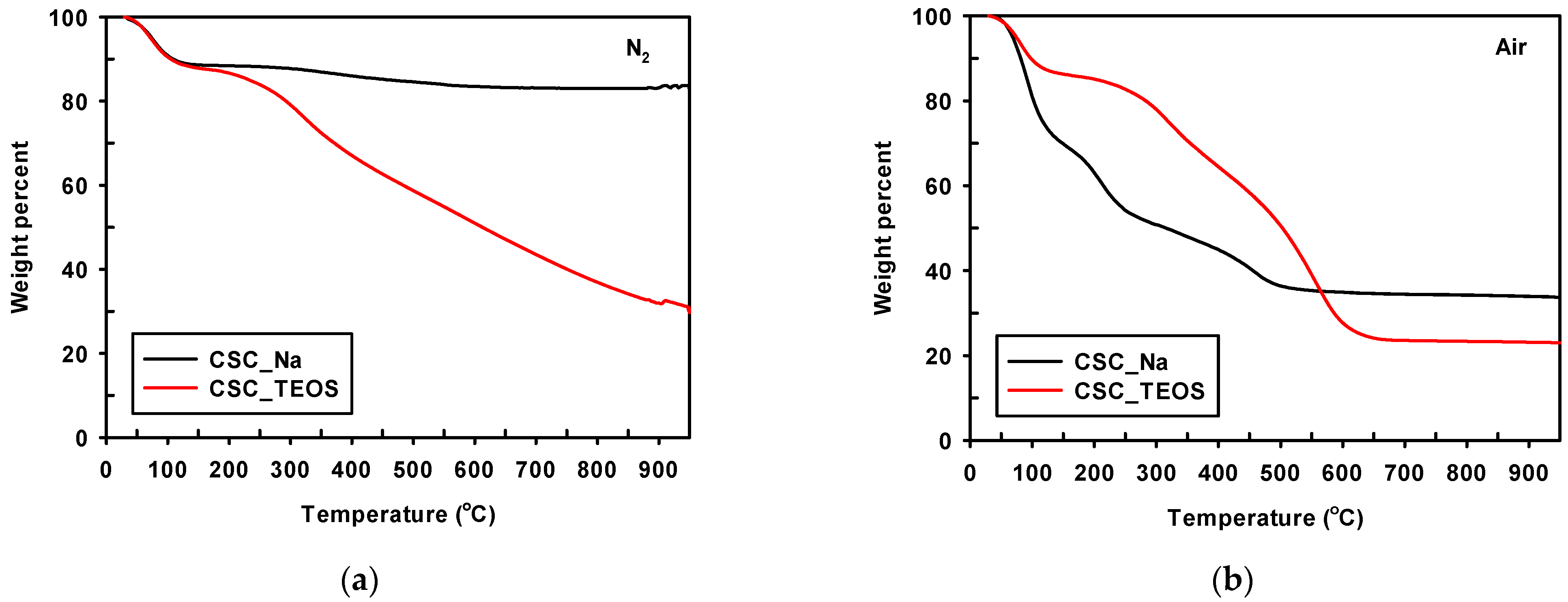
3.1.2. Physicochemical Properties of Composites under Optimum Conditions
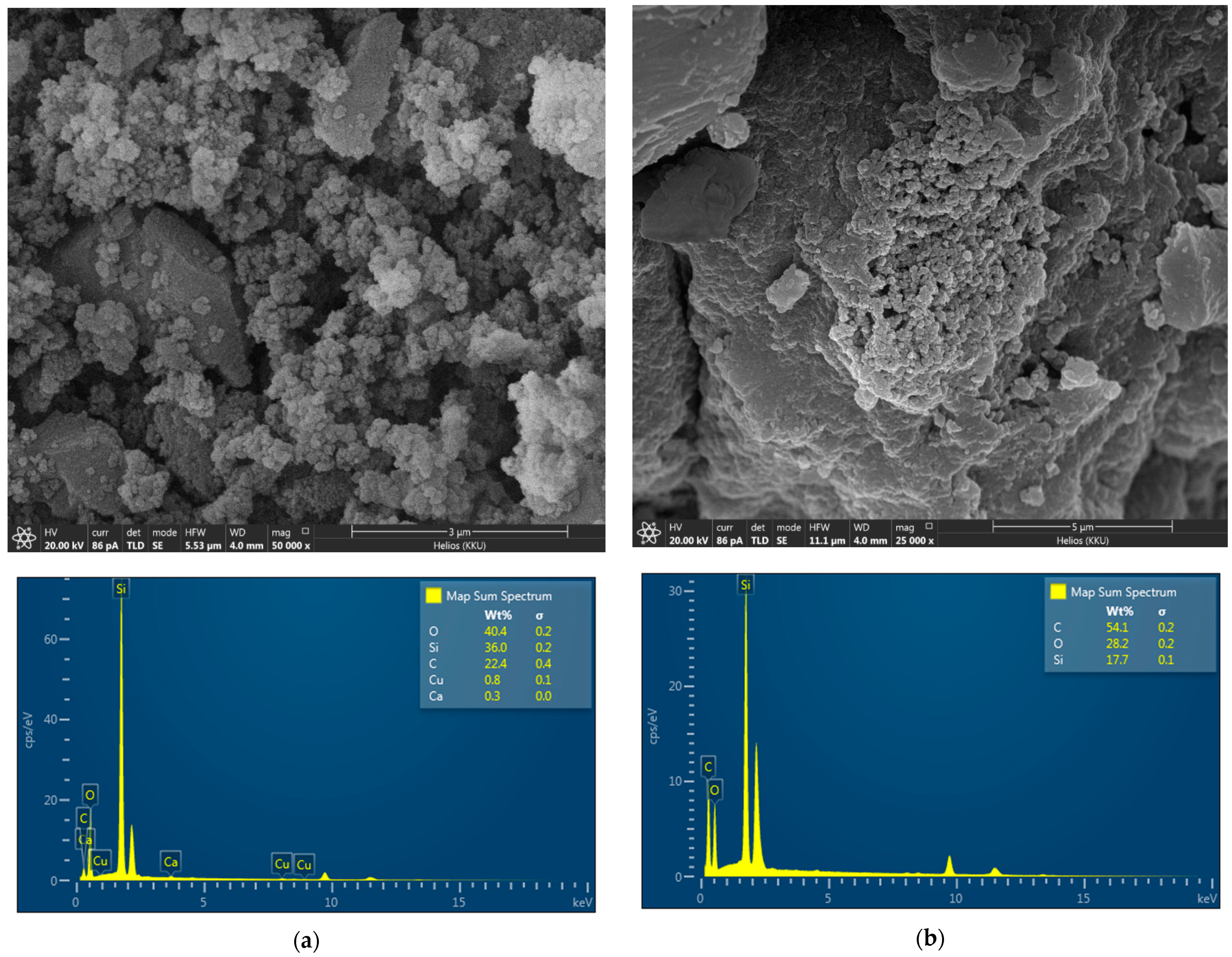
3.1.3. Surface Morphology
3.2. Adsorption Study
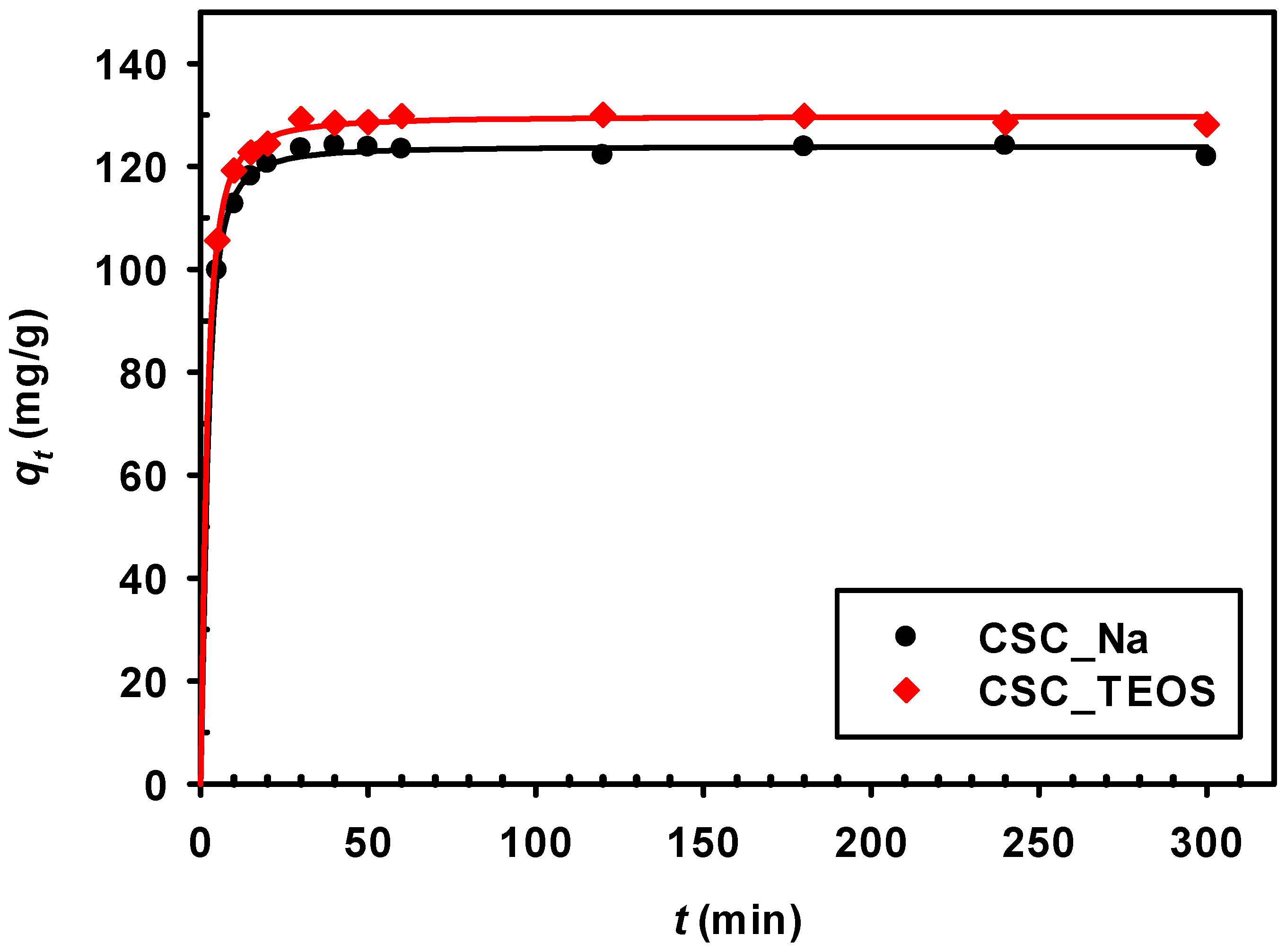
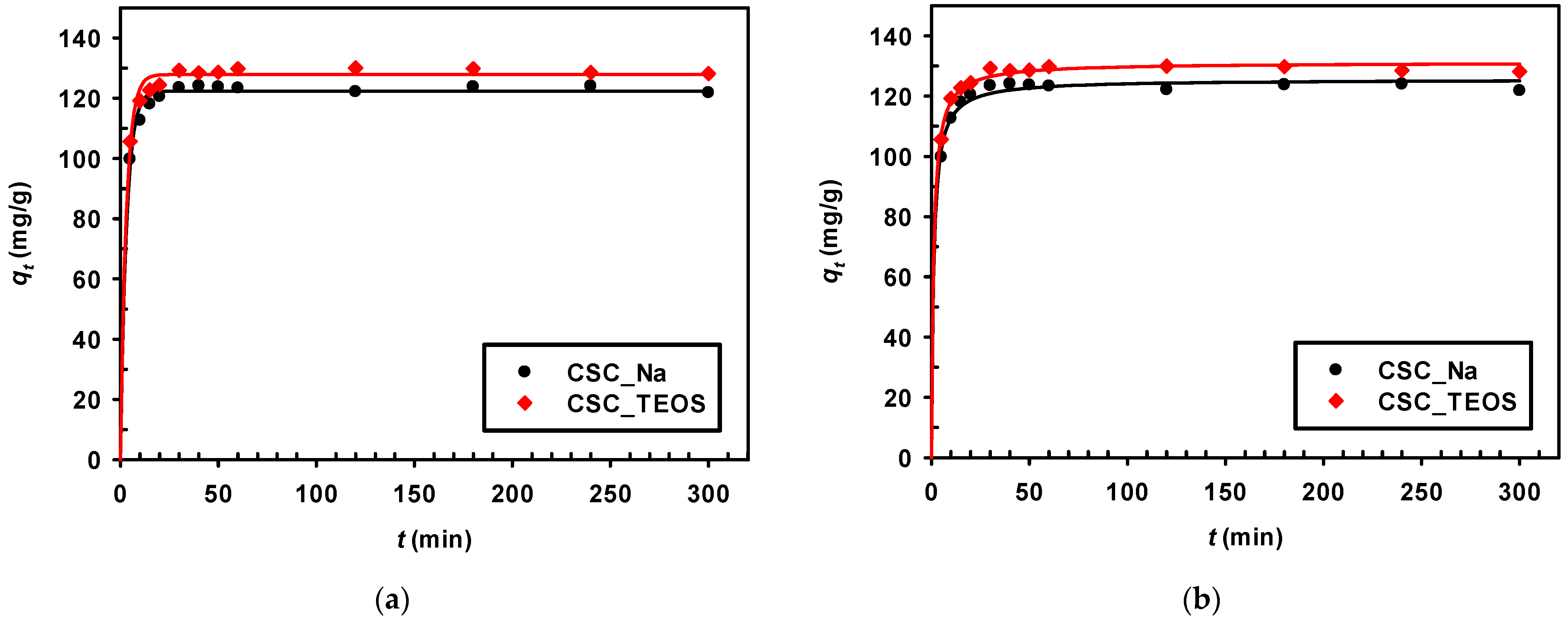
3.2.1. Effect of Adsorption Time
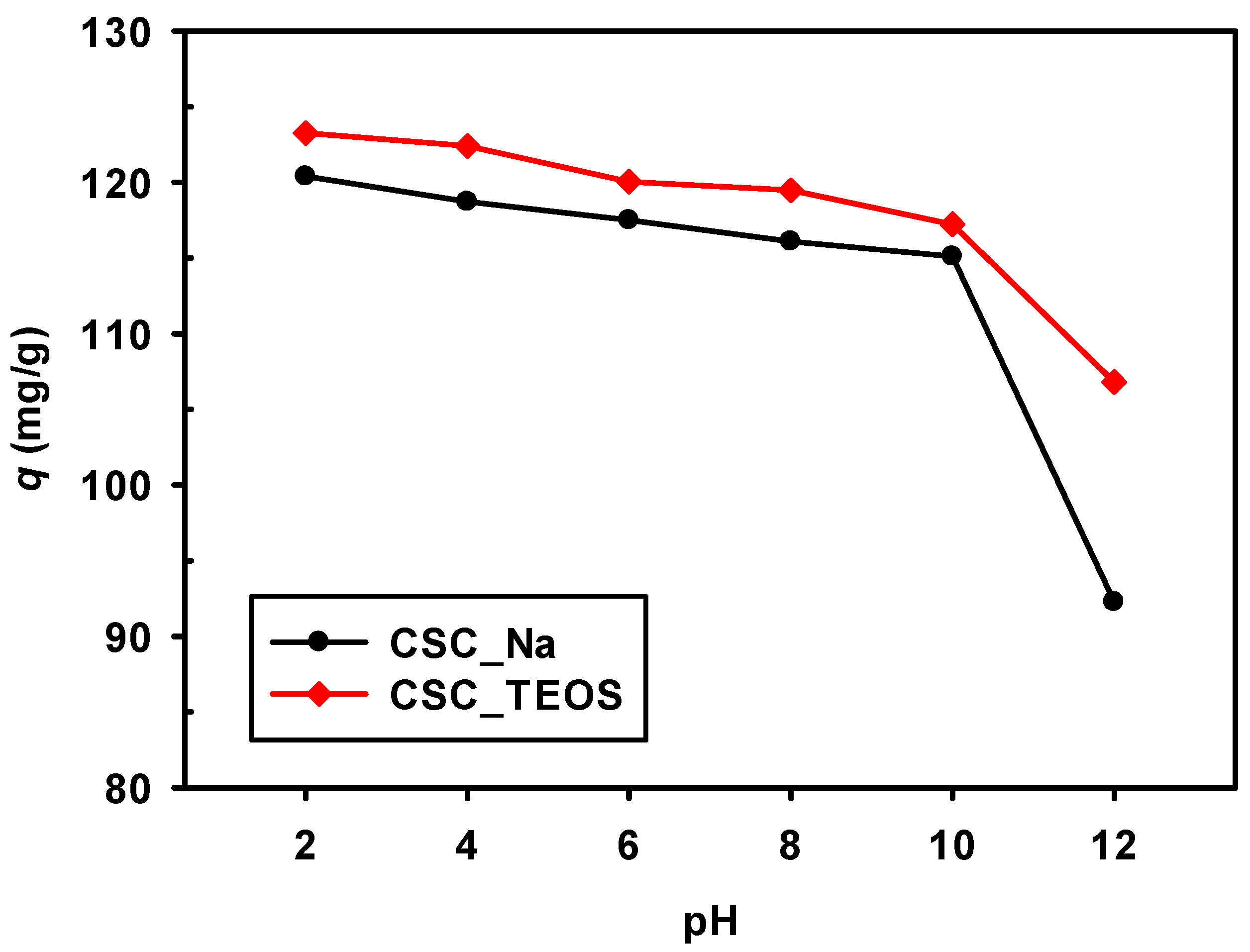
3.2.2. Effect of Initial pH
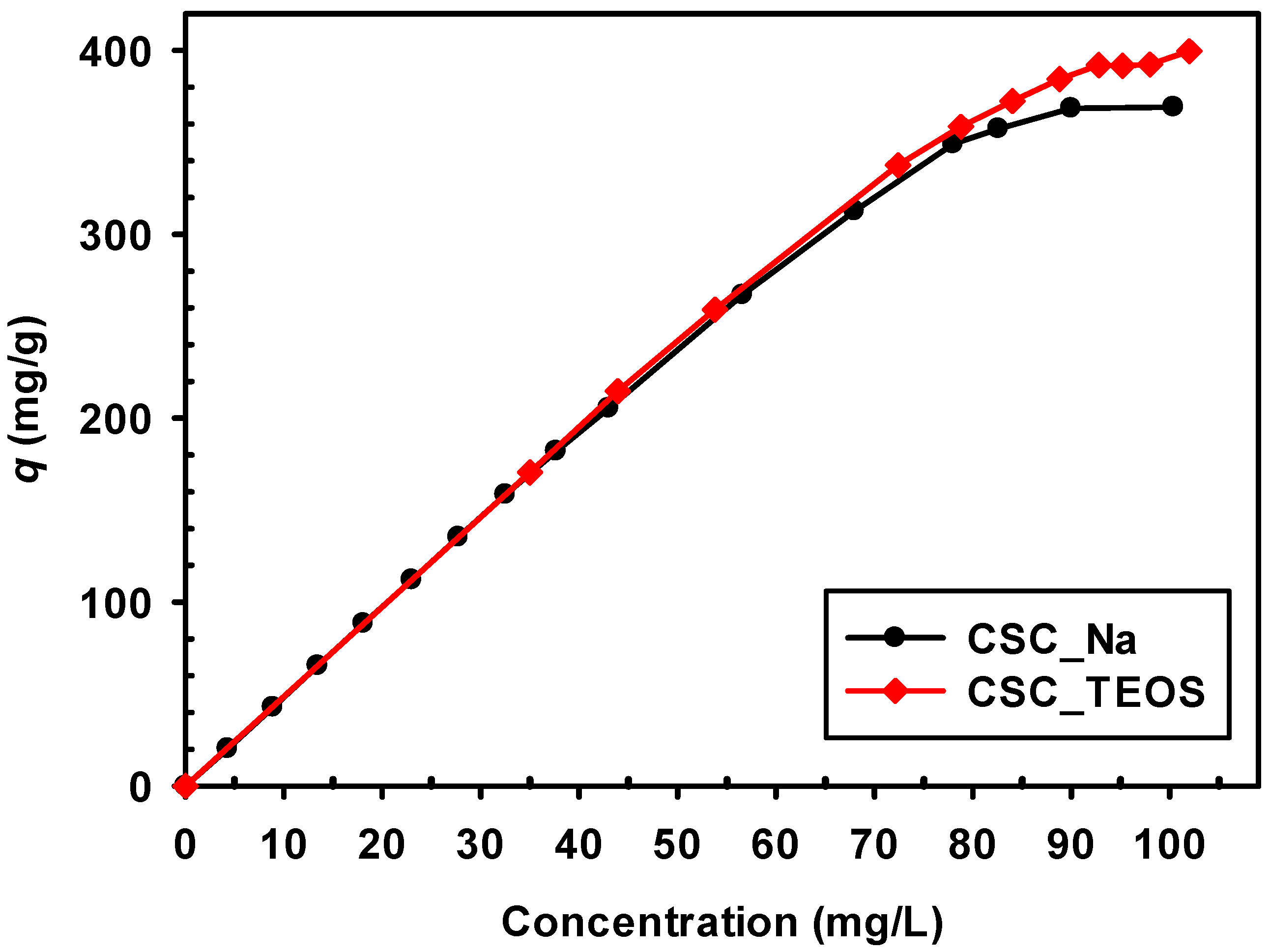
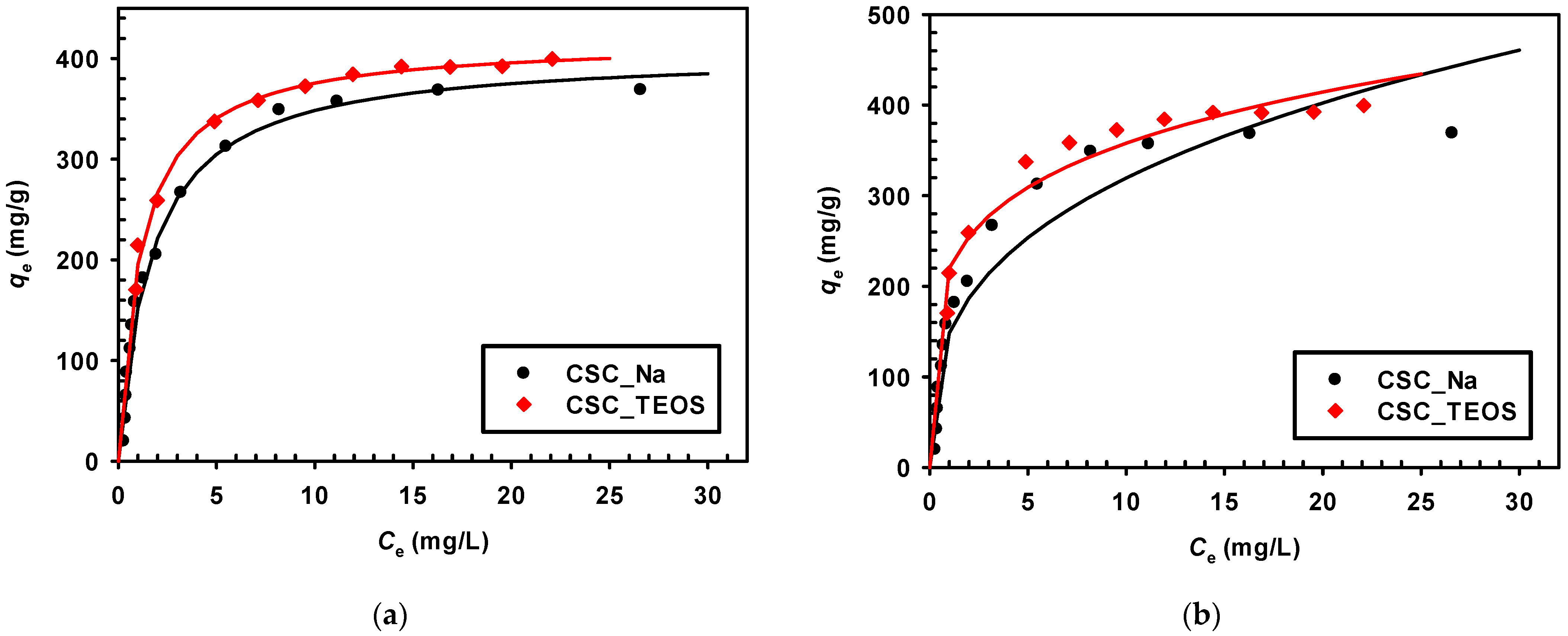
3.2.3. Effect of Initial Solution Concentration
3.2.4. Kinetic Models
3.2.5. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of Ibuprofen from Aqueous Media by Adsorption: A comprehensive review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, A.C.; dos Reis, G.S.; Pavan, F.A.; Lima, É.C.; Foletto, E.L.; Dotto, G.L. Improvement of Activated Carbon Characteristics by Sonication and Its Application for Pharmaceutical Contaminant Adsorption. Environ. Sci. Pollut. Res. 2018, 25, 24713–24725. [Google Scholar] [CrossRef] [PubMed]
- Ulfa, M.; Prasetyoko, D. Drug Loading-Release Behaviour of Mesoporous Materials SBA-15 and CMK-3 using ibuprofen molecules as drug model. J. Phys. Conf. Ser. 2019, 1153, 012065. [Google Scholar] [CrossRef]
- Shen, S.-C.; Ng, W.K.; Chia, L.S.O.; Dong, Y.-C.; Tan, R.B.H. Applications of Mesoporous Materials as Excipients for Innovative Drug Delivery and Formulation. Curr. Pharm. Des. 2013, 19, 6270–6289. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, H.; Huang, Y.; Wang, Y. Porous Carbon/silica Composite Monoliths Derived from Resorcinol-formaldehyde/TEOS. J. Non-Cryst. Solids 2010, 356, 971–976. [Google Scholar] [CrossRef]
- Wu, T.; Ke, Q.; Lu, M.; Pan, P.; Zhou, Y.; Gu, Z.; Cui, G. Recent Advances in Carbon-Silica Composites: Preparation, Properties, and Applications. Catalysts 2022, 12, 573. [Google Scholar] [CrossRef]
- Furtado, A.M.B.; Wang, Y.; LeVan, M.D. Carbon Silica Composites for Sulfur dioxide and Ammonia Adsorption. Micropor. Mesopor. Mater. 2013, 165, 48–54. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, J.; Huang, W.; Fang, J.; Sun, X.; Wang, X.; Liao, K. Preparation of Nano-Porous Carbon-Silica Composites and Its Adsorption Capacity to Volatile Organic Compounds. Processes 2020, 8, 372. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Vieira, J.P.F.; Contesini, F.J.; Mantelatto, P.E.; Zaiat, M.; da Cruz Pradella, J.G. High Value Added Lipids Produced by Microorganisms: A Potential Use of Sugarcane Vinasse. Critical Reviews in Biotechnology 2017, 37, 1048–1061. [Google Scholar] [CrossRef]
- Ponnikorn, T.; Knijnenburg, J.T.N.; Macquarrie, D.J.; Ngernyen, Y. Novel Mesoporous Carbon-Silica Composites from Vinasse for the Removal of Dyes from Aqueous Silk Dyeing Wastes. Eng. Appl. Sci. Res. 2022, 49, 707–719. [Google Scholar]
- Nandan, D.; Sreenivasulu, P.; Konathala, L.N.S.; Kumar, M.; Viswanadham, N. Acid Functionalized Carbon-Silica Composite and Its Application for Solketal Production. Micropor. Mesopor. Mater. 2013, 179, 182–190. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Y.; Dan, H.; Xu, X.; Yue, Q.; Yan, J.; Wang, W.; Gao, B. Effect of Washing Conditions on Adsorptive Properties of Mesoporous Silica Carbon Composites by In-situ Carbothermal Treatment. Sci. Total Environ. 2020, 716, 136770. [Google Scholar] [CrossRef] [PubMed]
- Noa-Bolaño, A.; Pérez-Ones, O.; Zumalacárregui-de Cárdenas, L.; Pérez-de los Ríos, J.L. Simulation of Concentration and Incineration as an Alternative for Vinasses’ treatment. Rev. Mex. Ing. Química 2020, 19, 1265–1275. [Google Scholar] [CrossRef]
- Abdus-Salam, N.; Buhari, M. Adsorption of Alizarin and Fluorescein Dyes on Adsorbent prepared from Mango Seed. Pac. J. Sci. Technol. 2014, 15, 232–244. [Google Scholar]
- Asgari, M.; Anisi, H.; Mohammadi, H.; Sadighi, S. Designing a Commercial Scale Pressure Swing Adsorber for Hydrogen Purification. Pet. Coal 2014, 56, 552–561. [Google Scholar]
- Shweta, K.; Jha, H. Rice Husk Extracted Lignin−TEOS biocomposites: Effects of Acetylation and Silane Surface Treatments for Application in Nickel Removal. Biotechnol. Rep. 2015, 7, 95–106. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Mokaya, R. Periodic Mesoporous Organosilica Mesophases are Versatile Precursors for the Direct Preparation of Mesoporous Silica/Carbon Composites, Carbon and Silicon Carbide Materials. J. Mater. Chem. 2006, 16, 3417–3425. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of Pharmaceutical Compounds by Activated Carbon Prepared from Agricultural By-product. Chem. Eng. J. 2012, 211-212, 310–317. [Google Scholar] [CrossRef]
- Ai, T.; Jiang, X.; Zhong, Z.; Li, D.; Dai, S. Methanol-modified Ultra-fine Magnetic Orange Peel Powder Biochar as an Effective Adsorbent for Removal of Ibuprofen and Sulfamethoxazole from Water. Adsorpt. Sci. Technol. 2020, 38, 304–321. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Othman, N.; Al-Gheethi, A.; bini Mohd Dzahir, N.H.; Asharuddin, S.M.; Alshalif, A.F.; Nasser, I.M.; Tajarudin, H.A.; AL-Towayti, F.A.H. Adsorption of Zn2+ from synthetic Wastewater Using Dried Watermelon Rind (D-WMR): An Overview of Nonlinear and Linear Regression and Error Analysis. Molecules 2021, 26, 6176. [Google Scholar] [CrossRef]
- Guo, L.; Li, G.; Liu, J.; Meng, Y.; Xing, G. Nonlinear Analysis of the Kinetics and Equilibrium for Adsorptive Removal of Cd(II) by starch Phosphate. J. Dispers. Sci. Technol. 2012, 33, 403–409. [Google Scholar] [CrossRef]
- Ni, W.; Xiao, X.; Li, Y.; Li, L.; Xue, J.; Gao, Y.; Ling, F. DETA Impregnated Attapulgite Hybrid ZIF-8 Composite as an Adsorbent for the Adsorption of Aspirin and Ibuprofen in Aqueous Solution. New J. Chem. 2021, 45, 5637–5644. [Google Scholar] [CrossRef]
- Salim, N.A.A.; Puteh, M.H.; Khamidun, M.H.; Fulazzaky, M.A.; Abdullah, N.H.; Yusoff, A.R.M.; Zaini, M.A.A.; Ahmad, N.; Lazim, Z.M.; Nuid, M. Interpretation of Isotherm Models for Adsorption of Ammonium onto Granular Activated Carbon. Biointerface Res. Appl. Chem. 2021, 11, 9227–9241. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Isotherm Adsorption Studies of Ni(II) ion Removal from Aqueous Solutions by Modified Carboxymethyl Cellulose Hydrogel. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012017. [Google Scholar] [CrossRef]
- Du, Y.-D.; Zhang, X.-Q.; Shu, L.; Feng, Y.; Lv, C.; Liu, H.-Q.; Xu, F.; Wang, Q.; Zhao, C.C.; Kong, Q. Safety Evaluation and Ibuprofen Removal via an Alternanthera philoxeroides-based biochar. Environ. Sci. Pollut. Res. 2021, 28, 40568–40586. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N.; Duclaux, L. Adsorption of Ibuprofen from Aqueous Solution on Chemically Surface-Modified Activated Carbon Cloths. Arab. J. Chem. 2017, 10, S3584–S3594. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, H.; Li, G.; Liao, C.; Jiang, G. Adsorption Removal of Ibuprofen and Naproxen from Aqueous Solution with Cu-doped Mil-101(Fe). Sci. Total Environ. 2021, 797, 149179. [Google Scholar] [CrossRef]

| Composite | SBET (m2/g) | Vmic (cm3/g) | Vmeso (cm3/g) | VT (cm3/g) | DP (nm) |
|---|---|---|---|---|---|
| CSC_Na | 313 | 0.12 | 0.27 | 0.39 | 5.00 |
| (31%) | (69%) | ||||
| CSC_TEOS | 456 | 0.20 | 0.10 | 0.30 | 2.62 |
| (67%) | (33%) |
| Composite | Yield (wt.%) | Moisture (wt.%) | Ash (wt.%) | pH | pHpzc | Bulk Density (g/cm3) |
|---|---|---|---|---|---|---|
| CSC_Na | 6.33 | 12.97 | 44.39 | 1.85 | 2.20 | 0.66 |
| TEOS | 4.00 | 16.67 | 30.08 | 2.82 | 2.70 | 0.86 |
| Adsorbent | qe,exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/min) | R2 | qe (mg/g) | k1 (g/(mg·min) | R2 | ||
| CSC_Na | 124 | 122.37 | 0.318 | 0.996 | 125.52 | 0.007 | 0.997 |
| CSC_TEOS | 130 | 127.86 | 0.332 | 0.995 | 131.14 | 0.007 | 0.998 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | KF ((mg/g)(L/mg)1/n) | 1/n | R2 | |
| CSC_Na | 406 | 0.601 | 0.992 | 148.560 | 0.333 | 0.869 |
| CSC_TEOS | 418 | 0.880 | 0.998 | 220.100 | 0.211 | 0.914 |
| Adsorbent | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| CSC_Na | 406 | Present study |
| CSC_TEOS | 418 | Present study |
| Sonicated activated carbon | 134 | [20] |
| Alternanthera philoxeroides-based biochar | 172 | [25] |
| ATP@ZIF-8-DETA-20 composite | 218 | [2] |
| Surface modified activated carbon cloth | 492 | [26] |
| Cu-doped Mil-101(Fe) | 497 | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngernyen, Y.; Siriketh, T.; Manyuen, K.; Thawngen, P.; Rodtoem, W.; Wannuea, K.; Knijnenburg, J.T.N.; Budsaereechai, S. Easy and Low-Cost Method for Synthesis of Carbon–Silica Composite from Vinasse and Study of Ibuprofen Removal. C 2022, 8, 51. https://doi.org/10.3390/c8040051
Ngernyen Y, Siriketh T, Manyuen K, Thawngen P, Rodtoem W, Wannuea K, Knijnenburg JTN, Budsaereechai S. Easy and Low-Cost Method for Synthesis of Carbon–Silica Composite from Vinasse and Study of Ibuprofen Removal. C. 2022; 8(4):51. https://doi.org/10.3390/c8040051
Chicago/Turabian StyleNgernyen, Yuvarat, Thitipong Siriketh, Kritsada Manyuen, Panta Thawngen, Wipha Rodtoem, Kritiyaporn Wannuea, Jesper T. N. Knijnenburg, and Supattra Budsaereechai. 2022. "Easy and Low-Cost Method for Synthesis of Carbon–Silica Composite from Vinasse and Study of Ibuprofen Removal" C 8, no. 4: 51. https://doi.org/10.3390/c8040051
APA StyleNgernyen, Y., Siriketh, T., Manyuen, K., Thawngen, P., Rodtoem, W., Wannuea, K., Knijnenburg, J. T. N., & Budsaereechai, S. (2022). Easy and Low-Cost Method for Synthesis of Carbon–Silica Composite from Vinasse and Study of Ibuprofen Removal. C, 8(4), 51. https://doi.org/10.3390/c8040051







