Decoration of Reduced Graphene Oxide with Magnesium Oxide during Reflux Reaction and Assessment of Its Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
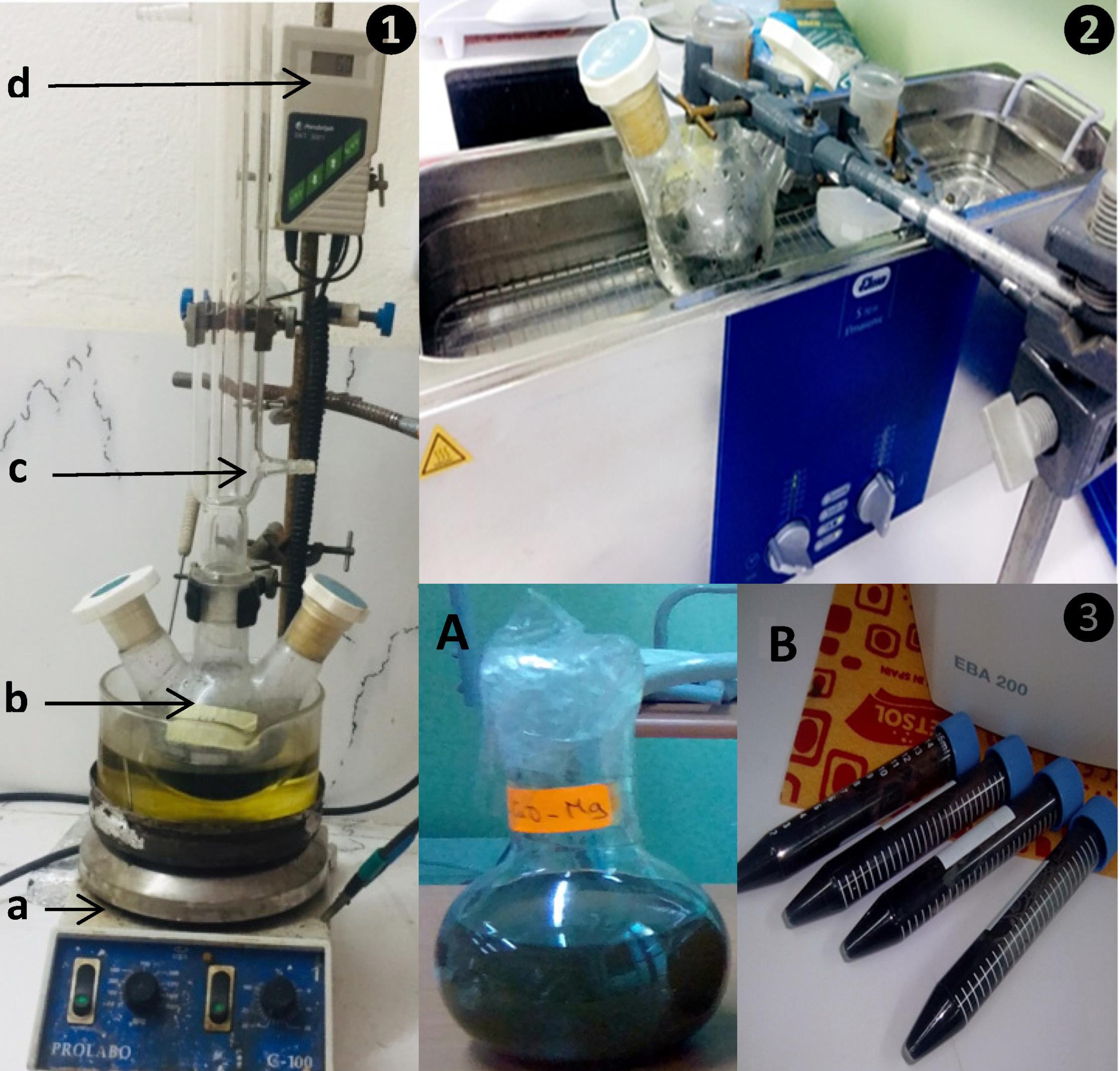
2.1. GO Synthesis
2.2. MgO–rGO Synthesis
2.3. Antioxidant Activity
2.3.1. DPPH Radical Scavenging Assay
2.3.2. Hydrogen Peroxide Scavenging Assay
2.3.3. Phosphomolybdenum Assay
2.3.4. Structural and Chemical Characterization
3. Results and Discussion
3.1. XRD and XPS Characterization
3.2. Antioxidant Properties
4. Conclusions
- -
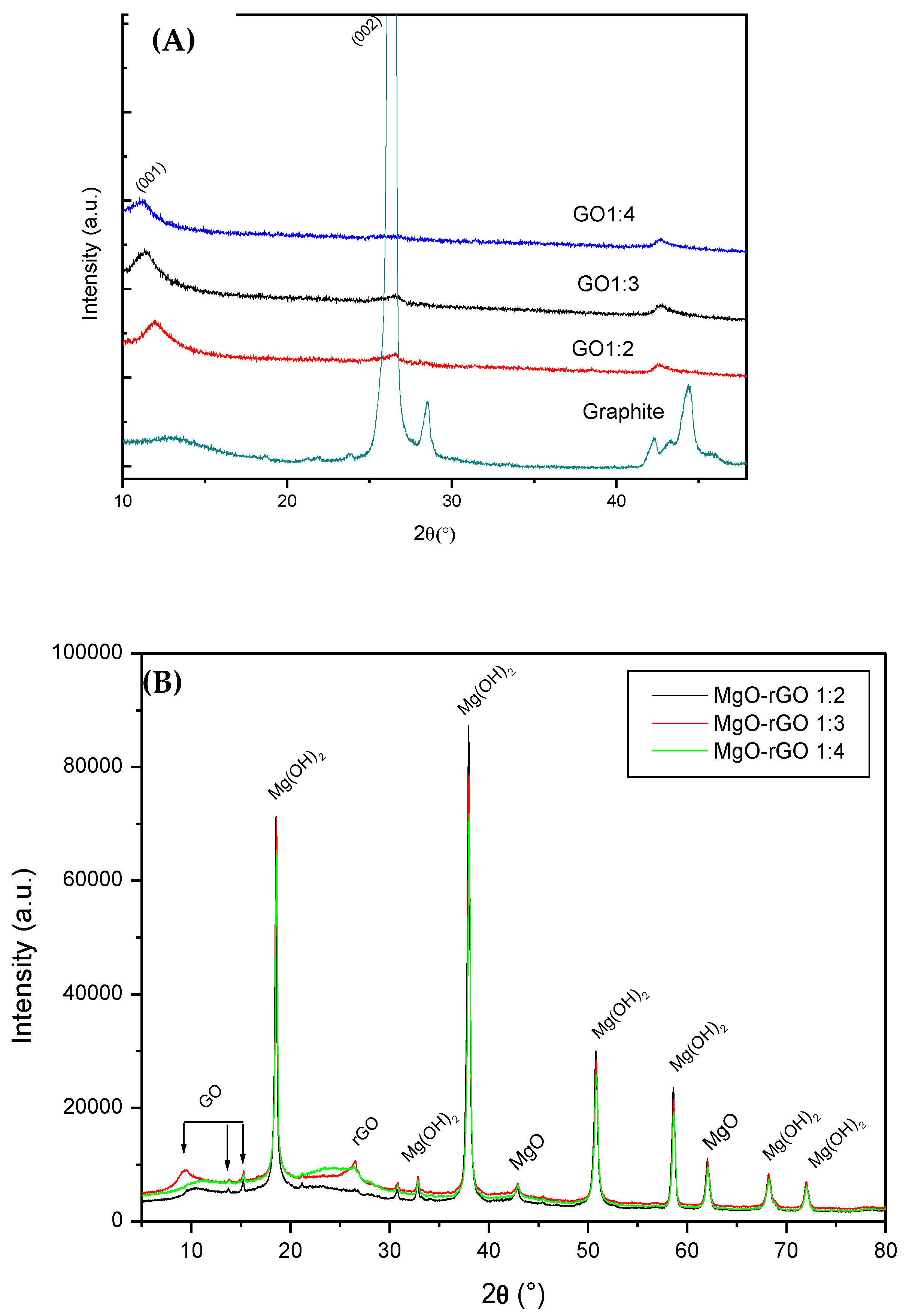
- Structural characterization of the obtained product, using X-ray diffraction confirms the formation of Mg(OH)2 phase in addition to MgO nanoparticles, resulting in a MgO–rGO–Mg(OH)2 nanocomposite.
- -
- XRD spectra of MgO–rGO1:2, MgO–rGO1:3, and MgO–rGO1:4 samples show the typical diffraction peaks of magnesium oxide and magnesium hydroxide. These diffraction peaks display a clear broadening, informing us about the nanometric size of Mg(OH)2 and MgO nanoparticles.
- -
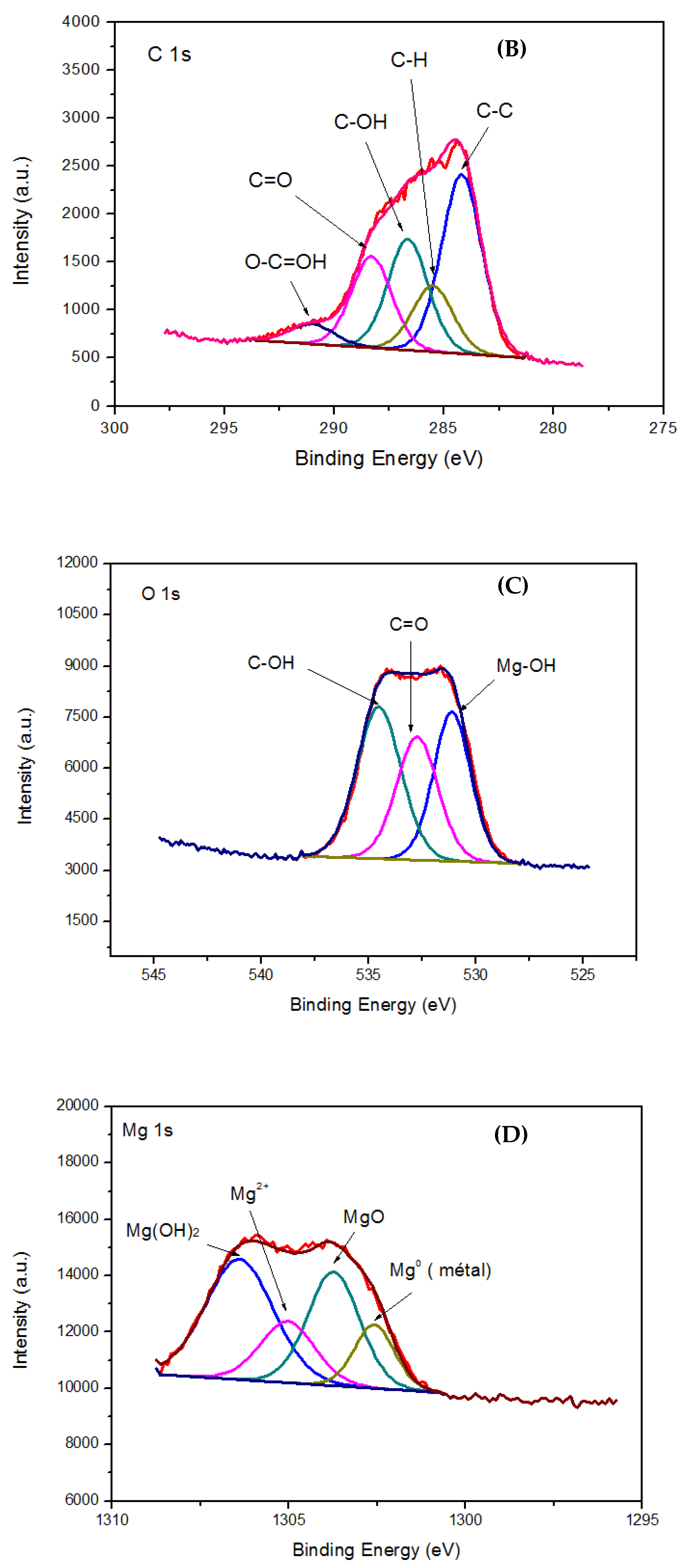
- All XPS wide spectra of the MgO–rGO nanocomposite show peaks related to C 1s, O 1s, and Mg 1s. The peak fitting of the high-resolution O 1s and Mg 1s core lines relative to the MgO–rGO1:4 and MgO–rGO1:3 samples display components related to MgO and Mg(OH)2. However, this latter component disappeared in the O 1s and Mg 1s spectra of the MgO–rGO1:2 nanocomposite. This is in agreement with the structural characterization results confirming the formation of nanocomposite MgO–rGO–Mg(OH)2.
- -
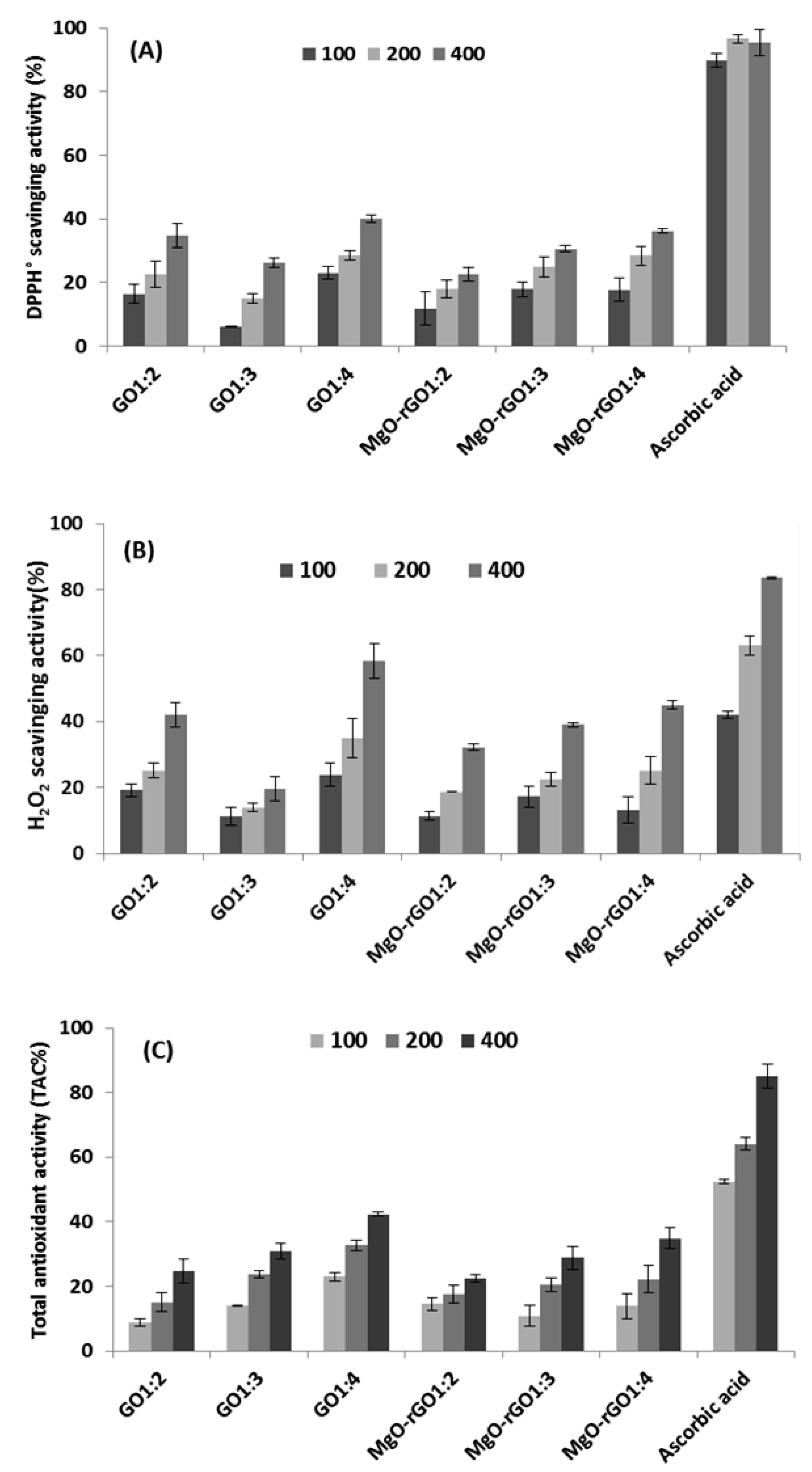
- The synthesized samples, i.e., GO1:2, GO1:3, GO1:4, MgO–rGO1:2, MgO–rGO1:3, and MgO–rGO1:4 nanocomposites were screened for antioxidant activity using DPPH radicals scavenging assay, H2O2 scavenging assay, and phosphomolybdate assay. The results suggest significant antioxidant activity in a concentration-dependent manner. Among the different synthesized nanoparticles, GO1:4 and MgO–rGO (1:4) showed the best antioxidant activity in all assays carried out. Current results suggest that GO1:4 is an excellent platform for radical-trapping antioxidants and could be useful as an antioxidant in environmental and pharmaceutical applications.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Razaul, K.M.; Hayami, S. Chemical, thermal, and light-driven reduction of graphene oxide: Approach to obtain graphene and its functional hybrids. In Graphene Materials—Advanced Applications; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef]
- Reshma, S.C.; Syama, S.; Mohanan, P.V. Nano-biointeractions of PEGylated and bare reduced graphene oxide on lung alveolar epithelial cells: A comparative in vitro study. Colloids Surf. B Biointerfaces 2016, 140, 104–116. [Google Scholar] [CrossRef]
- Udayabhanu, D.S.; Kumar, M.A.P.; Nagabhushana, H.; Sharma, S.C. Cinnamon supported facile green reduction of graphene oxide, its dye elimination and antioxidant activities. Mater. Lett. 2015, 151, 93–95. [Google Scholar]
- Thangamuthu, M.; Hsieh, K.Y.; Kumar, P.V.; Chen, G.Y. Graphene- and Graphene oxide-based nanocomposite platforms for electrochemical biosensing applications. Int. J. Mol. Sci. 2019, 20, 2975. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, S.K.; Ionov, A.; Mozhchil, R.N.; Naqvi, A.H. Simple synthesis of graphene nanocomposites MgO–rGO and Fe2O3–rGO for multifunctional applications. Appl. Phys. A 2018, 124, 365. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Han, M.; Muhammad, Y.; Wei, Y.; Zhu, Z.; Huang, J.; Li, J. A review on the development and application of graphene based materials for the fabrication of modified asphalt and cement. Constr. Build. Mater. 2021, 285, 122885. [Google Scholar] [CrossRef]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene oxide: Opportunities and challenges in biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Mohan, V.B.; Bhowmick, A.K.; Bhattacharyya, D. Metal/metal oxide decorated graphene synthesis and application as supercapacitor. J. Mater. Sci. 2020, 55, 6375–6400. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent advances in metal decorated nanomaterials and their various biological applications. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Karimi, N. Ultrasound assisted-phyto fabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2405–2423. [Google Scholar] [CrossRef] [PubMed]
- Baali, N.; Khecha, A.; Bensouici, A.; Speranza, G.; Hamdouni, N. Assessment of antioxidant activity of pure graphene oxide (Go) and ZnO-decorated reduced graphene oxide (rGO) using DPPH radical and H2O2 scavenging assays. Carbon 2019, 5, 75. [Google Scholar] [CrossRef]
- Ryou, J.; Hong, S. Firstprinciples study of carbon atoms adsorbed on MgO (100) related to graphene growth. Curr. Appl. Phys. 2013, 13, 327–330. [Google Scholar] [CrossRef]
- Liu, L.; Peng, F.; Zhang, D.; Li, M.; Huang, J.; Liu, X. A tightly bonded reduced graphene oxide coating on magnesium alloy with photothermal effect for tumor therapy. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Xu, H.; Pan, Y.; Hu, F.; Niu, B.; Zhang, Y. Anti-corrosion MgO nanoparticule-equipped graphene oxide nanosheet for efficient room-temperature H2S removal. J. Mater. Chem. A 2022, 10, 18308–18321. [Google Scholar] [CrossRef]
- Nan, H.Y.; Ni, Z.H.; Wang, J.; Zafar, Z.; Shi, Z.X.; Wang, Y.Y. The thermal stability of graphene in air investigated by Raman spectroscopy. J. Raman Spectrosc. 2013, 44, 1018–1021. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, J.H.; Han, J.T. Extraordinary thermal behavior of graphene oxide in air for electrode applications. Nanoscale Adv. 2021, 3, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, A.; Wang, W. Structural Evolution of Graphene Oxide and Its Thermal Stability during High Temperature Sinterung. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2022, 37, 342–349. [Google Scholar] [CrossRef]
- Li, C.; Lu, Y.; Yan, J.; Yu, W.; Zhao, R.; Du, S.; Niu, K. Effect of long-term ageing on graphene oxide: Structure and thermal decomposition. R. Soc. Open Sci. 2021, 8, 202309. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Purwajanti, S.; Huang, X.; Liu, Y.; Yang, Y.; Noonan, O.; Song, H.; Zhang, J.; Zhang, J.; Fu, J.; Liang, C.; et al. Mg(OH)2-MgO@reduced graphene oxide nanocomposites: The roles of composition and nanostructure on arsenite sorption. J. Mater. Chem. 2017, 5, 24484–24492. [Google Scholar] [CrossRef]
- Saoud, K.M.; Saeed, S.; Al-Soubaihi, R.M.; Bertino, M.F. Microwave assisted preparation of magnesium hydroxide nano-sheets. Am. J. Nanomater. 2014, 2, 21–25. [Google Scholar]
- Heo, Y.J.; Park, S.J. Facile Synthesis of MgO Modified Carbon Adsorbents with Microwave-Assisted Methods: Effect of MgO Particles and Porosities on CO2 Capture. Sci. Rep. 2017, 7, 5653. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Xu, M. Graphene oxide supported magnesium oxide as an efficient cathode catalyst for power generation and wastewater treatment in single chamber microbial fuel cells. Chem. Eng. J. 2017, 328, 106–116. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; Heer, W.; Bongiorno, A.; Riedo, E. Room-temperature metastability of multilayer graphene oxide films. Nat. Mater. 2012, 11, 544–549. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, J.; Lim, S.; Li, D.; Xiong, O.; Shen, Z.; Lin, J.; Yu, T. Modulating the electronic structures of graphene by controllable hydrogenation. Appl. Phys. Lett. 2010, 97, 233111. [Google Scholar] [CrossRef]
- Xia, W.; Wang, Y.; Bergstraber, B.; Kundu, S.; Muhler, M. Surface characterization of oxygen- functionalized multi-walled carbon nanotubes by high-resolution X-ray photoelectron spectroscopy and temperature-programmed de-sorption. Appl. Surf. Sci. 2007, 254, 247–250. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Lee, Y.P.; Lahaye, R.J.Y.P.; Park, M.-H.; An, K.H.; Kim, I.J.; Yang, C.-W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of graphitic AB stacking order of graphite oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoe-lectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, H.X.; Zhang, H.; Sasaoka, H.; Nishimur, K. Characterization and surface modification of carbon nanowalls. J. Mater. Chem. 2010, 20, 5070–5073. [Google Scholar] [CrossRef]
- Sullivan, M.G.; Schnyder, B.; Bärtsch, M.; Alliata, D.; Barbero, C.; Imhof, R.; Kötz, R. Electrochemically modified glassy carbon for capacitor electrodes characterization of thick anodic layers by cyclic voltammetry, differential electrochemical mass spectrometry, spectroscopic ellipsometry, X-ray photoelectron spectroscopy, ftir, and afm. J. Electrochem. Soc. 2000, 147, 2636–2643. [Google Scholar] [CrossRef]
- Elmi, C.; Guggenheim, S.; Gieré, R. Surface crystal chemistry of phyllosilicates using X-ray photoelectron spectroscopy: A Review. Clays Clay Miner. 2016, 64, 537–551. [Google Scholar] [CrossRef]
- Zhoua, L.; Zhang, S.; Lia, Z.; Liang, X.; Zhang, Z.; Liu, R.; Yun, J. Efficient degradation of phenol in aqueous solution by catalytic ozonation over MgO/AC. J. Water Process Eng. 2020, 36, 101168. [Google Scholar] [CrossRef]
- Haider, N.C.; Alonso, J.; Swartz, W.E. Valence and core electron spectra of Mg in MgO in evaporated thin films. Z. Nat. 2013, 1, 1486–1490. [Google Scholar]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Petlevski, R.; Flajs, D.; Kalođera, Z.; ZovkoKončić, M. Composition and antioxidant activity of aqueous and ethanolic Pelargonium radula extracts. S. Afr. J. Bot. 2013, 85, 17–22. [Google Scholar] [CrossRef]
- Fathy, R.M.; Mahfouz, A.Y. Eco-friendly graphene oxide-based magnesium oxide nanocomposite synthesis using fungal fermented by-products and gamma rays for outstanding antimicrobial, antioxidant, and anticancer activities. J. Nanostruct. Chem. 2021, 11, 301–321. [Google Scholar] [CrossRef]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippianodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Effic. Technol. 2017, 3, 506–515. [Google Scholar]
- Khan, I.; Sarfraz Ali, J.; Ul-Haq, I.; Zia, M. Biological and phytochemicals properties of Monothecalbuxifolia: An unexplored medicinal plant. Pharm. Chem. J. 2020, 54, 241–249. [Google Scholar] [CrossRef]
- Ikram, M.; Inayat, T.; Haider, A.; Ul-Hamid, A.; Haider, J.; Nabgan, W.; Saeed, A.; Shahbaz, A.; Hayat, S.; Ul-Ain, K.; et al. Graphene oxide-doped MgO nanostructures for highly efficient dye degradation and bactericidal Action. Nanoscale Res. Lett. 2021, 16, 56. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensouici, A.; Baali, N.; Bouloudenine, R.; Speranza, G. Decoration of Reduced Graphene Oxide with Magnesium Oxide during Reflux Reaction and Assessment of Its Antioxidant Properties. C 2022, 8, 49. https://doi.org/10.3390/c8040049
Bensouici A, Baali N, Bouloudenine R, Speranza G. Decoration of Reduced Graphene Oxide with Magnesium Oxide during Reflux Reaction and Assessment of Its Antioxidant Properties. C. 2022; 8(4):49. https://doi.org/10.3390/c8040049
Chicago/Turabian StyleBensouici, Aicha, Nacera Baali, Roumaissa Bouloudenine, and Giorgio Speranza. 2022. "Decoration of Reduced Graphene Oxide with Magnesium Oxide during Reflux Reaction and Assessment of Its Antioxidant Properties" C 8, no. 4: 49. https://doi.org/10.3390/c8040049
APA StyleBensouici, A., Baali, N., Bouloudenine, R., & Speranza, G. (2022). Decoration of Reduced Graphene Oxide with Magnesium Oxide during Reflux Reaction and Assessment of Its Antioxidant Properties. C, 8(4), 49. https://doi.org/10.3390/c8040049







