Synthetic, Photosynthetic, and Chemical Strategies to Enhance Carbon Dioxide Fixation
Abstract
1. Introduction
2. Engineering Photosynthetic Plants
2.1. Engineering C4 Cycle into C3 Plants
2.2. Engineering CAM into C3 Plants
3. Carbon Dioxide Fixation by Microbes
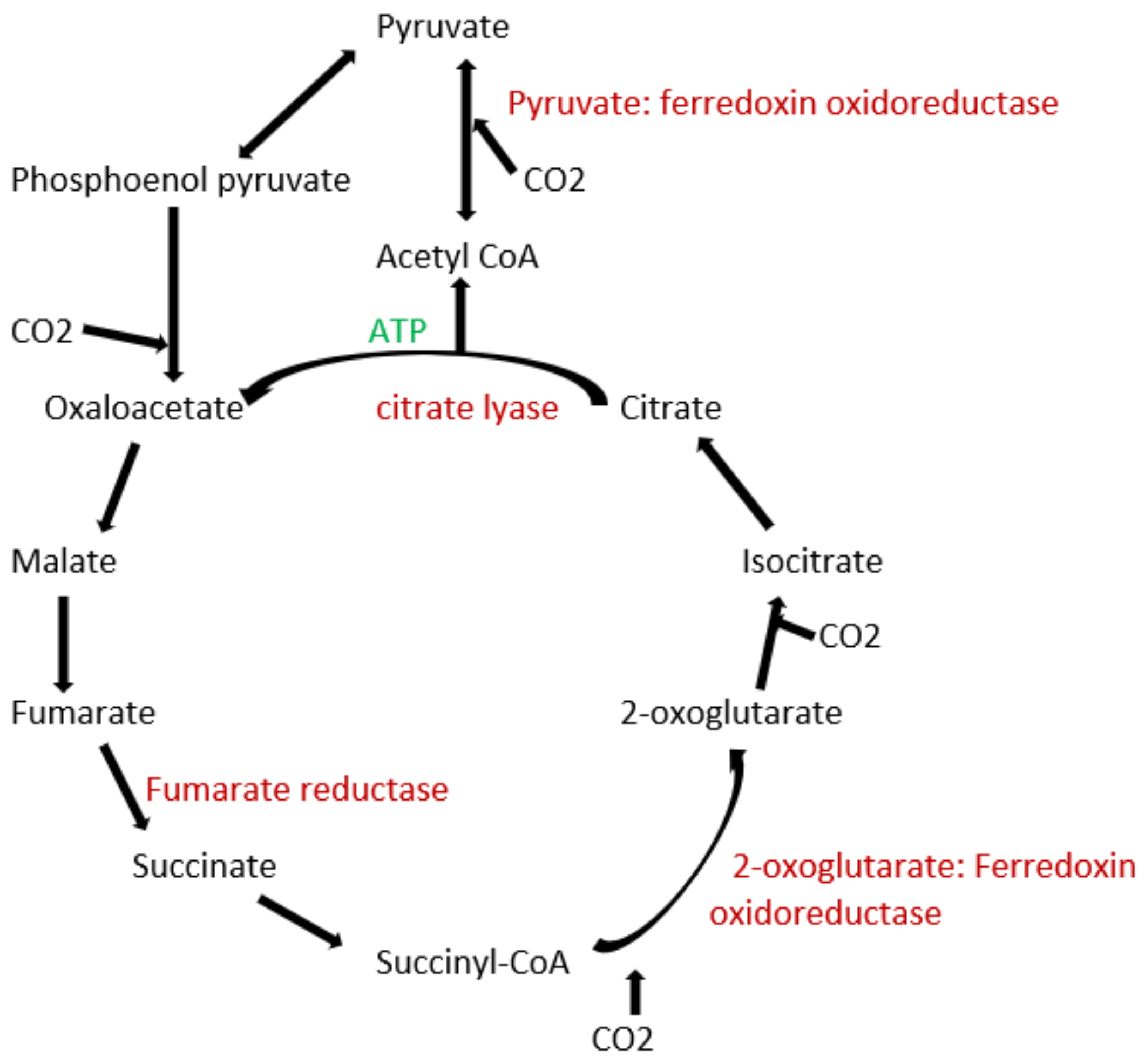
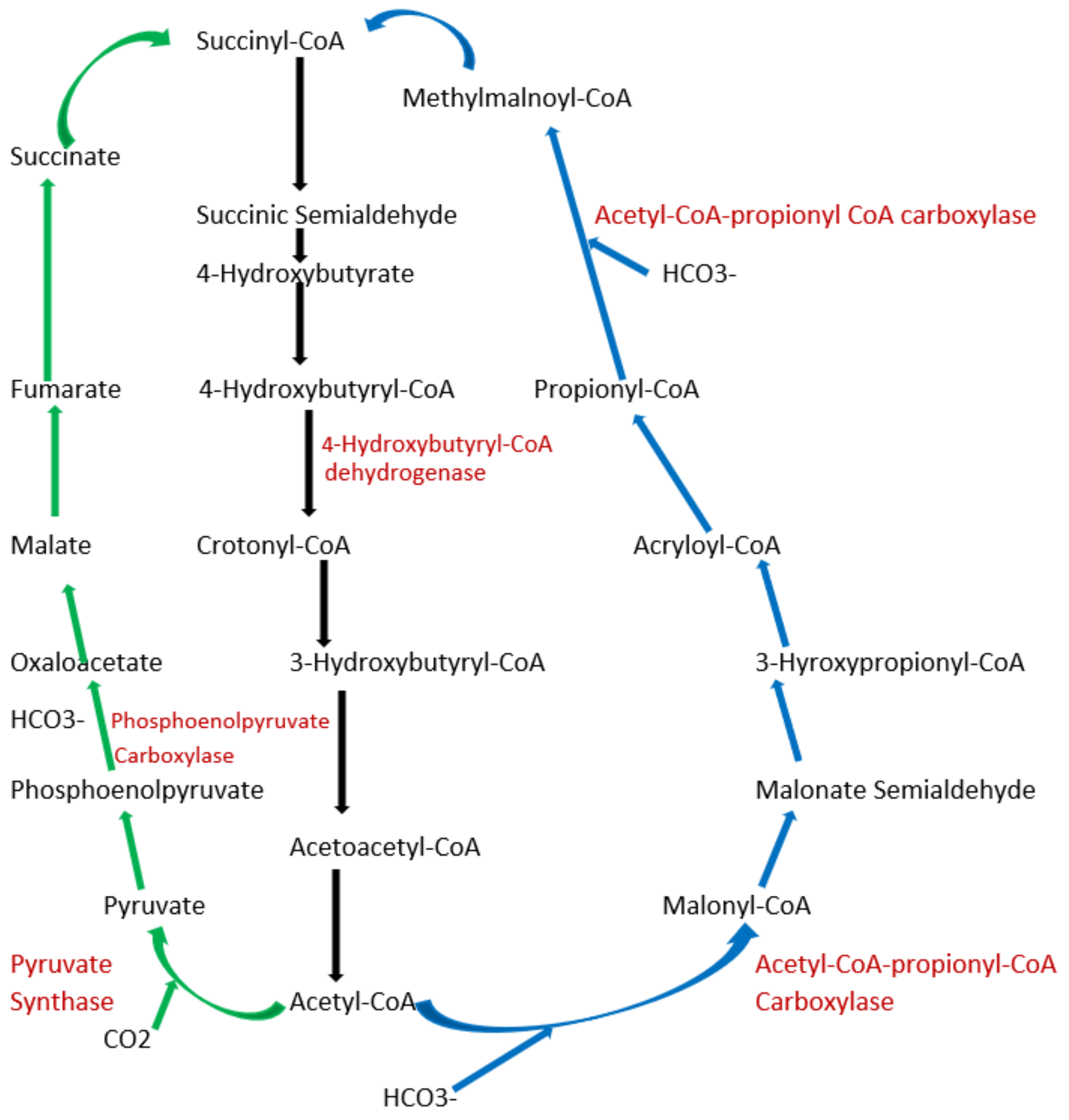
3.1. Archaea Bacteria
3.2. Acetogens
3.3. Methanogens
3.4. Algae
3.5. Proteobacteria
3.6. Engineering Microbes for Carbon Dioxide Fixation
4. Phytoplankton
4.1. Cyanobacteria
4.2. Dinoflagellates
4.3. Diatoms
4.4. What Are the Challenges in Genetically Modifing Diatoms?
5. Photosynthesis and Rubisco: An Evolutionary Perspective
5.1. Can Engineering Photosynthesis Provide Solution?
5.2. Why Is It Important to Look beyond Rubisco?
5.3. What Are the Other Natural Carbon-Fixing Metabolic Pathways?
6. Exploring Other Alternatives: Introduction to Synthetic Pathways
6.1. How to Use the Criteria Matrix to Select Synthetic Carbon Pathways?
6.1.1. Kinetic Analysis
6.1.2. Resource Consumption and Energetic Efficiency
6.1.3. Thermodynamic Analysis
6.1.4. Analysis of Distributed Thermodynamic Bottlenecks
6.1.5. Metabolic Regulation and Compatibility
6.1.6. Analysis of Metabolites
6.1.7. Application of Synthetic Pathways
7. Chemical Alternatives for Fixing Carbon Dioxide
7.1. Carbon Nanotube (CNT) Synthesis
7.2. Fixing Carbon Dioxide into Plastics
7.3. Metal-Organic Frameworks (MOFs)
7.4. Nanotechnology Based Carbon Dioxide Fixation
7.4.1. Metal-Based Nanocatalyst
7.4.2. Nanoparticles for Capturing Post-Combustion Carbon Dioxide
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irfan, M.; Bai, Y.; Zhou, L.; Kazmi, M.; Yuan, S.; Maurice Mbadinga, S.; Yang, S.Z.; Liu, J.F.; Sand, W.; Gu, J.D.; et al. Direct microbial transformation of carbon dioxide to value-added chemicals: A comprehensive analysis and application potentials. Bioresour. Technol. 2019, 288, 121401. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Opgenorth, P.H.; Wernick, D.G.; Rogers, S.; Wu, T.Y.; Higashide, W.; Malati, P.; Huo, Y.X.; Cho, K.M.; Liao, J.C. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 2012, 335, 1596. [Google Scholar] [CrossRef] [PubMed]
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.K.; González Rodriguez, H.; Ivanova, N.S. Autoecology and Ecophysiology of Woody Shrubs and Trees: Concepts and Applications; Wiley Blackwell: Chichester, UK, 2016. [Google Scholar]
- Kumar, V.; Sharma, A.; Soni, J.; Pawar, N. Physiological response of C3, C4 and CAM plants in changeable climate. India Pharma Innov. Int. J. 2017, 6, 70–79. [Google Scholar]
- Driever, S.M.; Kromdijk, J. Will C3 crops enhanced with the C4 CO2-concentrating mechanism live up to their full potential (yield)? J. Exp. Bot. 2013, 64, 3925–3935. [Google Scholar] [CrossRef]
- Bar-Even, A. Daring metabolic designs for enhanced plant carbon fixation. Plant Sci. 2018, 273, 71–83. [Google Scholar] [CrossRef]
- Aubry, S.; Brown, N.J.; Hibberd, J.M. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J. Exp. Bot. 2011, 62, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.L.; Mantegazza, O.; Weber, A.P.M. Engineering C4 photosynthesis into C3 chassis in the synthetic biology age. Plant J. 2016, 87, 51–65. [Google Scholar] [CrossRef]
- Borland, A.M.; Hartwell, J.; Weston, D.J.; Schlauch, K.A.; Tschaplinski, T.J.; Tuskan, G.A.; Yang, X.; Cushman, J.C. Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci. 2014, 19, 327–338. [Google Scholar] [CrossRef]
- Yang, X.; Cushman, J.C.; Borland, A.M.; Edwards, E.J.; Wullschleger, S.D.; Tuskan, G.A.; Owen, N.A.; Griffiths, H.; Smith, J.A.C.; De Paoli, H.C.; et al. A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol. 2015, 207, 491–504. [Google Scholar] [CrossRef]
- Fujita, E.; Muckerman, J.T.; Himeda, Y. Interconversion of CO2 and formic acid by bio-inspired Ir complexes with pendent bases. BBA Bioenerg. 2013, 1827, 1031–1038. [Google Scholar] [CrossRef]
- Lemaire, O.N.; Jespersen, M.; Wagner, T.; Scott, K. CO2-Fixation Strategies in Energy Extremophiles: What Can We Learn From Acetogens? Front. Microbiol. 2020, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Rismani-yazdi, H.; Stephanopoulos, G. Anaerobic CO2 Fixation by the Acetogenic Bacterium Moorella thermoacetica. AIChe J. 2013, 59, 3176–3183. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Vera, W.H.R.; Say, R.F. Autotrophic carbon fixation in archaea. Nat. Rev. Genet. 2010, 8, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Haiza, N.; Yasin, M.; Maeda, T.; Hu, A.; Yu, C.; Wood, K.T. CO2 sequestration by methanogens in activated sludge for methane production. Appl. Energy 2015, 142, 426–434. [Google Scholar] [CrossRef]
- Martin, W.F.; Thauer, R.K. Energy in Ancient Metabolism. Cell 2017, 168, 953–955. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Genet. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Moal, G.; Lagoutte, B. Biochimica et Biophysica Acta Photo-induced electron transfer from photosystem I to NADP +: Characterization and tentative simulation of the in vivo environment. BBA Bioenerg. 2012, 1817, 1635–1645. [Google Scholar] [CrossRef]
- Peters, J.W.; Beratan, D.N.; Bothner, B.; Dyer, R.B.; Harwood, C.S.; Heiden, Z.M.; Hille, R.; Jones, A.K.; King, P.W.; Lu, Y.; et al. A new era for electron bifurcation. Curr. Opin. Chem. Biol. 2018, 47, 32–38. [Google Scholar] [CrossRef]
- Wagner, T.; Ermler, U.; Shima, S. The methanogenic CO2 reducing-and-fixing enzyme is bifunctional and contains 46 [4Fe-4S] clusters. Struct. Biol. 2015, 592, 1406–1416. [Google Scholar]
- Wang, S.; Huang, H.; Kahnt, J.; Mueller, A.P.; Köpke, M.; Thauer, K. NADP-Specific Electron-Bifurcating [FeFe]-Hydrogenase in a Functional Complex with Formate Dehydrogenase in Clostridium autoethanogenum Grown on CO. Bacteriology 2013, 195, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G. Variations of the Acetyl-eoA Pathway in Diversely Related Microorganisms That Are Not Acetogens. In Acetogenesis; Chapman & Hall Microbiology Series; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Mueller-cajar, O.; Stotz, M.; Wendler, P.; Hartl, F.U.; Bracher, A.; Hayer-hartl, M. Structure and function of the AAA 1 protein CbbX, a red-type Rubisco activase. Nature 2011, 479, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Maier, U.; Fraunholz, M.; Zauner, S.; Penny, S.; Douglas, S. A Nucleomorph-Encoded CbbX and the Phylogeny of RuBisCo Regulators. Mol. Biol. Evol. 1995, 17, 576–583. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Palmer, J.D. Rampant Horizontal Transfer and Duplication Eubacteria and Plastids of Rubisco Genes in eubacteria and plastids. Mol. Biol. Evol. 1996, 13, 873–882. [Google Scholar] [CrossRef]
- Singh, U.B.; Ahluwalia, A.S. Microalgae: A promising tool for carbon sequestration. Mitig. Adapt. Strat. Glob. Chang. 2012, 18, 73–95. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2014, 184, 190–201. [Google Scholar] [CrossRef]
- Pimentel, D.; Whitecraft, M.; Scott, Z.R.; Zhao, L.; Satkiewicz, P.; Scott, T.J.; Phillips, J.; Szimak, D.; Singh, G.; Gonzalez, D.O.; et al. Will Limited Land, Water, and Energy Control Human Population Numbers in the Future? Hum. Ecol. 2010, 38, 599–611. [Google Scholar] [CrossRef]
- Hu, M.; Wirsen, C.O.; Fuchs, G.; Taylor, C.D.; Sievert, S.M. Evidence for Autotrophic CO2 Fixation via the Reductive Tricarboxylic Acid Cycle by Members of the ε Subdivision of Proteobacteria. Am. Soc. Microbiol. 2005, 187, 3020–3027. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Loganathan, N.; Tsai, Y.C.; Mueller-cajar, O. Characterization of the heterooligomeric red-type rubisco activase from red algae. Proc. Natl. Acad. Sci. USA 2016, 113, 14019–14024. [Google Scholar] [CrossRef] [PubMed]
- Gerotto, C.; Norici, A.; Giordano, M. Toward Enhanced Fixation of CO2 in Aquatic Biomass: Focus on Microalgae. Front. Energy Res. 2020, 8, 213. [Google Scholar] [CrossRef]
- Gong, F.; Liu, G.; Zhai, X.; Zhou, J.; Cai, Z.; Li, Y. Quantitative analysis of an engineered CO2-fixing Escherichia coli reveals great potential of heterotrophic CO2 fixation. Biotechnol. Biofuels 2015, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Schwander, T.; Burgener, S.; Erb, T.J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 2016, 354, 900–904. [Google Scholar] [CrossRef]
- Singh, R.; Singh, R.; Li, J.; Sung, B.H.; Cho, B.; Kim, D.R.; Kim, S.C.; Kalia, C.; Zhang, Y.P.; Zhao, H.; et al. Insights into cell-free conversion of CO2 to chemicals by a multienzyme cascade reaction. ACS Catal. 2018, 8, 11085–11093. [Google Scholar] [CrossRef]
- Shi, T.; Liu, S.; Zhang, Y.P.J. CO2 fixation for malate synthesis energized by starch via in vitro metabolic engineering. Metab. Eng. 2019, 55, 152–160. [Google Scholar] [CrossRef]
- Basen, M.; Geiger, I.; Henke, L. A Genetic System for the Thermophilic Acetogenic Bacterium. Appl. Environ. Microbiol. 2018, 84, e02210-17. [Google Scholar] [CrossRef]
- Mcgrath, J.M.; Long, S.P. Can the Cyanobacterial Carbon-Concentrating Mechanism Increase Photosynthesis in Crop Species? A Theoretical Analysis. Plant Physiol. 2014, 164, 2247–2261. [Google Scholar] [CrossRef]
- Rae, B.D.; Long, B.M.; Förster, B.; Nguyen, N.D.; Velanis, C.N.; Atkinson, N.; Hee, W.Y.; Mukherjee, B.; Price, G.D.; Mccormick, A.J. Progress and challenges of engineering a biophysical carbon dioxide-concentrating mechanism into higher plants. J. Exp. Bot. 2017, 68, 3717–3737. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Munive, A.; Moulin, L. Nodulation of legumes by members of the β -subclass of Proteobacteria. Nature 2001, 411, 948–950. [Google Scholar]
- Oelkers, H.E.; Cole, R.D. Carbon Dioxide Sequestration A Solution to a Global Problem. Elements 2008, 4, 305–310. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and Use of Bicarbonate in Plants: Current Knowledge and Challenges Ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef] [PubMed]
- Kruk, C.; Mazzeo, N.; Lacerot, G.; Reynolds, C.S. Classification schemes for phytoplankton: A local validation of a functional approach to the analysis of species temporal replacement. J. Plankton Res. 2002, 24, 901–912. [Google Scholar] [CrossRef]
- Brierley, A.S. Plankton. Curr. Biol. 2017, 27, R478–R483. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.; Butler, T.O.; Shuhaili, F.; Vaidyanathan, S. Diatoms for Carbon Sequestration and Bio-Based Manufacturing. Biology 2020, 9, 217. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant. Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Mona, S.; Malyan, S.K.; Saini, N.; Deepak, B.; Pugazhendhi, A.; Kumar, S.S. Chemosphere Towards sustainable agriculture with carbon sequestration, and greenhouse gas mitigation using algal biochar. Chemosphere 2021, 275, 129856. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Bioresource Technology Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Salois, P.; Markovic, P.; Hastings, J.W. A nuclear-encoded form II RuBisCO in dinoflagellates. Science 1995, 268, 1622–1624. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.M.; Andrews, T.J. The CO2 specificity of single-subunit ribulose-bisphosphate carboxylase from the dinoflagellate. Funct. Plant Biol. 1998, 25, 131–138. [Google Scholar] [CrossRef]
- Lapointe, M.; Mackenzie, T.D.; Morse, D. An external delta-carbonic anhydrase in a free-living marine dinoflagellate may circumvent diffusion-limited carbon acquisition. Plant Physiol. 2008, 147, 1427–1436. [Google Scholar] [CrossRef]
- Nimmo, I.C.; Barbrook, A.C.; Lassadi, I.; Chen, J.E.; Geisler, K.; Smith, A.G.; Aranda, M.; Purton, S.; Waller, R.F.; Nisbet, R.E.R.; et al. Genetic transformation of the dinoflagellate chloroplast. eLife 2019, 8, e45292. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Spalding, M.D.; Brown, B.E. Warm-water coral reefs and climate change. Science 2015, 350, 769–771. [Google Scholar] [CrossRef]
- Slavov, C.; Schrameyer, V.; Reus, M.; Ralph, P.J.; Hill, R.; Büchel, C.; Larkum, A.W.; Holzwarth, A.R. “Super-quenching” state protects Symbiodinium from thermal stress—Implications for coral bleaching. Biochim. Biophys. Acta 2016, 1857, 840–847. [Google Scholar] [CrossRef]
- Rehman, A.U.; Szabó, M.; Deák, Z.; Sass, L.; Larkum, A.; Ralph, P.; Vass, I. Symbiodinium sp. cells produce light-induced intra- and extracellular singlet oxygen, which mediates photodamage of the photosynthetic apparatus and has the potential to interact with the animal host in coral symbiosis. New Phytol. 2016, 212, 472–484. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 1999, 96, 8007–8012. [Google Scholar] [CrossRef]
- Howe, C.J.; Nisbet, R.E.; Barbrook, A.C. The remarkable chloroplast genome of dinoflagellates. J. Exp. Bot. 2008, 59, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Te, M.R.; Lohuis; Miller, D.J. Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): Expression of GUS in microalgae using heterologous promoter constructs. Plant J. 1998, 13, 427–435. [Google Scholar] [CrossRef]
- Walker, T.L.; Collet, C.; Purton, S. Algal transgenics in the genomic era1. J. Phycol. 2005, 41, 1077–1093. [Google Scholar] [CrossRef]
- Ortiz-Matamoros, M.F.; Villanueva, M.A.; Islas-Flores, T. Transient transformation of cultured photosynthetic dinoflagellates (Symbiodinium spp.) with plant-targeted vectors. Cienc. Mar. 2015, 41, 21–32. [Google Scholar] [CrossRef][Green Version]
- Jensen, E.L.; Yangüez, K.; Carrière, F.; Gontero, B. Storage Compound Accumulation in Diatoms as Response to Elevated CO2 Concentration. Biology 2019, 9, 5. [Google Scholar] [CrossRef]
- Burkhardt, S.; Amoroso, G.; Riebesell, U.; Sültemeyer, D. CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr. 2001, 46, 1378–1391. [Google Scholar] [CrossRef]
- Clement, R.; Jensen, E.; Prioretti, L.; Maberly, S.C.; Gontero, B. Diversity of CO2-concentrating mechanisms and responses to CO2 concentration in marine and freshwater diatoms. J. Exp. Bot. 2017, 68, 3925–3935. [Google Scholar] [CrossRef]
- Granum, E.; Raven, J.A.; Leegood, R.C. How do marine diatoms fix 10 billion tonnes of inorganic carbon per year? Can. J. Bot. 2005, 83, 898–908. [Google Scholar] [CrossRef]
- Hopkinson, B.M.; Dupont, C.L.; Matsuda, Y. The physiology and genetics of CO2 concentrating mechanisms in model diatoms. Curr. Opin. Plant Biol. 2016, 31, 51–57. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Li, Y.; Wang, Y.; Xu, M.; Zhou, B.; Lu, K.; Wang, Y. The Bloom-Forming Dinoflagellate Karenia mikimotoi Adopts Different Growth Modes When Exposed to Short or Long Period of Seawater Acidification. Toxins 2021, 13, 629. [Google Scholar] [CrossRef]
- Pierella Karlusich, J.J.; Bowler, C.; Biswas, H. Carbon Dioxide Concentration Mechanisms in Natural Populations of Marine Diatoms: Insights from Tara Oceans. Front. Plant Sci. 2021, 12, 657821. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Mitra, A.; Greenwell, H.C.; Sui, J. Monster potential meets potential monster: Pros and cons of deploying genetically modified microalgae for biofuels production. Interface Focus 2013, 3, 20120037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rothschild, L.J. The evolution of photosynthesis…again? Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2787–2801. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D. Models describing the kinetics of ribulose biphosphate carboxylase-oxygenase. Arch. Biochem. Biophys. 1979, 193, 456–468. [Google Scholar] [CrossRef]
- Farquhar, J.; Zerkle, A.L.; Bekker, A. Geological constraints on the origin of oxygenic photosynthesis. Photosynth. Res. 2011, 107, 11–36. [Google Scholar] [CrossRef]
- Berg, I.A. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 2011, 77, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Bar-Even, A.; Noor, E.; Milo, R. A survey of carbon fixation pathways through a quantitative lens. J. Exp. Bot. 2012, 63, 2325–2342. [Google Scholar] [CrossRef]
- Iñiguez, C.; Capó-Bauçà, S.; Niinemets, Ü.; Stoll, H.; Aguiló-Nicolau, P.; Galmés, J. Evolutionary trends in RuBisCO kinetics and their co-evolution with CO2 concentrating mechanisms. Plant J. 2020, 101, 897–918. [Google Scholar] [CrossRef]
- Liu, D.; Ramya RC, S.; Mueller-Cajar, O. Surveying the expanding prokaryotic Rubisco multiverse. FEMS Microbiol. Lett. 2017, 364, fnx156. [Google Scholar] [CrossRef]
- Tabita, F.R.; Satagopan, S.; Hanson, T.E.; Kreel, N.E.; Scott, S.S. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 2008, 59, 1515–1524. [Google Scholar] [CrossRef]
- Lee, J.W.; Mets, L.; Greenbau, E. Improvement of photosynthetic CO2 fixation at high light intensity through reduction of chlorophyll antenna size. Appl. Biochem. Biotechnol. 2002, 98–100, 37–48. [Google Scholar] [CrossRef]
- Lefebvre, S.; Lawson, T.; Fryer, M.; Zakhleniuk, O.V.; Lloyd, J.C.; Raines, C.A. Increased sedoheptulose-1, 7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 2005, 138, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. Variation in the k cat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J. Exp. Bot. 2002, 53, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kebeish, R.; Niessen, M.; Thiruveedhi, K.; Bari, R.; Hirsch, H.-J.; Rosenkranz, R.; Stäbler, N.; Schönfeld, B.; Kreuzaler, F.; Peterhänsel, C. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 2007, 25, 593–599. [Google Scholar] [CrossRef]
- Raines, C.A. Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ. 2006, 29, 331–339. [Google Scholar] [CrossRef]
- Tcherkez, G.G.; Farquhar, G.D.; Andrews, T.J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef]
- Kapralov, M.V.; Filatov, D.A. Widespread positive selection in the photosynthetic Rubisco enzyme. BMC Evol. Biol. 2007, 7, 73. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Savir, Y.; Noor, E.; Milo, R.; Tlusty, T. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proc. Natl. Acad. Sci. USA 2010, 107, 3475–3480. [Google Scholar] [CrossRef]
- Evans, M.; Buchanan, B.B.; Arnon, D.I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl. Acad. Sci. USA 1966, 55, 928. [Google Scholar] [CrossRef]
- Huber, H.; Gallenberger, M.; Jahn, U.; Eylert, E.; Berg, I.A.; Kockelkorn, D.; Eisenreich, W.; Fuchs, G. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. USA 2008, 105, 7851–7856. [Google Scholar] [CrossRef] [PubMed]
- Herter, S.; Fuchs, G.; Bacher, A.; Eisenreich, W. A bicyclic autotrophic CO2 fixation pathway in Chloroflexus aurantiacus. J. Biol. Chem. 2002, 277, 20277–20283. [Google Scholar] [CrossRef] [PubMed]
- Bar-Even, A.; Noor, E.; Lewis, N.E.; Milo, R. Design and analysis of synthetic carbon fixation pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 8889–8894. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Papin, J.A.; Stelling, J.; Price, N.D.; Klamt, S.; Schuster, S.; Palsson, B.O. Comparison of network-based pathway analysis methods. Trends Biotechnol. 2004, 22, 400–405. [Google Scholar] [CrossRef]
- Schilling, C.H.; Letscher, D.; Palsson, B.O. Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway-oriented perspective. J. Biol. 2000, 203, 229–248. [Google Scholar] [CrossRef]
- Schuster, S.; Dandekar, T.; Fell, D.A. Detection of elementary flux modes in biochemical networks: A promising tool for pathway analysis and metabolic engineering. Trends Biotechnol. 1999, 17, 53–60. [Google Scholar] [CrossRef]
- Lorsch, J.R. Practical Steady-State Enzyme Kinetics. In Methods in Enzymology; Elsevier: Amsterdam, The Netherland, 2014; pp. 3–15. [Google Scholar] [CrossRef]
- Manichaikul, A.; Ghamsari, L.; Hom, E.F.; Lin, C.; Murray, R.R.; Chang, R.L.; Balaji, S.; Hao, T.; Shen, Y.; Chavali, A.K.; et al. Metabolic network analysis integrated with transcript verification for sequenced genomes. Nat. Methods 2009, 6, 589–592. [Google Scholar] [CrossRef]
- Mittenthal, J.E.; Clarke, B.; Waddell, T.G.; Fawcett, G. A new method for assembling metabolic networks, with application to the Krebs citric acid cycle. J. Biol. 2001, 208, 361–382. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhang, X.; Sun, X.; Ma, Y. Shape-controlled synthesis of nanocarbons through direct conversion of carbon dioxide. Sci. Rep. 2013, 3, 3534. [Google Scholar] [CrossRef]
- Licht, S.; Douglas, A.; Ren, J.; Carter, R.; Lefler, M.; Pint, C.L. Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes. ACS Cent. Sci. 2016, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Lim, W.-G.; Kang, D.; Park, J.H.; Lee, H.; Lee, J.; Lee, J.W. Transformation of carbon dioxide into carbon nanotubes for enhanced ion transport and energy storage. Nanoscale 2020, 12, 7822–7833. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; He, M.; Zhao, D.; Li, Z.; Shang, T. Synthesis of carbon nanorods by reduction of carbon bisulfide. J. Alloy. Compd. 2010, 507, 38–41. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Ji, D.; Liu, Y.; Li, L.; Yuan, D.; Zhang, Z.; Ren, J.; Lefler, M.; Wang, B.; et al. One-pot synthesis of nanostructured carbon materials from carbon dioxide via electrolysis in molten carbonate salts. Carbon 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef]
- Motiei, M.; Hacohen, Y.R.; Calderon-Moreno, J.; Gedanken, A. Preparing carbon nanotubes and nested fullerenes from supercritical CO2 by a chemical reaction. J. Am. Chem. Soc. 2001, 123, 8624–8625. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Choudhury, H.A.; Wang, D.; El-Halwagi, M.M.; Weitz, E.; Elbashir, N.O. A novel CO2 utilization technology for the synergistic co-production of multi-walled carbon nanotubes and syngas. Sci. Rep. 2021, 11, 1417. [Google Scholar] [CrossRef]
- Daiyan, R.; Lovell, E.C.; Huang, B.; Zubair, M.; Leverett, J.; Zhang, Q.; Lim, S.; Horlyck, J.; Tang, J.; Lu, X.; et al. Uncovering Atomic-Scale Stability and Reactivity in Engineered Zinc Oxide Electrocatalysts for Controllable Syngas Production. Adv. Energy Mater. 2020, 10, 2001381. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal–Organic Framework-Based Catalysts: Chemical Fixation of CO2 with Epoxides Leading to Cyclic Organic Carbonates. Front. Energy Res. 2015, 2, 63. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Phang, H.S.; Li, Y.; Zhao, Y. Highly Effective Carbon Fixation via Catalytic Conversion of CO2 by an Acylamide-Containing Metal–Organic Framework. Chem. Mater. 2017, 29, 9256–9261. [Google Scholar] [CrossRef]
- Tran, Y.B.N.; Nguyen, P.T.K.; Luong, Q.T.; Nguyen, K.D. Series of M-MOF-184 (M = Mg, Co, Ni, Zn, Cu, Fe) Metal–Organic Frameworks for Catalysis Cycloaddition of CO2. Inorg. Chem. 2020, 59, 16747–16759. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cui, C.; Shi, C.; Wu, Z.S.; Yang, J.; Cheng, R.; Guang, T.; Wang, H.; Lu, H.; Wang, X. High-Energy-Density Hydrogen-Ion-Rocking-Chair Hybrid Supercapacitors Based on Ti3C2Tx MXene and Carbon Nanotubes Mediated by Redox Active Molecule. ACS Nano 2019, 13, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Gaur, R.; Yadav, M.; Goswami, A.; Zbořil, R.; Gawande, M.B. An efficient copper-based magnetic nanocatalyst for the fixation of carbon dioxide at atmospheric pressure. Sci. Rep. 2019, 8, 1901. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, G.A.; Alamiry, M.A.H.; Šiller, L. Nickel Nanoparticles for Enhancing Carbon Capture. J. Nanomater. 2015, 2015, 581785. [Google Scholar] [CrossRef]
- Kumar, R.; Mangalapuri, R.; Ahmadi, M.H.; Vo, D.-V.N.; Solanki, R.; Kumar, P. The role of nanotechnology on post-combustion CO2 absorption in process industries. Int. J. Low-Carbon Technol. 2020, 15, 361–367. [Google Scholar] [CrossRef]
- Normile, D. Round and Round: A Guide to the Carbon Cycle. Science 2009, 325, 1642–1643. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.H.; Bambach, R.K.; Payne, J.L.; Pruss, S.; Fischer, W.W. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 2007, 256, 295–313. [Google Scholar] [CrossRef]
- Ahmed, A.; Ahmed, Q.; Odelade, K.A. Microbial Inoculants for Improving Carbon Sequestration in Agroecosystems to Mitigate Climate Change Microbial Inoculants for Improving Carbon Sequestration in Agroecosystems to Mitigate Climate Change. In Handbook of Climate Change Resilience; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-ghanim, K.A.; Ahmed, Z.; Al-mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks. J. King Saud Univ.-Sci. 2020, 33, 101282. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Koide, R.T. Soil Biology & Biochemistry Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter. Soil Biol. Biochem. 2014, 77, 150–157. [Google Scholar] [CrossRef]
- Pal, A.; Pandey, S. Role of Glomalin in Improving Soil Fertility: A Review. Int. J. Plant Soil Sci. 2014, 3, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-baracaldo, P.; Bianchini, G.; Wilson, J.D.; Knoll, A.H. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 2021, 30, 143–157. [Google Scholar] [CrossRef] [PubMed]

| Microorganism | Description | Pathway | Advantage | Disadvantage | Reference |
|---|---|---|---|---|---|
| Methanogens | Prokaryotic, anaerobic, archaea | Reductive acetyl-CoA pathway | Efficient carbon capture, require low energy for carbon fixation, used for waste water treatment | Require specific conditions for growth, slow growth rate, oxygen-sensitive | [13,16] |
| Acetogens | Prokaryotic, anaerobic, bacteria | Reductive acetyl-CoA pathway | Efficient carbon capture, require low energy for fixation | Require specific conditions for growth, slow growth rate, oxygen-sensitive | [13,39] |
| Cyanobacteria | Prokaryotic, aerobic, bacteria | Calvin cycle | Carbon-concentrating mechanism present | Use inefficient RubisCO for carbon-fixation | [39,40] |
| Algae | Eukaryotic, single- or multi-cellular | Calvin cycle | Quick conversion of carbon for biomass and useful byproducts, may contain carbon-concentrating mechanisms, red algae contain better rubisco activases than other species. | Use inefficient RubisCO for carbon-fixation | [22,29,41] |
| Proteobacteria | Usually, Gram-negative bacteria, includes nitrogen-fixing bacteria, pathogenic | Reductive acetyl-CoA cycle, Calvin cycle | Different pathways across species, production of bioplastics and medicines as byproducts | Some require high pH, certain may use RubisCO | [35,42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, S.; Abraham, J.; Jordan, N.; Lindsay, M.; Chauhan, N. Synthetic, Photosynthetic, and Chemical Strategies to Enhance Carbon Dioxide Fixation. C 2022, 8, 18. https://doi.org/10.3390/c8010018
Ray S, Abraham J, Jordan N, Lindsay M, Chauhan N. Synthetic, Photosynthetic, and Chemical Strategies to Enhance Carbon Dioxide Fixation. C. 2022; 8(1):18. https://doi.org/10.3390/c8010018
Chicago/Turabian StyleRay, Supriyo, Jason Abraham, Nyiah Jordan, Mical Lindsay, and Neha Chauhan. 2022. "Synthetic, Photosynthetic, and Chemical Strategies to Enhance Carbon Dioxide Fixation" C 8, no. 1: 18. https://doi.org/10.3390/c8010018
APA StyleRay, S., Abraham, J., Jordan, N., Lindsay, M., & Chauhan, N. (2022). Synthetic, Photosynthetic, and Chemical Strategies to Enhance Carbon Dioxide Fixation. C, 8(1), 18. https://doi.org/10.3390/c8010018





