Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries
Abstract
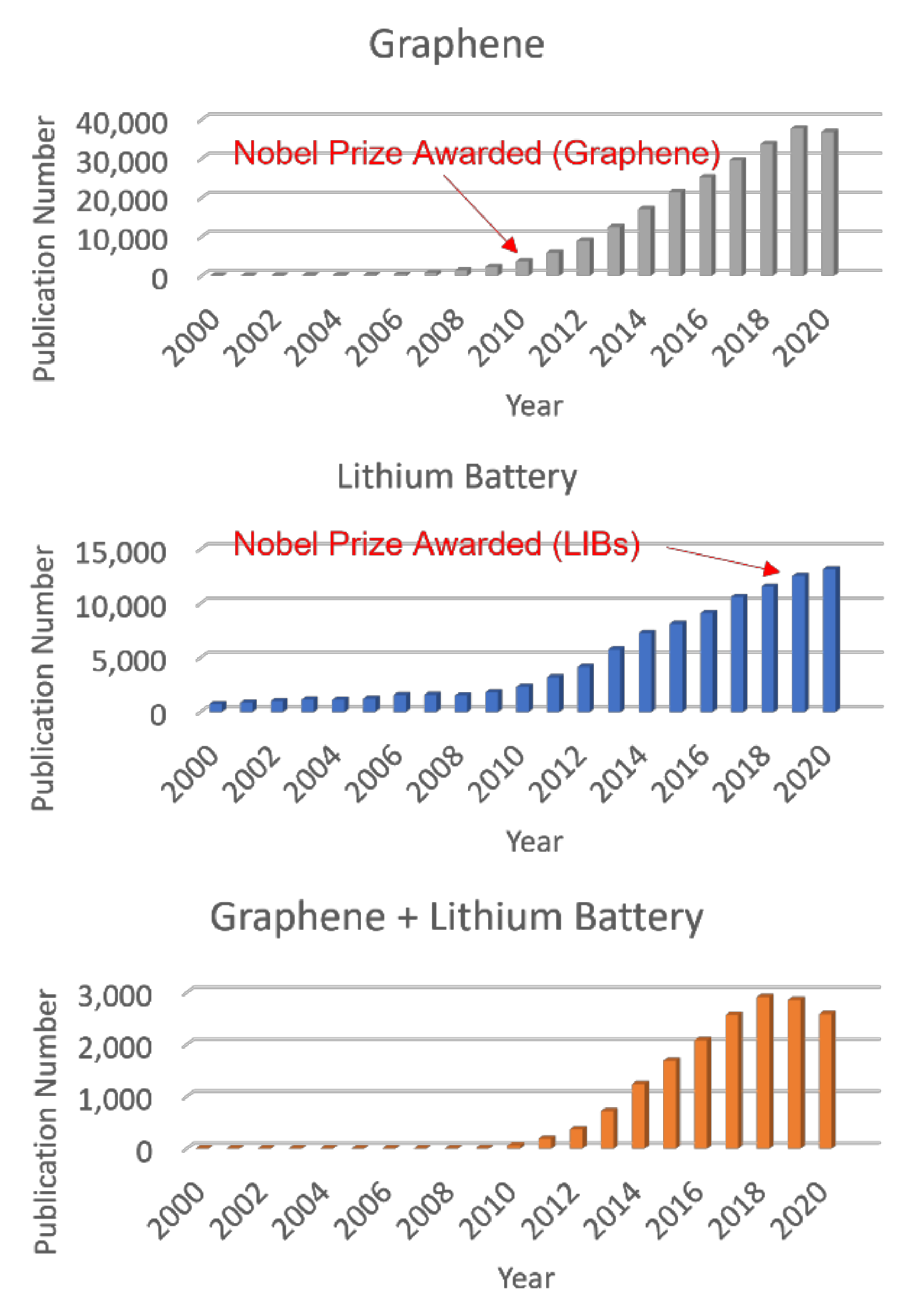
:1. Introduction
2. Designs and Methods of Integrating Graphene into Battery Components
3. Graphene-Enhanced Anode Materials
3.1. Graphene-Enhanced Alloy-Type Anode Materials
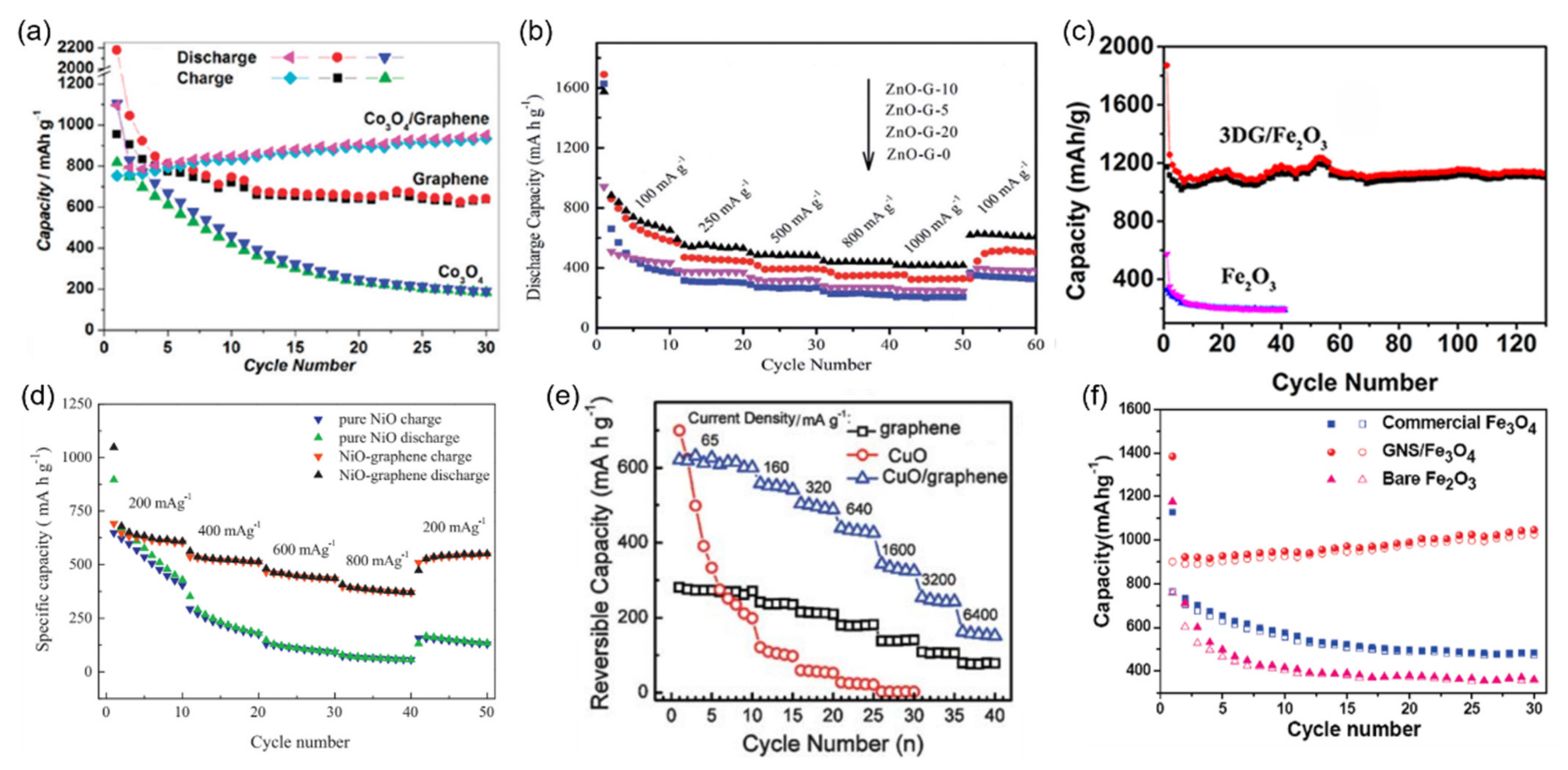
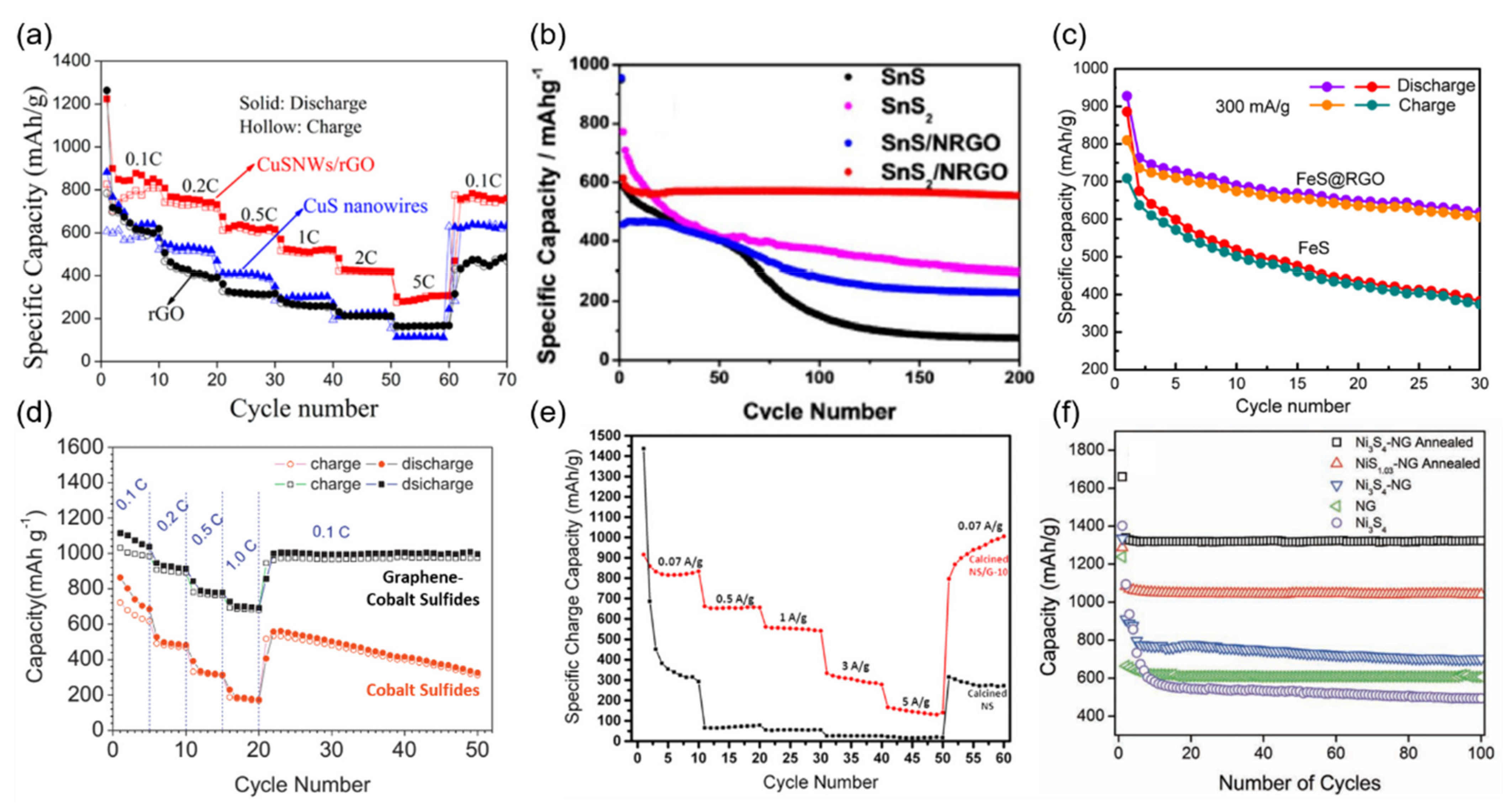
3.2. Graphene-Enhanced Conversion-Type Anode Materials
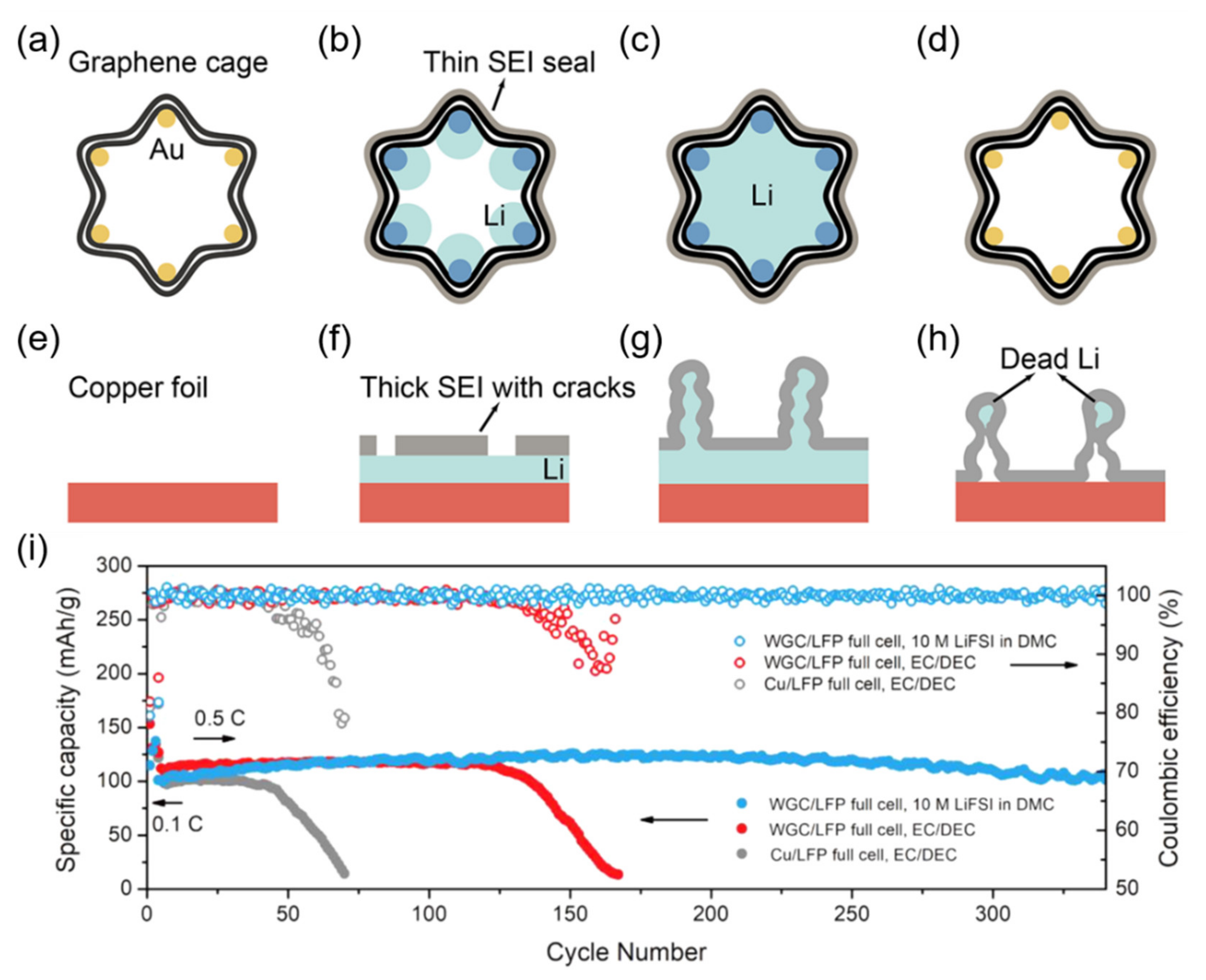
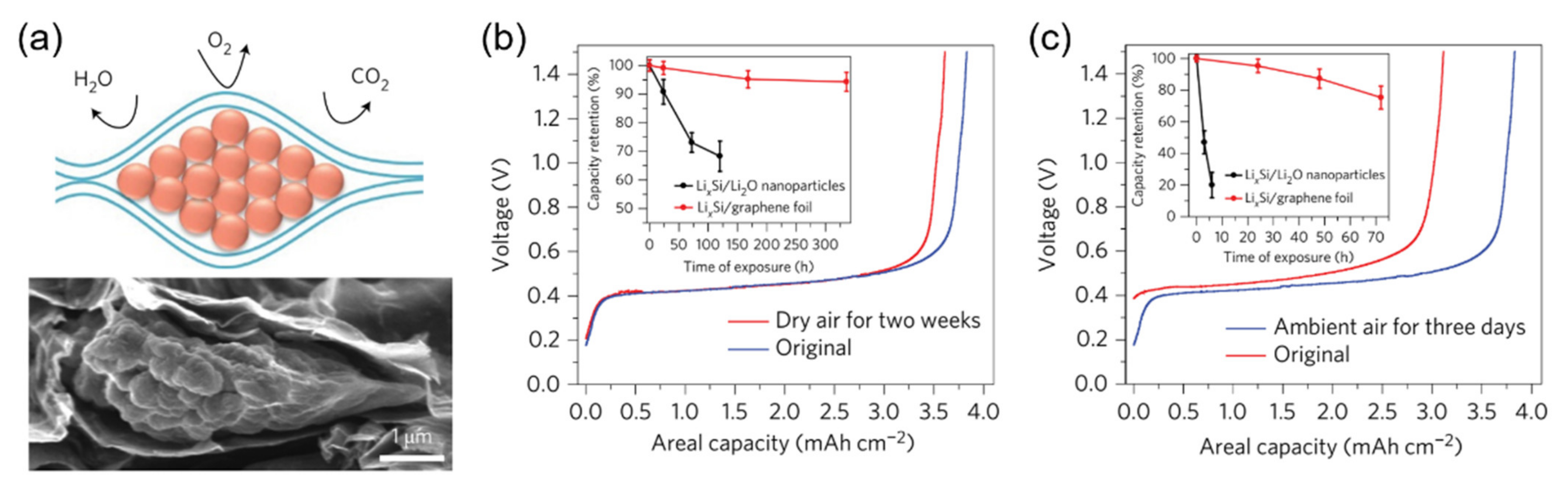
3.3. Graphene-Enhanced Lithium Metal and Lithiated Anode Materials
4. Graphene-Enhanced Cathode Materials
4.1. Graphene-Enhanced Cathode Materials for Lithium-Ion Batteries
4.2. Graphene-Enhanced Sulfur Cathode Materials
5. Graphene-Enhanced Separators
6. Graphene-Enhanced Current Collectors
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, B.Z.; Huang, W.C. Nano-Scaled Graphene Plates. U.S. Patent 7,071,258 B1, 4 July 2006. [Google Scholar]
- The Nobel Prize in Physics 2010. Available online: https://www.nobelprize.org/prizes/physics/2010/summary/ (accessed on 28 July 2021).
- The Nobel Prize in Chemistry 2019. Available online: https://www.nobelprize.org/prizes/chemistry/2019/summary/ (accessed on 25 August 2021).
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A Review of Advanced and Practical Lithium Battery Materials. J. Mater. Chem. 2011, 21, 9938. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for Batteries, Supercapacitors and Beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Kausar, A. Applications of Polymer/Graphene Nanocomposite Membranes: A Review. Mater. Res. Innov. 2019, 23, 276–287. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. MATERIALS SCIENCE: Graphene-Based Materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The Role of Graphene for Electrochemical Energy Storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Wu, S.; Ge, R.; Lu, M.; Xu, R.; Zhang, Z. Graphene-Based Nano-Materials for Lithium–Sulfur Battery and Sodium-Ion Battery. Nano Energy 2015, 15, 379–405. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on Graphene and Its Derivatives: Synthesis Methods and Potential Industrial Implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, K. Comprehensive Review on the Use of Graphene-Based Substrates for Regenerative Medicine and Biomedical Devices. ACS Appl. Mater. Interfaces 2016, 8, 26431–26457. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Ju Kim, H.; Ho Jung, D. A Comprehensive Review of Graphene Nanocomposites: Research Status and Trends. J. Nanomater. 2013, 2013, 763953. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/Graphene Composites: A Review. J. Mater. Sci. 2019, 54, 12236–12289. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, Z.; Huang, L.; Essa, K.; Bilotti, E.; Zhang, H.; Peijs, T.; Hao, L. Additive Manufacturing High Performance Graphene-Based Composites: A Review. Compos. Part Appl. Sci. Manuf. 2019, 124, 105483. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A Comprehensive Review on Graphene-Based Anti-Corrosive Coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A Brief Review of Corrosion Protective Films and Coatings Based on Graphene and Graphene Oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Kong, W.; Kum, H.; Bae, S.-H.; Shim, J.; Kim, H.; Kong, L.; Meng, Y.; Wang, K.; Kim, C.; Kim, J. Path towards Graphene Commercialization from Lab to Market. Nat. Nanotechnol. 2019, 14, 927–938. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, H.-M. The Global Growth of Graphene. Nat. Nanotechnol. 2014, 9, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mo, X.; Law, W.-C.; Chan, K.C. 3D Printed Graphene/Nickel Electrodes for High Areal Capacitance Electrochemical Storage. J. Mater. Chem. A 2019, 7, 4055–4062. [Google Scholar] [CrossRef]
- Ghany, N.A.A.; Elsherif, S.A.; Handal, H.T. Revolution of Graphene for Different Applications: State-of-the-Art. Surf. Interfaces 2017, 9, 93–106. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Priydarshi, M.K.; Gao, T.; Song, X.; Zhang, Y.; Liu, Z. Graphene Glass from Direct CVD Routes: Production and Applications. Adv. Mater. 2016, 28, 10333–10339. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Banks, C.E. The Electrochemistry of CVD Graphene: Progress and Prospects. Phys. Chem. Chem. Phys. 2012, 14, 8264. [Google Scholar] [CrossRef]
- Porwal, H.; Grasso, S.; Reece, M.J. Review of Graphene–Ceramic Matrix Composites. Adv. Appl. Ceram. 2013, 112, 443–454. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Graphene via Sonication Assisted Liquid-Phase Exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide Using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Krishnan, D.; Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.J.; Jang, H.D.; Huang, J. Energetic Graphene Oxide: Challenges and Opportunities. Nano Today 2012, 7, 137–152. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Functional Composite Materials Based on Chemically Converted Graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A Review on the Separators of Liquid Electrolyte Li-Ion Batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E.; Holze, R. Carbon Anode Materials for Lithium Ion Batteries. J. Power Sources 2003, 114, 228–236. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-J. A Review of the Electrochemical Performance of Alloy Anodes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Wang, F.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Engineering of Carbon and Other Protective Coating Layers for Stabilizing Silicon Anode Materials. Carbon Energy 2019, 1, 219–245. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S. Chemomechanical Modeling of Lithiation-Induced Failure in High-Volume-Change Electrode Materials for Lithium Ion Batteries. Npj Comput. Mater. 2017, 3, 7. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable Silicon–Carbon Nanocables Sandwiched between Reduced Graphene Oxide Sheets as Lithium Ion Battery Anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Chae, S.; Jeong, S.; Oh, P.; Cho, J. Elastic a -Silicon Nanoparticle Backboned Graphene Hybrid as a Self-Compacting Anode for High-Rate Lithium Ion Batteries. ACS Nano 2014, 8, 8591–8599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Facile Synthesis of Silicon Nanoparticles Inserted into Graphene Sheets as Improved Anode Materials for Lithium-Ion Batteries. Chem. Commun. 2012, 48, 2198. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Zhong, C.; Chou, S.-L.; Liu, H.-K. Flexible Free-Standing Graphene-Silicon Composite Film for Lithium-Ion Batteries. Electrochem. Commun. 2010, 12, 1467–1470. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/Nanosized Silicon Composites for Lithium Battery Anodes with Improved Cycling Stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
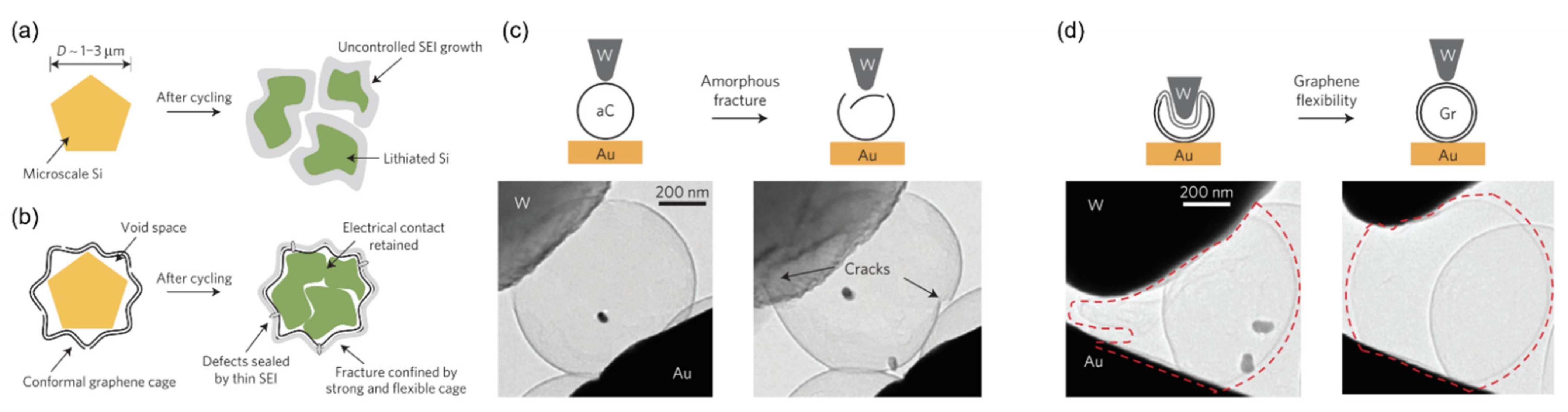
- Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y. Growth of Conformal Graphene Cages on Micrometre-Sized Silicon Particles as Stable Battery Anodes. Nat. Energy 2016, 1, 15029. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Wang, B.; Zhang, Y.; Ning, J.; Xiao, Z.; Zhang, X.; Chang, Y.; Zhi, L. High-Performance Silicon Battery Anodes Enabled by Engineering Graphene Assemblies. Nano Lett. 2015, 15, 6222–6228. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon Nanoparticles–Graphene Paper Composites for Li Ion Battery Anodes. Chem. Commun. 2010, 46, 2025. [Google Scholar] [CrossRef] [PubMed]
- Son, I.H.; Hwan Park, J.; Kwon, S.; Park, S.; Rümmeli, M.H.; Bachmatiuk, A.; Song, H.J.; Ku, J.; Choi, J.W.; Choi, J.; et al. Silicon Carbide-Free Graphene Growth on Silicon for Lithium-Ion Battery with High Volumetric Energy Density. Nat. Commun. 2015, 6, 7393. [Google Scholar] [CrossRef] [Green Version]
- Eom, K.; Joshi, T.; Bordes, A.; Do, I.; Fuller, T.F. The Design of a Li-Ion Full Cell Battery Using a Nano Silicon and Nano Multi-Layer Graphene Composite Anode. J. Power Sources 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Ji, J.; Ji, H.; Zhang, L.L.; Zhao, X.; Bai, X.; Fan, X.; Zhang, F.; Ruoff, R.S. Graphene-Encapsulated Si on Ultrathin-Graphite Foam as Anode for High Capacity Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4673–4677. [Google Scholar] [CrossRef]
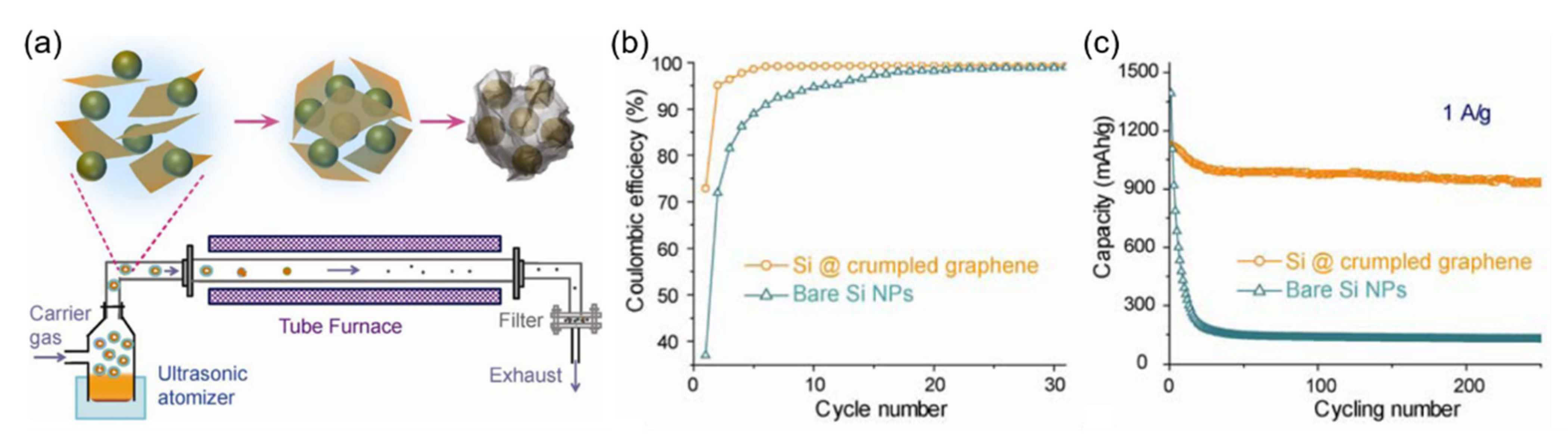
- Luo, J.; Zhao, X.; Wu, J.; Jang, H.D.; Kung, H.H.; Huang, J. Crumpled Graphene-Encapsulated Si Nanoparticles for Lithium Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sun, J.; Yu, Z.; Yin, Y.; Xin, S.; Yu, S.; Guo, Y. SiOx Encapsulated in Graphene Bubble Film: An Ultrastable Li-Ion Battery Anode. Adv. Mater. 2018, 30, 1707430. [Google Scholar] [CrossRef]
- Shi, L.; Pang, C.; Chen, S.; Wang, M.; Wang, K.; Tan, Z.; Gao, P.; Ren, J.; Huang, Y.; Peng, H.; et al. Vertical Graphene Growth on SiO Microparticles for Stable Lithium Ion Battery Anodes. Nano Lett. 2017, 17, 3681–3687. [Google Scholar] [CrossRef]
- Chen, K.-S.; Balla, I.; Luu, N.S.; Hersam, M.C. Emerging Opportunities for Two-Dimensional Materials in Lithium-Ion Batteries. ACS Energy Lett. 2017, 2, 2026–2034. [Google Scholar] [CrossRef]
- Mo, R.; Rooney, D.; Sun, K.; Yang, H.Y. 3D Nitrogen-Doped Graphene Foam with Encapsulated Germanium/Nitrogen-Doped Graphene Yolk-Shell Nanoarchitecture for High-Performance Flexible Li-Ion Battery. Nat. Commun. 2017, 8, 13949. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Du, J. Facile Synthesis of Germanium–Graphene Nanocomposites and Their Application as Anode Materials for Lithium Ion Batteries. CrystEngComm 2012, 14, 397–400. [Google Scholar] [CrossRef]
- Ren, J.-G.; Wu, Q.-H.; Tang, H.; Hong, G.; Zhang, W.; Lee, S.-T. Germanium–Graphene Composite Anode for High-Energy Lithium Batteries with Long Cycle Life. J. Mater. Chem. A 2013, 1, 1821–1826. [Google Scholar] [CrossRef]
- Yuan, F.-W.; Tuan, H.-Y. Scalable Solution-Grown High-Germanium-Nanoparticle-Loading Graphene Nanocomposites as High-Performance Lithium-Ion Battery Electrodes: An Example of a Graphene-Based Platform toward Practical Full-Cell Applications. Chem. Mater. 2014, 26, 2172–2179. [Google Scholar] [CrossRef]
- Qin, J.; He, C.; Zhao, N.; Wang, Z.; Shi, C.; Liu, E.-Z.; Li, J. Graphene Networks Anchored with Sn@Graphene as Lithium Ion Battery Anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, X.; Lian, P.; Yang, W.; Wang, H. Superior Cycle Performance of Sn@C/Graphene Nanocomposite as an Anode Material for Lithium-Ion Batteries. J. Solid State Chem. 2011, 184, 1400–1404. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Chui, Y.-S.; Wu, Q.-H.; Chen, X.; Zhang, W. Three-Dimensional Sn–Graphene Anode for High-Performance Lithium-Ion Batteries. Nanoscale 2013, 5, 10599. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Son, Y.; Park, C.; Cho, J.; Choi, H.C. Catalyst-Free Direct Growth of a Single to a Few Layers of Graphene on a Germanium Nanowire for the Anode Material of a Lithium Battery. Angew. Chem. Int. Ed. 2013, 52, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Lou, X.W. Nanostructured Conversion-Type Anode Materials for Advanced Lithium-Ion Batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Seo, D.-H.; Kim, S.-W.; Kim, J.; Kang, K. Highly Reversible Co3O4/Graphene Hybrid Anode for Lithium Rechargeable Batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Jiang, T.; Bu, F.; Feng, X.; Shakir, I.; Hao, G.; Xu, Y. Porous Fe2O3 Nanoframeworks Encapsulated within Three-Dimensional Graphene as High-Performance Flexible Anode for Lithium-Ion Battery. ACS Nano 2017, 11, 5140–5147. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.; Cheng, H.-M. Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured Reduced Graphene Oxide/Fe2O3 Composite as a High-Performance Anode Material for Lithium Ion Batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef]
- Mai, Y.J.; Shi, S.J.; Zhang, D.; Lu, Y.; Gu, C.D.; Tu, J.P. NiO–Graphene Hybrid as an Anode Material for Lithium Ion Batteries. J. Power Sources 2012, 204, 155–161. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.-L.; Shu, C.-Y.; Guo, Y.-G.; Wang, C.-R. Synthesis of CuO/Graphene Nanocomposite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 10661. [Google Scholar] [CrossRef]
- Mai, Y.J.; Wang, X.L.; Xiang, J.Y.; Qiao, Y.Q.; Zhang, D.; Gu, C.D.; Tu, J.P. CuO/Graphene Composite as Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 2306–2311. [Google Scholar] [CrossRef]
- Li, L.; Raji, A.-R.O.; Tour, J.M. Graphene-Wrapped MnO2 -Graphene Nanoribbons as Anode Materials for High-Performance Lithium Ion Batteries. Adv. Mater. 2013, 25, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Yu, M.; Wang, A.; Wang, Y.; Li, C.; Shi, G. An Alumina Stabilized ZnO–Graphene Anode for Lithium Ion Batteries via Atomic Layer Deposition. Nanoscale 2014, 6, 11419–11424. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yin, X.; Zhang, M.; Hao, Q.; Wang, Y.; Wang, T. In Situ Synthesis of SnO2/Graphene Nanocomposite and Their Application as Anode Material for Lithium Ion Battery. Mater. Lett. 2010, 64, 2076–2079. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. High Reversible Capacity of SnO2/Graphene Nanocomposite as an Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 4532–4539. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, L.; Yang, M.; Song, X.; Zhao, H.; Jia, Z.; Sun, K.; Liu, G. One-Pot Synthesis of Copper Sulfide Nanowires/Reduced Graphene Oxide Nanocomposites with Excellent Lithium-Storage Properties as Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 15726–15734. [Google Scholar] [CrossRef] [Green Version]
- Youn, D.H.; Stauffer, S.K.; Xiao, P.; Park, H.; Nam, Y.; Dolocan, A.; Henkelman, G.; Heller, A.; Mullins, C.B. Simple Synthesis of Nanocrystalline Tin Sulfide/N-Doped Reduced Graphene Oxide Composites as Lithium Ion Battery Anodes. ACS Nano 2016, 10, 10778–10788. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Lin, Q.; Yuan, B.; Chen, G.; Xie, P.; Li, Y.; Xu, Y.; Deng, S.; Smirnov, S.; Luo, H. Reduced Graphene Oxide Wrapped FeS Nanocomposite for Lithium-Ion Battery Anode with Improved Performance. ACS Appl. Mater. Interfaces 2013, 5, 5330–5335. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, T.; Wang, Z.; Chang, K.; Chen, W. Synthesis and Electrochemical Performances of Cobalt Sulfides/Graphene Nanocomposite as Anode Material of Li-Ion Battery. J. Power Sources 2013, 235, 122–128. [Google Scholar] [CrossRef]
- Peng, S.; Han, X.; Li, L.; Zhu, Z.; Cheng, F.; Srinivansan, M.; Adams, S.; Ramakrishna, S. Unique Cobalt Sulfide/Reduced Graphene Oxide Composite as an Anode for Sodium-Ion Batteries with Superior Rate Capability and Long Cycling Stability. Small 2016, 12, 1359–1368. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Yang, X.; Yang, J.; Chen, X.; Ying, J.Y. Graphene-Wrapped Nickel Sulfide Nanoprisms with Improved Performance for Li-Ion Battery Anodes and Supercapacitors. Nano Energy 2016, 26, 425–437. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Hou, Y. Nickel Sulfide/Nitrogen-Doped Graphene Composites: Phase-Controlled Synthesis and High Performance Anode Materials for Lithium Ion Batteries. Small 2013, 9, 1321–1328. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Wu, W.; Wu, F.; Li, L.; Qian, J.; Xu, R.; Wu, H.; Albishri, H.M.; Al-Bogami, A.S.; et al. Free-Standing Hierarchically Sandwich-Type Tungsten Disulfide Nanotubes/Graphene Anode for Lithium-Ion Batteries. Nano Lett. 2014, 14, 5899–5904. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, X.; Shen, X.; Zhang, R.; Liu, H.; Zhang, Q. A Review of Composite Lithium Metal Anode for Practical Applications. Adv. Mater. Technol. 2020, 5, 1900806. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Aurbach, D. A Short Review of Failure Mechanisms of Lithium Metal and Lithiated Graphite Anodes in Liquid Electrolyte Solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.-R.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. Int. Ed. 2017, 56, 7764–7768. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, Y.; Liu, Y.; Lin, D.; Zhu, C.; Chen, G.; Yang, A.; Yan, K.; Chen, H.; et al. Wrinkled Graphene Cages as Hosts for High-Capacity Li Metal Anodes Shown by Cryogenic Electron Microscopy. Nano Lett. 2019, 19, 1326–1335. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, G.; Yan, K.; Xie, J.; Li, Y.; Liao, L.; Jin, Y.; Liu, K.; Hsu, P.-C.; Wang, J.; et al. Air-Stable and Freestanding Lithium Alloy/Graphene Foil as an Alternative to Lithium Metal Anodes. Nat. Nanotechnol. 2017, 12, 993–999. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Peng, H.-J.; Huang, J.-Q.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Dual-Phase Lithium Metal Anode Containing a Polysulfide-Induced Solid Electrolyte Interphase and Nanostructured Graphene Framework for Lithium–Sulfur Batteries. ACS Nano 2015, 9, 6373–6382. [Google Scholar] [CrossRef]
- Dong, L.; Nie, L.; Liu, W. Water-Stable Lithium Metal Anodes with Ultrahigh-Rate Capability Enabled by a Hydrophobic Graphene Architecture. Adv. Mater. 2020, 32, 1908494. [Google Scholar] [CrossRef]
- Huang, G.; Han, J.; Zhang, F.; Wang, Z.; Kashani, H.; Watanabe, K.; Chen, M. Lithiophilic 3D Nanoporous Nitrogen-Doped Graphene for Dendrite-Free and Ultrahigh-Rate Lithium-Metal Anodes. Adv. Mater. 2019, 31, 1805334. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered Reduced Graphene Oxide with Nanoscale Interlayer Gaps as a Stable Host for Lithium Metal Anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Liu, S.; Wang, A.; Li, Q.; Wu, J.; Chiou, K.; Huang, J.; Luo, J. Crumpled Graphene Balls Stabilized Dendrite-Free Lithium Metal Anodes. Joule 2018, 2, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, R.; Thomas, A.V.; Datta, D.; Singh, E.; Li, J.; Eksik, O.; Shenoy, V.B.; Koratkar, N. Defect-Induced Plating of Lithium Metal within Porous Graphene Networks. Nat. Commun. 2014, 5, 3710. [Google Scholar] [CrossRef] [Green Version]
- Pu, J.; Li, J.; Shen, Z.; Zhong, C.; Liu, J.; Ma, H.; Zhu, J.; Zhang, H.; Braun, P.V. Interlayer Lithium Plating in Au Nanoparticles Pillared Reduced Graphene Oxide for Lithium Metal Anodes. Adv. Funct. Mater. 2018, 28, 1804133. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, C.J.; Lu, P.; Dong, Y.; Wen, P.; Wu, Z.-S. Conducting and Lithiophilic MXene/Graphene Framework for High-Capacity, Dendrite-Free Lithium–Metal Anodes. ACS Nano 2019, 13, 14308–14318. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, X.; Yang, Y.-W.; Huang, J.; Liu, X.; Luo, J. Horizontal Centripetal Plating in the Patterned Voids of Li/Graphene Composites for Stable Lithium-Metal Anodes. Chem 2018, 4, 2192–2200. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhai, P.; Legut, D.; Wang, L.; Liu, X.; Li, B.; Dong, C.; Fan, Y.; Gong, Y.; Zhang, Q. S-Doped Graphene-Regional Nucleation Mechanism for Dendrite-Free Lithium Metal Anodes. Adv. Energy Mater. 2019, 9, 1804000. [Google Scholar] [CrossRef]
- Xue, P.; Liu, S.; Shi, X.; Sun, C.; Lai, C.; Zhou, Y.; Sui, D.; Chen, Y.; Liang, J. A Hierarchical Silver-Nanowire-Graphene Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Lithium-Metal Composite Anodes. Adv. Mater. 2018, 30, 1804165. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Spachis, L.; Zisis, G.; Raptis, I.; Papanikolaou, N.; Vavouliotis, A.; Penedo, R.; Fernandes, N.; Dimoulas, A. Layer-by-Layer Assembled Graphene Coatings on Polyurethane Films as He Permeation Barrier. Prog. Org. Coat. 2021, 150, 105984. [Google Scholar] [CrossRef]
- Lochala, J.A.; Zhang, H.; Wang, Y.; Okolo, O.; Li, X.; Xiao, J. Practical Challenges in Employing Graphene for Lithium-Ion Batteries and Beyond. Small Methods 2017, 1, 1700099. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Liu, Y.; Huang, Z.-D.; He, Y.; Kim, J.-K. Percolation Threshold of Graphene Nanosheets as Conductive Additives in Li4Ti5O12 Anodes of Li-Ion Batteries. Nanoscale 2013, 5, 2100. [Google Scholar] [CrossRef]
- Jiang, K.-C.; Xin, S.; Lee, J.-S.; Kim, J.; Xiao, X.-L.; Guo, Y.-G. Improved Kinetics of LiNi1/3Mn1/3Co1/3O2 Cathode Material through Reduced Graphene Oxide Networks. Phys. Chem. Chem. Phys. 2012, 14, 2934. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Zhu, J.; Wang, B.; Guo, C.; Wang, D. The Composite Electrode of LiFePO4 Cathode Materials Modified with Exfoliated Graphene from Expanded Graphite for High Power Li-Ion Batteries. J. Mater. Chem. A 2014, 2, 2822–2829. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Liu, W.-R. Effects of Graphene Nanosheets with Different Lateral Sizes as Conductive Additives on the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li Ion Batteries. Polymers 2020, 12, 1162. [Google Scholar] [CrossRef]
- Tian, R.; Alcala, N.; O’Neill, S.J.K.; Horvath, D.V.; Coelho, J.; Griffin, A.J.; Zhang, Y.; Nicolosi, V.; O’Dwyer, C.; Coleman, J.N. Quantifying the Effect of Electronic Conductivity on the Rate Performance of Nanocomposite Battery Electrodes. ACS Appl. Energy Mater. 2020, 3, 2966–2974. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, X.; King, S.T.; Quilty, C.D.; Zhu, Y.; Takeuchi, K.J.; Takeuchi, E.S.; Bock, D.C.; Wang, L.; Marschilok, A.C.; et al. Unveiling the Dimensionality Effect of Conductive Fillers in Thick Battery Electrodes for High-Energy Storage Systems. Appl. Phys. Rev. 2020, 7, 041405. [Google Scholar] [CrossRef]
- Sun, D.; Tan, Z.; Tian, X.; Ke, F.; Wu, Y.; Zhang, J. Graphene: A Promising Candidate for Charge Regulation in High-Performance Lithium-Ion Batteries. Nano Res. 2021. [Google Scholar] [CrossRef]
- Robertson, A.W.; Allen, C.S.; Wu, Y.A.; He, K.; Olivier, J.; Neethling, J.; Kirkland, A.I.; Warner, J.H. Spatial Control of Defect Creation in Graphene at the Nanoscale. Nat. Commun. 2012, 3, 1144. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.-H.; Zhang, J.; Jin, X.; Liu, J.-Y.; Li, Q.; Li, M.-H.; Cai, W.; Wu, D.-Y.; Zhan, D.; Ren, B. Quantitative Correlation between Defect Density and Heterogeneous Electron Transfer Rate of Single Layer Graphene. J. Am. Chem. Soc. 2014, 136, 16609–16617. [Google Scholar] [CrossRef]
- Du, Z.; Ai, W.; Sun, C.; Zou, C.; Zhao, J.; Chen, Y.; Dong, X.; Liu, J.; Sun, G.; Yu, T.; et al. Engineering the Li Storage Properties of Graphene Anodes: Defect Evolution and Pore Structure Regulation. ACS Appl. Mater. Interfaces 2016, 8, 33712–33722. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Xu, F.; Yin, J.; Ren, H.; Zhuo, Q.; Long, Z.; Zhang, P. Preparation of Nano-Structured LiFePO4/Graphene Composites by Co-Precipitation Method. Electrochem. Commun. 2010, 12, 10–13. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Liu, Z.; Xiao, C.; Dong, S.; Han, P.; Zhang, Z.; Zhang, X.; Bi, C.; Cui, G. A Facile Method of Preparing Mixed Conducting LiFePO4/Graphene Composites for Lithium-Ion Batteries. Solid State Ion. 2010, 181, 1685–1689. [Google Scholar] [CrossRef]
- Su, C.; Bu, X.; Xu, L.; Liu, J.; Zhang, C. A Novel LiFePO4/Graphene/Carbon Composite as a Performance-Improved Cathode Material for Lithium-Ion Batteries. Electrochim. Acta 2012, 64, 190–195. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhu, Y.; Liu, Z. Graphene Modified LiFePO4 Cathode Materials for High Power Lithium Ion Batteries. J. Mater. Chem. 2011, 21, 3353. [Google Scholar] [CrossRef]
- Toprakci, O.; Toprakci, H.A.K.; Ji, L.; Lin, Z.; Gu, R.; Zhang, X. LiFePO4 Nanoparticles Encapsulated in Graphene-Containing Carbon Nanofibers for Use as Energy Storage Materials. J. Renew. Sustain. Energy 2012, 4, 013121. [Google Scholar] [CrossRef]
- Lim, J.; Gim, J.; Song, J.; Nguyen, D.T.; Kim, S.; Jo, J.; Mathew, V.; Kim, J. Direct Formation of LiFePO4/Graphene Composite via Microwave-Assisted Polyol Process. J. Power Sources 2016, 304, 354–359. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and Graphene-Based Composites as Li-Ion Battery Electrode Materials and Their Application in Full Cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent Progress in the Synthesis of Graphene and Derived Materials for next Generation Electrodes of High Performance Lithium Ion Batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Akbar, S.; Rehan, M.; Liu, H.; Rafique, I.; Akbar, H. A Brief Review on Graphene Applications in Rechargeable Lithium Ion Battery Electrode Materials. Carbon Lett. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Venkateswara Rao, C.; Leela Mohana Reddy, A.; Ishikawa, Y.; Ajayan, P.M. LiNi1/3Co1/3Mn1/3O2 –Graphene Composite as a Promising Cathode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2011, 3, 2966–2972. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L. Chemical Stability and Long-Term Cell Performance of Low-Cobalt, Ni-Rich Cathodes Prepared by Aqueous Processing for High-Energy Li-Ion Batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Shim, J.-H.; Kim, C.-Y.; Cho, S.-W.; Missiul, A.; Kim, J.-K.; Ahn, Y.J.; Lee, S. Effects of Heat-Treatment Atmosphere on Electrochemical Performances of Ni-Rich Mixed-Metal Oxide (LiNi0.80Co0.15Mn0.05O2) as a Cathode Material for Lithium Ion Battery. Electrochim. Acta 2014, 138, 15–21. [Google Scholar] [CrossRef]
- Jan, S.S.; Nurgul, S.; Shi, X.; Xia, H.; Pang, H. Improvement of Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Material by Graphene Nanosheets Modification. Electrochim. Acta 2014, 149, 86–93. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.-H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.-J. Graphene Collage on Ni-Rich Layered Oxide Cathodes for Advanced Lithium-Ion Batteries. Nat. Commun. 2021, 12, 2145. [Google Scholar] [CrossRef]
- He, X.; Han, G.; Lou, S.; Du, L.; Xu, X.; Du, C.; Cheng, X.; Zuo, P.; Ma, Y.; Huo, H.; et al. Improved Electrochemical Performance of LiNi0.8Co0.15Al0.05O2 Cathode Material by Coating of Graphene Nanodots. J. Electrochem. Soc. 2019, 166, A1038–A1044. [Google Scholar] [CrossRef]
- Yao, F.; Güneş, F.; Ta, H.Q.; Lee, S.M.; Chae, S.J.; Sheem, K.Y.; Cojocaru, C.S.; Xie, S.S.; Lee, Y.H. Diffusion Mechanism of Lithium Ion through Basal Plane of Layered Graphene. J. Am. Chem. Soc. 2012, 134, 8646–8654. [Google Scholar] [CrossRef] [Green Version]
- Persson, K.; Sethuraman, V.A.; Hardwick, L.J.; Hinuma, Y.; Meng, Y.S.; van der Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium Diffusion in Graphitic Carbon. J. Phys. Chem. Lett. 2010, 1, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lin, Z.; Zhong, X.; Huang, X.; Weiss, N.O.; Huang, Y.; Duan, X. Holey Graphene Frameworks for Highly Efficient Capacitive Energy Storage. Nat. Commun. 2014, 5, 4554. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, P.; Wang, Z.; Qi, F.; Liu, J. Synthesis and Electrochemical Properties of Graphene-Modified LiCo1/3Ni 1/3Mn1/3O2 Cathodes for Lithium Ion Batteries. RSC Adv. 2014, 4, 2568–2572. [Google Scholar] [CrossRef]
- Wu, F.; Yan, Y.; Wang, R.; Cai, H.; Tong, W.; Tang, H. Synthesis of LiNi1/3Mn1/3Co1/3O2@graphene for Lithium-Ion Batteries via Self-Assembled Polyelectrolyte Layers. Ceram. Int. 2017, 43, 7668–7673. [Google Scholar] [CrossRef]
- Luo, W.; Liu, L.; Li, X.; Yu, J.; Fang, C. Templated Assembly of LiNi0·8Co0·15Al0·05O2/Graphene Nano Composite with High Rate Capability and Long-Term Cyclability for Lithium Ion Battery. J. Alloys Compd. 2019, 810, 151786. [Google Scholar] [CrossRef]
- Song, B.; Lai, M.O.; Liu, Z.; Liu, H.; Lu, L. Graphene-Based Surface Modification on Layered Li-Rich Cathode for High-Performance Li-Ion Batteries. J. Mater. Chem. A 2013, 1, 9954. [Google Scholar] [CrossRef]
- Ju, J.-H.; Cho, S.-W.; Hwang, S.-G.; Yun, S.-R.; Lee, Y.; Jeong, H.M.; Hwang, M.-J.; Kim, K.M.; Ryu, K.-S. Electrochemical Performance of Li[Co0.1Ni0.15Li0.2Mn0.55]O2 Modified by Carbons as Cathode Materials. Electrochim. Acta 2011, 56, 8791–8796. [Google Scholar] [CrossRef]
- Jiang, K.-C.; Wu, X.-L.; Yin, Y.-X.; Lee, J.-S.; Kim, J.; Guo, Y.-G. Superior Hybrid Cathode Material Containing Lithium-Excess Layered Material and Graphene for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 4858–4863. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Papandrea, B.; Xu, X.; Xu, Y.; Chen, C.-Y.; Lin, Z.; Wang, G.; Luo, Y.; Liu, M.; Huang, Y.; Mai, L.; et al. Three-Dimensional Graphene Framework with Ultra-High Sulfur Content for a Robust Lithium–Sulfur Battery. Nano Res. 2016, 9, 240–248. [Google Scholar] [CrossRef]
- Chong, W.G.; Huang, J.-Q.; Xu, Z.-L.; Qin, X.; Wang, X.; Kim, J.-K. Lithium-Sulfur Battery Cable Made from Ultralight, Flexible Graphene/Carbon Nanotube/Sulfur Composite Fibers. Adv. Funct. Mater. 2017, 27, 1604815. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhuang, T.-Z.; Zhang, Q.; Peng, H.-J.; Chen, C.-M.; Wei, F. Permselective Graphene Oxide Membrane for Highly Stable and Anti-Self-Discharge Lithium–Sulfur Batteries. ACS Nano 2015, 9, 3002–3011. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Wang, X.; Liu, M.; Ye, F.; Wang, J.; Qiu, Y.; Li, W.; Zhang, Y. Dense Integration of Graphene and Sulfur through the Soft Approach for Compact Lithium/Sulfur Battery Cathode. Nano Energy 2015, 12, 468–475. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.-Q.; Zhang, Q.; Zhu, L.; Wang, H.-F.; Shi, J.-L.; Wei, F. CaO-Templated Growth of Hierarchical Porous Graphene for High-Power Lithium-Sulfur Battery Applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, Y.; Chen, J.; Zhou, H.; Huang, Y. Macroporous Free-Standing Nano-Sulfur/Reduced Graphene Oxide Paper as Stable Cathode for Lithium-Sulfur Battery. Nano Energy 2015, 11, 678–686. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Yang, Y.; Kushima, A.; Chen, J.; Huang, Y.; Li, J. Slurryless Li 2 S/Reduced Graphene Oxide Cathode Paper for High-Performance Lithium Sulfur Battery. Nano Lett. 2015, 15, 1796–1802. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium–Sulfur Battery Cathode Material with High Capacity and Cycling Stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, L.; Zhang, F.; Huang, Y.; Chen, Y. Sulfur-Infiltrated Graphene-Based Layered Porous Carbon Cathodes for High-Performance Lithium–Sulfur Batteries. ACS Nano 2014, 8, 5208–5215. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, L.-L.; Liu, S.; Li, G.-R.; Gao, X.-P. A High-Efficiency Sulfur/Carbon Composite Based on 3D Graphene Nanosheet@Carbon Nanotube Matrix as Cathode for Lithium-Sulfur Battery. Adv. Energy Mater. 2017, 7, 1602543. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, L.-C.; Wang, D.-W.; Li, L.; Pei, S.; Gentle, I.R.; Li, F.; Cheng, H.-M. Fibrous Hybrid of Graphene and Sulfur Nanocrystals for High-Performance Lithium–Sulfur Batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Nestler, T.; Schmid, R.; Münchgesang, W.; Bazhenov, V.; Schilm, J.; Leisegang, T.; Meyer, D.C. Separators—Technology Review: Ceramic Based Separators for Secondary Batteries. AIP Conf. Proc. 2014, 1597, 155–184. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Lin, Y.; Wu, K.; Lu, S. Multi-Functional Ceramic-Coated Separator for Lithium-Ion Batteries Safety Tolerance Improvement. Ceram. Int. 2020, 46, 24689–24697. [Google Scholar] [CrossRef]
- Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; He, W.; et al. Inhibiting Polysulfide Shuttling with a Graphene Composite Separator for Highly Robust Lithium-Sulfur Batteries. Joule 2018, 2, 2091–2104. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Chen, Y.; Li, P.; He, J.; Zhao, Y.; Wang, Z.; Liu, J.; Qi, F.; Zheng, B.; Zhou, J.; et al. Enhanced Performance of Lithium Sulfur Battery with a Reduced Graphene Oxide Coating Separator. J. Electrochem. Soc. 2015, 162, A1624–A1629. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Gao, Y.; Wang, Y.; Wang, S.; Gao, Q.; Jiao, Z.; Zhao, B.; Chen, Z. Inhibiting the Shuttle Effect of Li–S Battery with a Graphene Oxide Coating Separator: Performance Improvement and Mechanism Study. J. Power Sources 2017, 342, 929–938. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Huang, J.; Cheng, X.; Yuan, Z.; Wei, F.; Zhang, Q. Janus Separator of Polypropylene-Supported Cellular Graphene Framework for Sulfur Cathodes with High Utilization in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1500268. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.-K.; Kannan, A.G.; Kim, D.-W. Effective Suppression of Dendritic Lithium Growth Using an Ultrathin Coating of Nitrogen and Sulfur Codoped Graphene Nanosheets on Polymer Separator for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23700–23707. [Google Scholar] [CrossRef]
- Song, X.; Wang, S.; Chen, G.; Gao, T.; Bao, Y.; Ding, L.-X.; Wang, H. Fe-N-Doped Carbon Nanofiber and Graphene Modified Separator for Lithium-Sulfur Batteries. Chem. Eng. J. 2018, 333, 564–571. [Google Scholar] [CrossRef]
- Zhai, P.-Y.; Peng, H.-J.; Cheng, X.-B.; Zhu, L.; Huang, J.-Q.; Zhu, W.; Zhang, Q. Scaled-up Fabrication of Porous-Graphene-Modified Separators for High-Capacity Lithium–Sulfur Batteries. Energy Storage Mater. 2017, 7, 56–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; Lv, W.; He, Y.-B.; Wang, C.; Yun, Q.; Li, B.; Kang, F.; Yang, Q.-H. Dense Coating of Li4Ti5O12 and Graphene Mixture on the Separator to Produce Long Cycle Life of Lithium-Sulfur Battery. Nano Energy 2016, 30, 1–8. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Wang, D.-W.; Shan, X.; Pei, S.; Li, F.; Cheng, H.-M. A Flexible Sulfur-Graphene-Polypropylene Separator Integrated Electrode for Advanced Li-S Batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, C.; Lu, Y.; Zang, J.; Jiang, M.; Kim, D.; Zhang, X. Highly Porous Polyacrylonitrile/Graphene Oxide Membrane Separator Exhibiting Excellent Anti-Self-Discharge Feature for High-Performance Lithium–Sulfur Batteries. Carbon 2016, 101, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, T.-Z.; Huang, J.-Q.; Peng, H.-J.; He, L.-Y.; Cheng, X.-B.; Chen, C.-M.; Zhang, Q. Rational Integration of Polypropylene/Graphene Oxide/Nafion as Ternary-Layered Separator to Retard the Shuttle of Polysulfides for Lithium-Sulfur Batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Manthiram, A. Lithium–Sulphur Batteries with a Microporous Carbon Paper as a Bifunctional Interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-S.; Manthiram, A. A New Approach to Improve Cycle Performance of Rechargeable Lithium–Sulfur Batteries by Inserting a Free-Standing MWCNT Interlayer. Chem. Commun. 2012, 48, 8817. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Chung, S.-H.; Manthiram, A. Ultra-Lightweight PANiNF/MWCNT-Functionalized Separators with Synergistic Suppression of Polysulfide Migration for Li–S Batteries with Pure Sulfur Cathodes. J. Mater. Chem. A 2015, 3, 18829–18834. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chung, S.-H.; Manthiram, A. Effective Stabilization of a High-Loading Sulfur Cathode and a Lithium-Metal Anode in Li-S Batteries Utilizing SWCNT-Modulated Separators. Small 2016, 12, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-H.; Manthiram, A. High-Performance Li–S Batteries with an Ultra-Lightweight MWCNT-Coated Separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef]
- Wang, M.; Tang, M.; Chen, S.; Ci, H.; Wang, K.; Shi, L.; Lin, L.; Ren, H.; Shan, J.; Gao, P.; et al. Graphene-Armored Aluminum Foil with Enhanced Anticorrosion Performance as Current Collectors for Lithium-Ion Battery. Adv. Mater. 2017, 29, 1703882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, M.S.; Jow, T.R. Self-Discharge of Li/LixMn2O4 Batteries in Relation to Corrosion of Aluminum Cathode Substrates. J. Power Sources 2001, 102, 16–20. [Google Scholar] [CrossRef]
- Myung, S.-T.; Hitoshi, Y.; Sun, Y.-K. Electrochemical Behavior and Passivation of Current Collectors in Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 9891. [Google Scholar] [CrossRef]
- Ma, T.; Xu, G.; Li, Y.; Wang, L.; He, X.; Zheng, J.; Engelhard, M.H.; Zapol, P.; Curtiss, L.A.; Jorne, J.; et al. Revisiting the Corrosion of Aluminum Current Collector in Lithium-Ion Batteries. J. Phys. Chem. Lett. 2017, 8, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lin, K.; Qin, X.; Zhang, L.; Li, T.; Lv, F.; Xia, Y.; Han, W.; Kang, F.; Li, B. Electrosprayed Robust Graphene Layer Constructing Ultrastable Electrode Interface for High-Voltage Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 37034–37046. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.I.; Wee, J.-H.; Kim, C.H.; Ahn, B.W.; Lee, J.W.; Shu, S.J.; Terrones, M.; Kim, Y.A.; Yang, C.-M. Few-Layer Graphene Coated Current Collectors for Safe and Powerful Lithium Ion Batteries. Carbon 2019, 153, 495–503. [Google Scholar] [CrossRef]
- Richard Prabakar, S.J.; Hwang, Y.-H.; Bae, E.G.; Lee, D.K.; Pyo, M. Graphene Oxide as a Corrosion Inhibitor for the Aluminum Current Collector in Lithium Ion Batteries. Carbon 2013, 52, 128–136. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Wang, J.; Li, R.; Sun, X. Free-Standing Graphene–Carbon Nanotube Hybrid Papers Used as Current Collector and Binder Free Anodes for Lithium Ion Batteries. J. Power Sources 2013, 237, 41–46. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Gao, J.; Fang, M.; Tian, G.; Wang, J.; Fan, S. Graphene-Coated Plastic Film as Current Collector for Lithium/Sulfur Batteries. J. Power Sources 2013, 239, 623–627. [Google Scholar] [CrossRef]
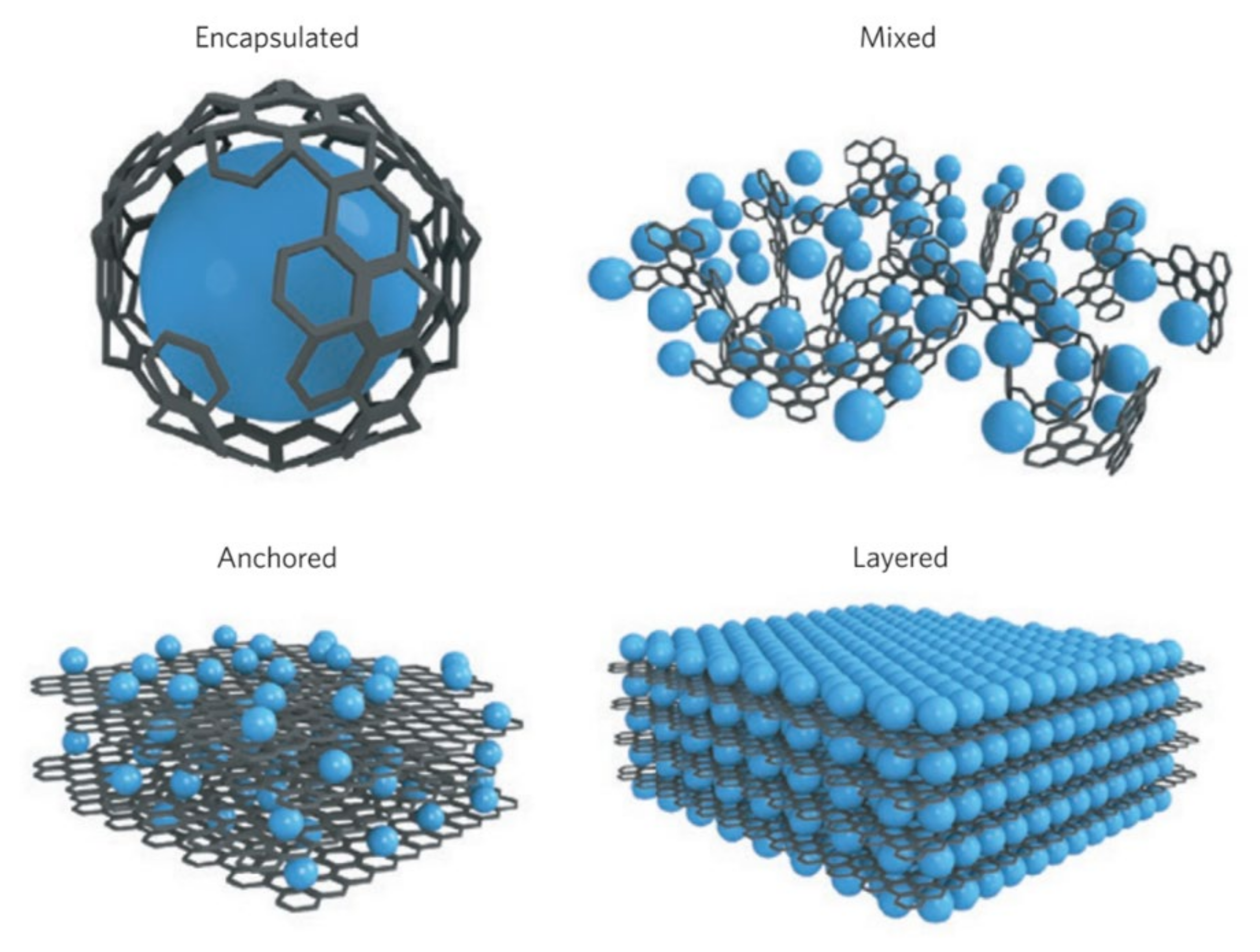



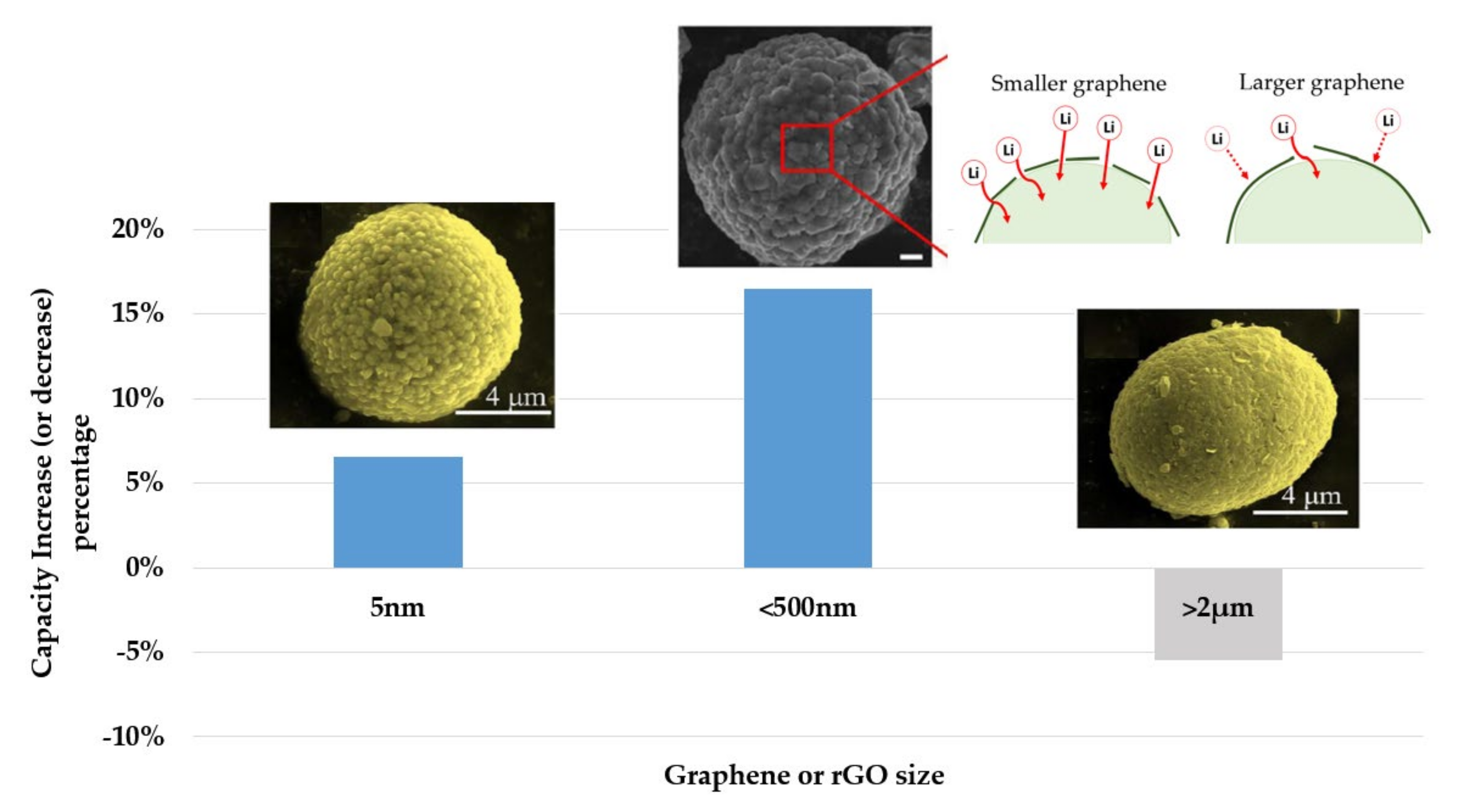
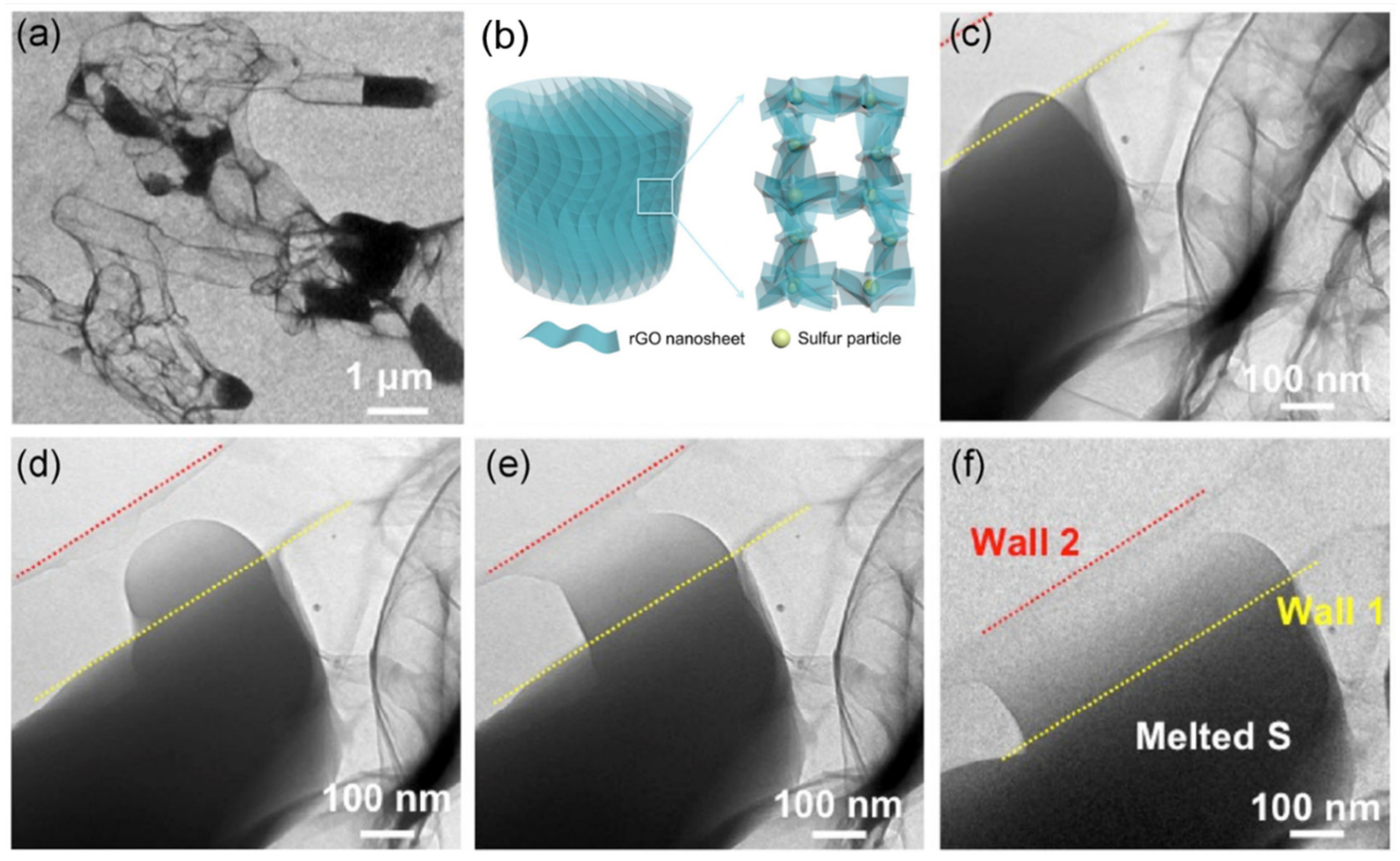
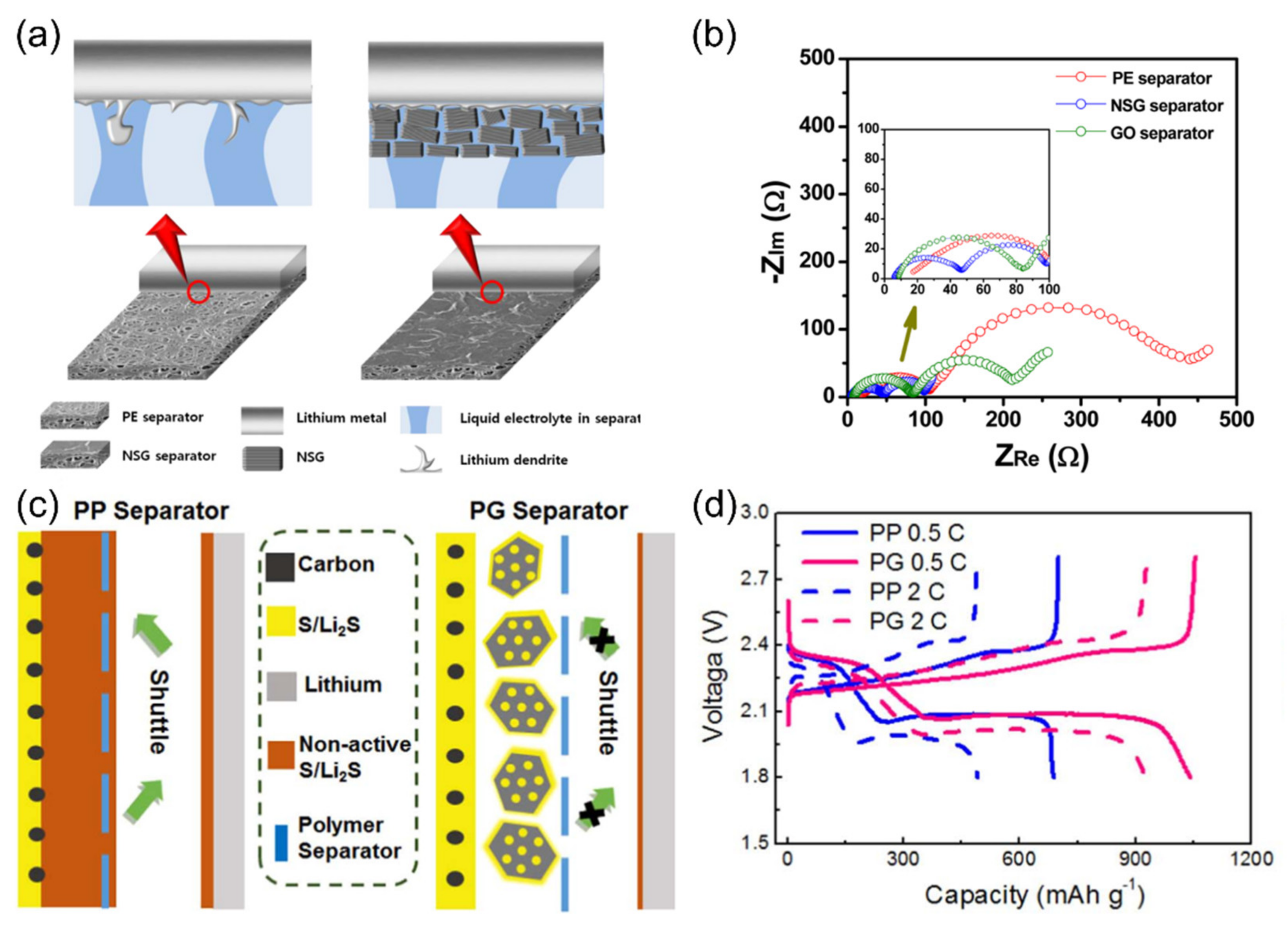
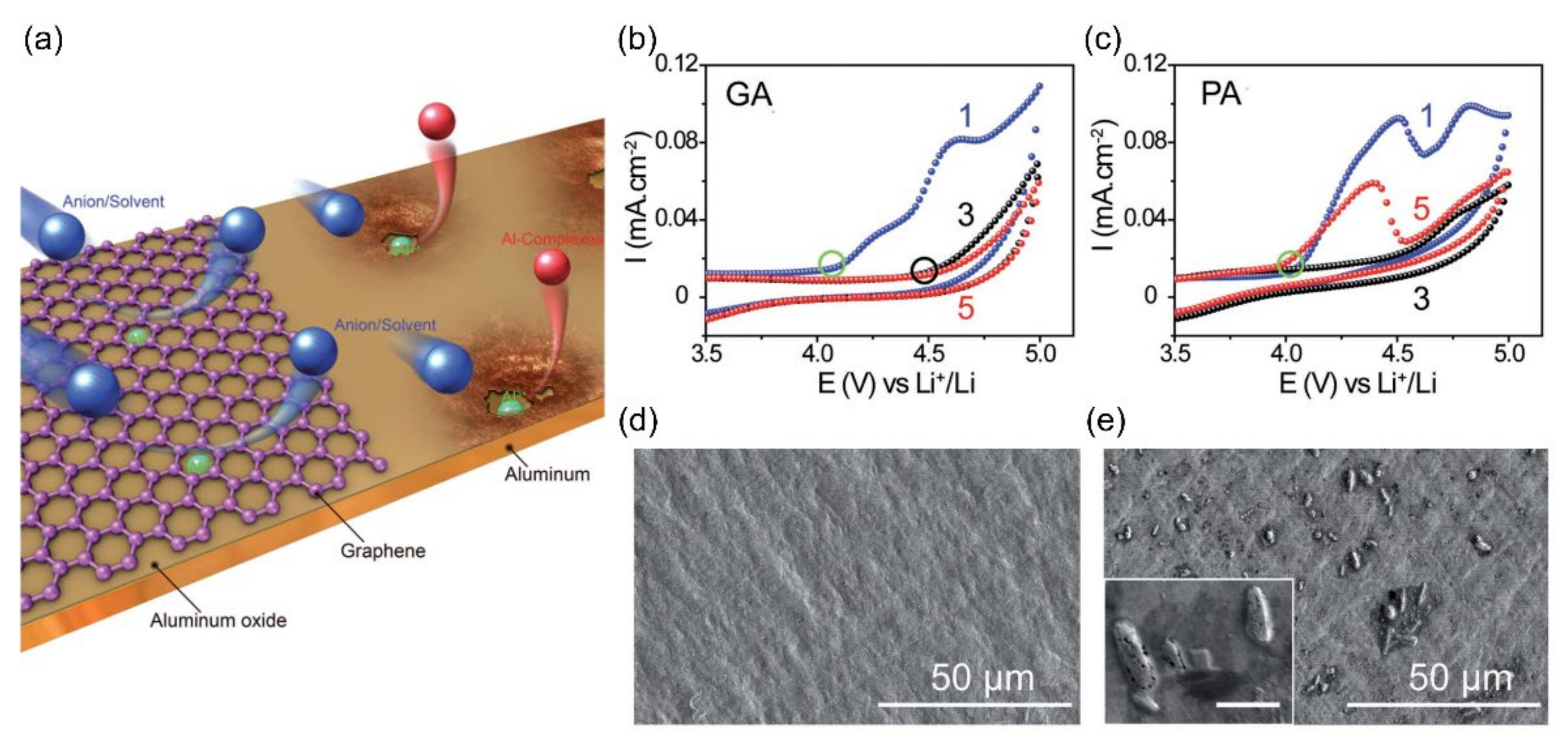
| Name | Graphene Content | Structure Type | Capacity 1 (Control Sample) | Capacity 1 (Composite) | Ref. |
|---|---|---|---|---|---|
| NMC811/rGO | 5.5 wt.% | Mixed | 158 | 185 | [134] |
| NMC811/Graphene | 0.5 wt.% | Encapsulated | 175 | 190 | [135] |
| NMC333/rGO | 10 wt.% | Mixed | 158 | 175 | [131] |
| NMC333/rGO | 9 wt.% (before reduction) | Encapsulated | 112 | 163 | [140] |
| NMC333/Graphene | 2.3 wt.% | Encapsulated | 123 | 135 | [141] |
| NCA/rGO | 0.5 wt.% | Mixed | 168 | 180 | [142] |
| NCA/Graphene | 0.5 wt.% | Encapsulated | 119 | 188 | [135] |
| NCA/Graphene nanodot | 0.5 wt.% | Encapsulated | 150 | 175 | [136] |
| LLNMC/rGO | 1.5 wt.% | Encapsulated | 150 | 230 | [143] |
| LLNMC/Graphene | 0.5 wt.% | Anchored | 140 | 215 | [144] |
| LLNMC/rGO | 4.9 wt.% | Encapsulated | 75 | 160 | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-H.; Ho, T.-H.; Su, Y.-S. Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries. C 2021, 7, 65. https://doi.org/10.3390/c7030065
Chang H-H, Ho T-H, Su Y-S. Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries. C. 2021; 7(3):65. https://doi.org/10.3390/c7030065
Chicago/Turabian StyleChang, Hao-Hsun, Tseng-Hsiang Ho, and Yu-Sheng Su. 2021. "Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries" C 7, no. 3: 65. https://doi.org/10.3390/c7030065
APA StyleChang, H.-H., Ho, T.-H., & Su, Y.-S. (2021). Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries. C, 7(3), 65. https://doi.org/10.3390/c7030065







