A Comparative Study of Aromatization Catalysts: The Advantage of Hybrid Oxy/Carbides and Platinum-Catalysts Based on Carbon Gels
Abstract
1. Introduction
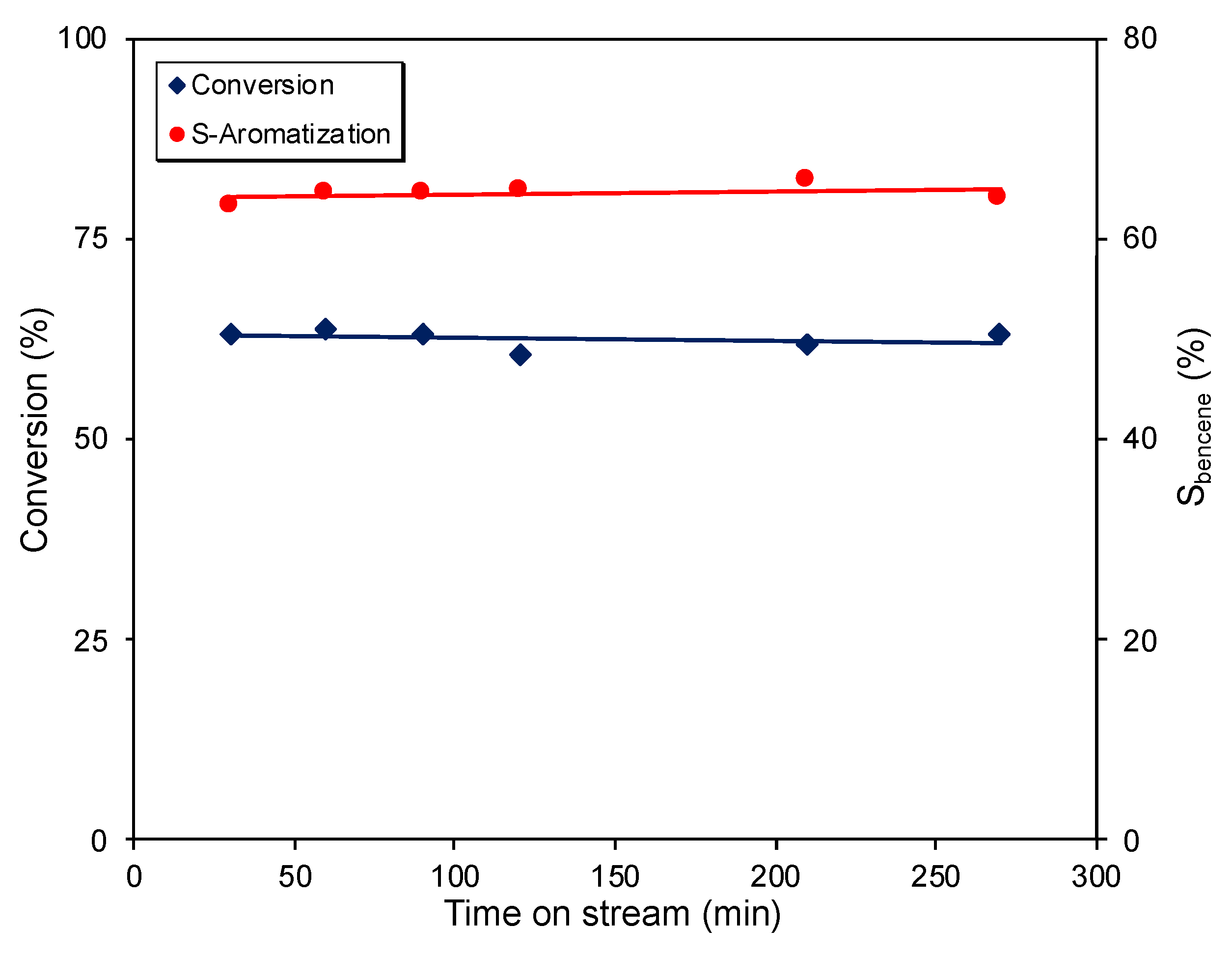
2. Results and Discussion
3. Materials and Methods
- (a)
- Preparation of carbon-gel supports (Figure 7): Resorcinol (R) and formaldehyde (F) in a molar ratio of R/F= ½ and Na2CO3, as polymerization catalyst (C) are dissolved in the appropriate amounts of distilled water (W). Solutions are cast into glass molds, sealed and cured for 1 day at room temperature, 2 days at 50 °C and 5 days at 80 °C. After that, gel rods are cut in pellets and introduced in acetone to remove the water inside the pores. Finally, gels were supercritically dried with carbon dioxide to obtain the corresponding organic RF aerogels or dried in the oven at 120 °C obtaining the corresponding xerogels. Pyrolysis of organic gels to obtain the derivative carbon gels is carried out in a tubular furnace under N2 flow by heating up to different temperatures (500 or 1000 °C) with a heating rate between 0.5 and 1.5 °C min−1.
- (a)
- Metal-doped carbon gels were obtained directly by replacing Na2CO3 by the corresponding metal precursor in the starting solution [30,38]. In this case, ammonium molybdate or tungstate was used. The rest of the synthesis procedure was maintained as in procedure (a). In such a way, simultaneous carbonization of the RF organic fraction and decomposition of the precursor salts occurs during the same thermal process, leading directly to materials suitable to be used in catalysis.
- (b)
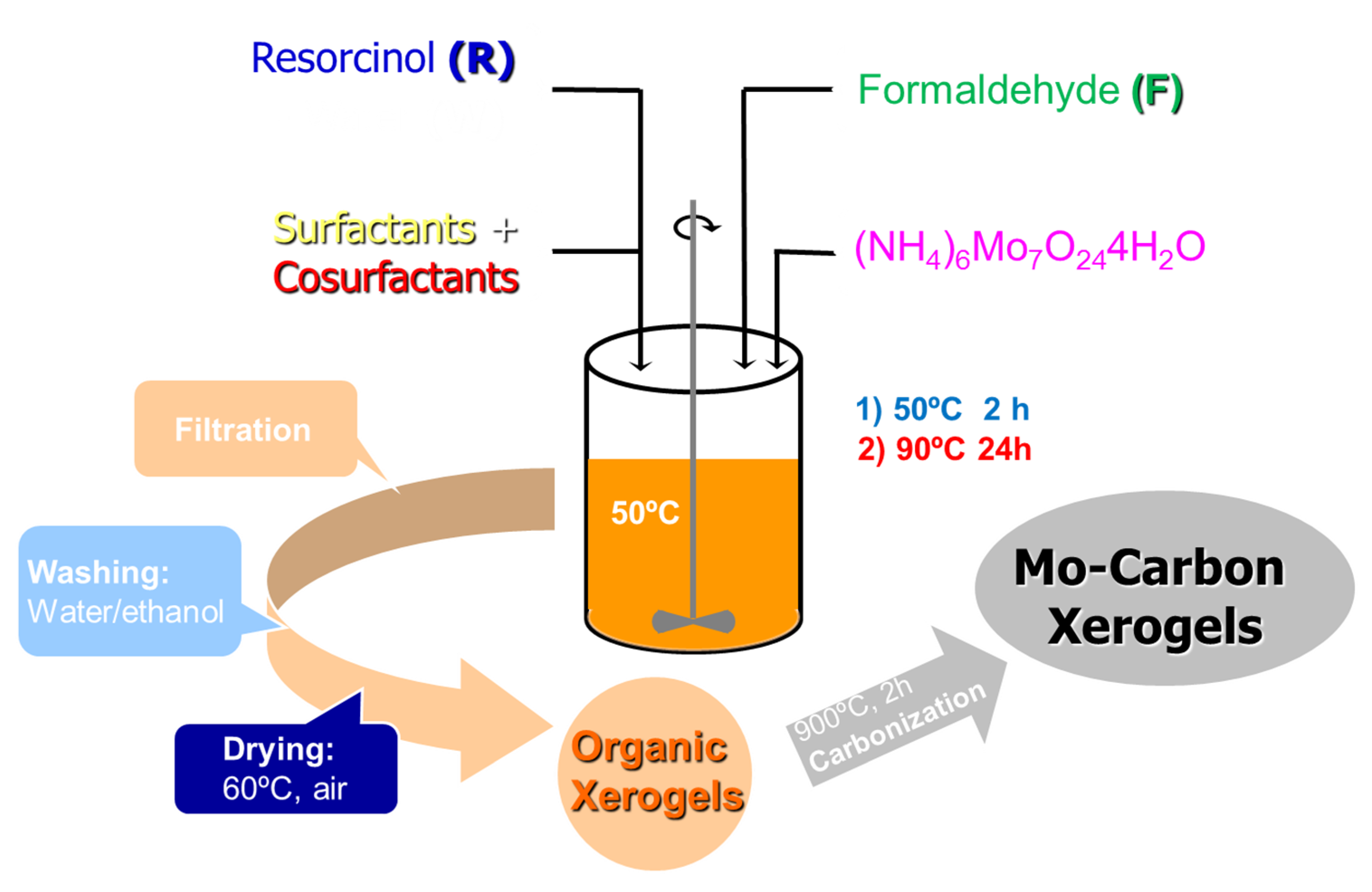
- Metal-supported carbon xerogels were obtained in powder following a procedure in two steps [29]; firstly, the polymerization of RF in the presence/absence of surfactants is carried out in a batch reactor at 50 °C for 2 h; after the morphology of the organic RF is defined, the solid in suspension is doped with metal precursors, then the temperature of the bath is increased up to 90 °C for an additional 24 h, allowing the complete polymerization of the organic fraction. Finally, solids are recovered by filtration, properly washed, dried in an oven and finally carbonized at 900 °C. The procedure is summarized in Figure 8.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derouane, E.G.; Vanderveken, D.J. Structural recognition and preorganization in zeolite catalysis: Direct aromatization of n-hexane on zeolite L-based catalysts. Appl. Catal. 1988, 45, L15–L22. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of short chain alkanes on zeolite catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Mahale, R.S.; Parikh, P.A. Aromatization of n-hexane: Synergism afforded by C1-C3 alcohols. Chem. Eng. Sci. 2020, 217, 115519. [Google Scholar] [CrossRef]
- Nezam, I.; Zhou, W.; Gusmão, G.S.; Realff, M.J.; Wang, Y.; Medford, A.J.; Jones, C.W. Direct aromatization of CO2 via combined CO2 hydrogenation and zeolite-based acid catalysis. J. CO2 Util. 2021, 45, 101405. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Cui, J.; Yang, Y.; Hu, C.-L.; Liu, B.-J. Preparation of Pt/CeL reforming catalyst and its performance in the aromatization of naphtha. J. Fuel Chem. Technol. 2019, 47, 318–322. [Google Scholar] [CrossRef]
- Besoukhanova, C.; Barthomeuf, D.; Breysse, M.; Bernard, J.R. Unusual Properties of Platinum Alkaline Zeolites in N-Hexane Dehydrocyclisation and Benzene Hydrogenation. In Studies in Surface Science and Catalysis; Seiyama, T., Tanabe, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; Volume 7, pp. 1410–1411. [Google Scholar]
- Tauster, S.J.; Steger, J.J. Molecular die catalysis: Hexane aromatization over Pt/KL. J. Catal. 1990, 125, 387–389. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J.; Silva, J.M.; Ribeiro, F.R.; Ribeiro, M.F. Influence of the exchanged cation in coke deposition during n-hexane reactions on Pt/Mβ zeolite catalysts. Catal. Lett. 1997, 48, 69–73. [Google Scholar] [CrossRef]
- Bécue, T.; Maldonado-Hodar, F.J.; Antunes, A.P.; Silva, J.M.; Ribeiro, M.F.; Massiani, P.; Kermarec, M. Influence of Cesium in Pt/NaCsβ on the Physico-Chemical and Catalytic Properties of the Pt Clusters in the Aromatization of n-Hexane. J. Catal. 1999, 181, 244–255. [Google Scholar] [CrossRef]
- Maldonado, F.J.; Bécue, T.; Silva, J.M.; Ribeiro, M.F.; Massiani, P.; Kermarec, M. Influence of the Alkali in Pt/Alkali-β Zeolite on the Pt Characteristics and Catalytic Activity in the Transformation of n-Hexane. J. Catal. 2000, 195, 342–351. [Google Scholar] [CrossRef]
- Wongnongwa, Y.; Kidkhunthod, P.; Sukkha, U.; Pengpanich, S.; Thavornprasert, K.-A.; Phupanit, J.; Kungwan, N.; Feng, G.; Keawin, T.; Jungsuttiwong, S. Local structure elucidation and reaction mechanism of light naphtha aromatization over Ga embedded H-ZSM-5 zeolite: Combined DFT and experimental study. Microporous Mesoporous Mater. 2020, 306, 110414. [Google Scholar] [CrossRef]
- Uslamin, E.A.; Saito, H.; Sekine, Y.; Hensen, E.J.M.; Kosinov, N. Different mechanisms of ethane aromatization over Mo/ZSM-5 and Ga/ZSM-5 catalysts. Catal. Today 2020. [Google Scholar] [CrossRef]
- Fadaeerayeni, S.; Shan, J.; Sarnello, E.; Xu, H.; Wang, H.; Cheng, J.; Li, T.; Toghiani, H.; Xiang, Y. Nickel/gallium modified HZSM-5 for ethane aromatization: Influence of metal function on reactivity and stability. Appl. Catal. A Gen. 2020, 601, 117629. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; He, N.; Sheng, S.; Wang, G.; Guo, H. Pt supported on Zn modified silicalite-1 zeolite as a catalyst for n-hexane aromatization. J. Energy Chem. 2019, 34, 96–103. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Catalysis on nano-carbon materials: Going where to? Catal. Today 2012, 186, 1–6. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Tailoring the surface chemistry and porosity of activated carbons: Evidence of reorganization and mobility of oxygenated surface groups. Carbon 2014, 68, 520–530. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Nanostructured porous carbons for electrochemical energy conversion and storage. Surf. Coat. Technol. 2018, 350, 307–312. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Design of low-temperature Pt-carbon combustion catalysts for VOC’s treatments. J. Hazard. Mater. 2010, 183, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Calvino-Casilda, V.; López-Peinado, A.J.; Durán-Valle, C.J.; Martín-Aranda, R.M. Last Decade of Research on Activated Carbons as Catalytic Support in Chemical Processes. Catal. Rev. 2010, 52, 325–380. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Bai, T.; Chen, T.; Fan, W. Coking kinetics and influence of reaction-regeneration on acidity, activity and deactivation of Zn/HZSM-5 catalyst during methanol aromatization. J. Energy Chem. 2015, 24, 108–118. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Transformations of n-heptane over Pt/activated carbon catalysts. Appl. Catal. A Gen. 1994, 119, 271–278. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Bachiller-Baeza, B.; Rodríguez-Ramos, I. Catalytic properties of carbon-supported ruthenium catalysts for n-hexane conversion. Appl. Catal. A Gen. 1998, 173, 231–238. [Google Scholar] [CrossRef]
- Trunschke, A.; Hoang, D.L.; Radnik, J.; Brzezinka, K.W.; Brückner, A.; Lieske, H. Transition metal oxide/carbon composite catalysts for n-alkane aromatization: Structure and catalytic properties. Appl. Catal. A Gen. 2001, 208, 381–392. [Google Scholar] [CrossRef]
- Hoang, D.L.; Preiss, H.; Parlitz, B.; Krumeich, F.; Lieske, H. Zirconia/carbon composites as monofunctional catalysts in C6+ alkane aromatization. Appl. Catal. A Gen. 1999, 182, 385–397. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J. Advances in the development of nanostructured catalysts based on carbon gels. Catal. Today 2013, 218–219, 43–50. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J. Metal-doped carbon aerogels as catalysts for the aromatization of n-hexane. Appl. Catal. A Gen. 2011, 408, 156–162. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J. Platinum supported on carbon aerogels as catalysts for the n-hexane aromatization. Catal. Commun. 2012, 17, 89–94. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J.; Jirglová, H.; Morales-Torres, S.; Pérez-Cadenas, A.F. Influence of surfactants on the physicochemical properties and catalytic behaviour of Mo-doped carbon xerogels. Catal. Today 2018, 301, 217–225. [Google Scholar] [CrossRef]
- Tshabalala, T.; Scurrell, M. Aromatization of n-hexane over Ga, Mo and Zn modified H-ZSM-5 zeolite catalysts. Catal. Commun. 2015, 72. [Google Scholar] [CrossRef]
- Huyen, P.T.; Trinh, V.D.; Teresa Portilla, M.; Martínez, C. Influence of boron promotion on the physico-chemical properties and catalytic behavior of Zn/ZSM-5 in the aromatization of n-hexane. Catal. Today 2020. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Moreno-Castilla, C. Molybdenum Carbide Formation in Molybdenum-Doped Organic and Carbon Aerogels. Langmuir 2005, 21, 10850–10855. [Google Scholar] [CrossRef]
- Delporte, P.; Pham-Huu, C.; Ledoux, M.J. Effect of the reaction temperature and hydrocarbon partial pressure on the activity of carbon-modified MoO3 for n-hexane isomerization. Appl. Catal. A Gen. 1997, 149, 151–180. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Huu, C.P.; Guille, J.; Dunlop, H. Compared activities of platinum and high specific surface area Mo2C and WC catalysts for reforming reactions: I. Catalyst activation and stabilization: Reaction of n-hexane. J. Catal. 1992, 134, 383–398. [Google Scholar] [CrossRef]
- Rahman, M.; Sridhar, A.; Khatib, S.J. Impact of the presence of Mo carbide species prepared ex situ in Mo/HZSM-5 on the catalytic properties in methane aromatization. Appl. Catal. A Gen. 2018, 558, 67–80. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Maldonado-Hódar, F.J. Influence of Electrostatic Interactions During the Resorcinol-Formaldehyde Polymerization on the Characteristics of Mo-Doped Carbon Gels. Processes 2020, 8, 746. [Google Scholar] [CrossRef]
- Pekala, R.W. U.S. Patent 4997804, 1991.
- Maldonado-Hódar, F.J.; Ferro-García, M.A.; Rivera-Utrilla, J.; Moreno-Castilla, C. Synthesis and textural characteristics of organic aerogels, transition-metal-containing organic aerogels and their carbonized derivatives. Carbon 1999, 37, 1199–1205. [Google Scholar] [CrossRef]
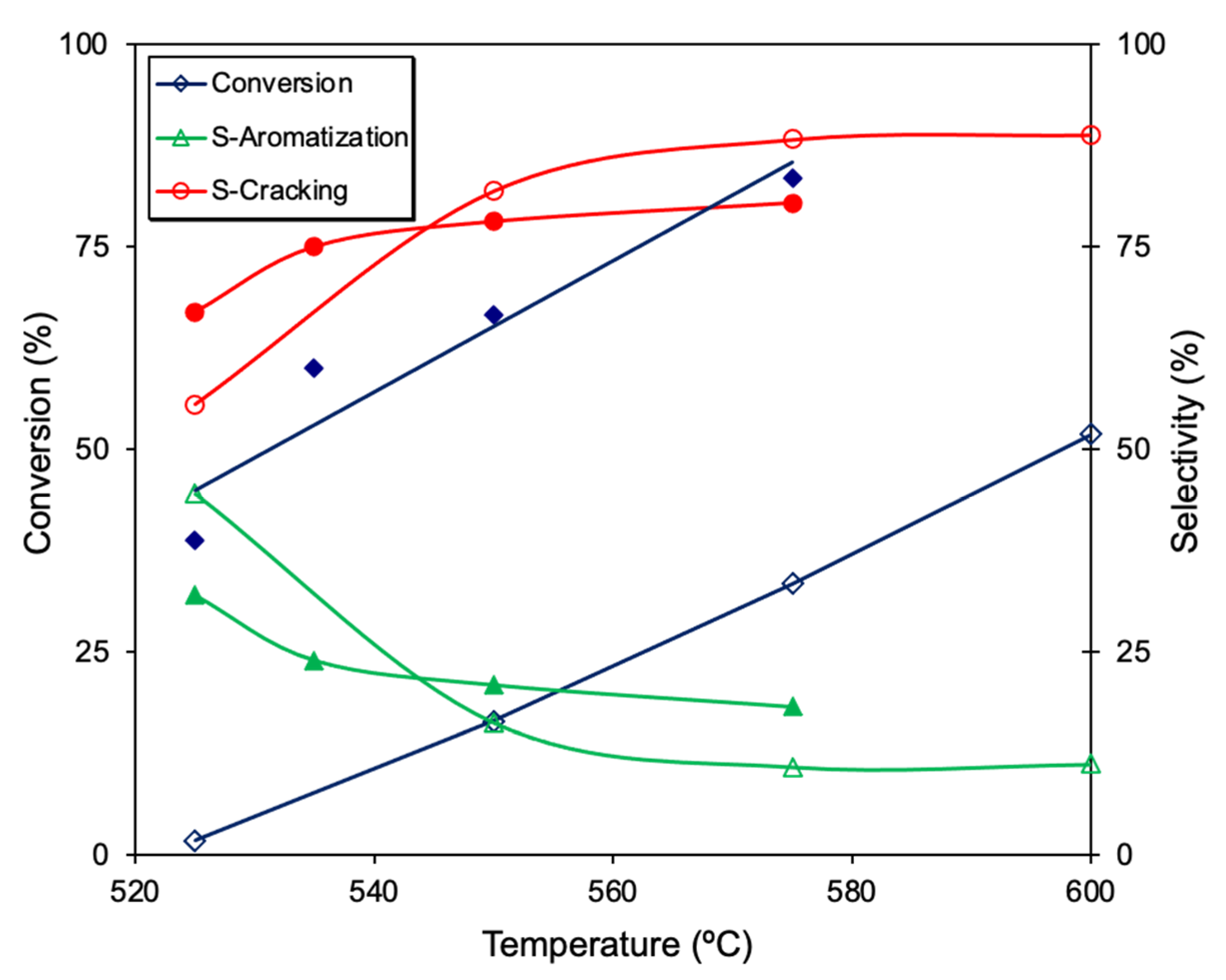
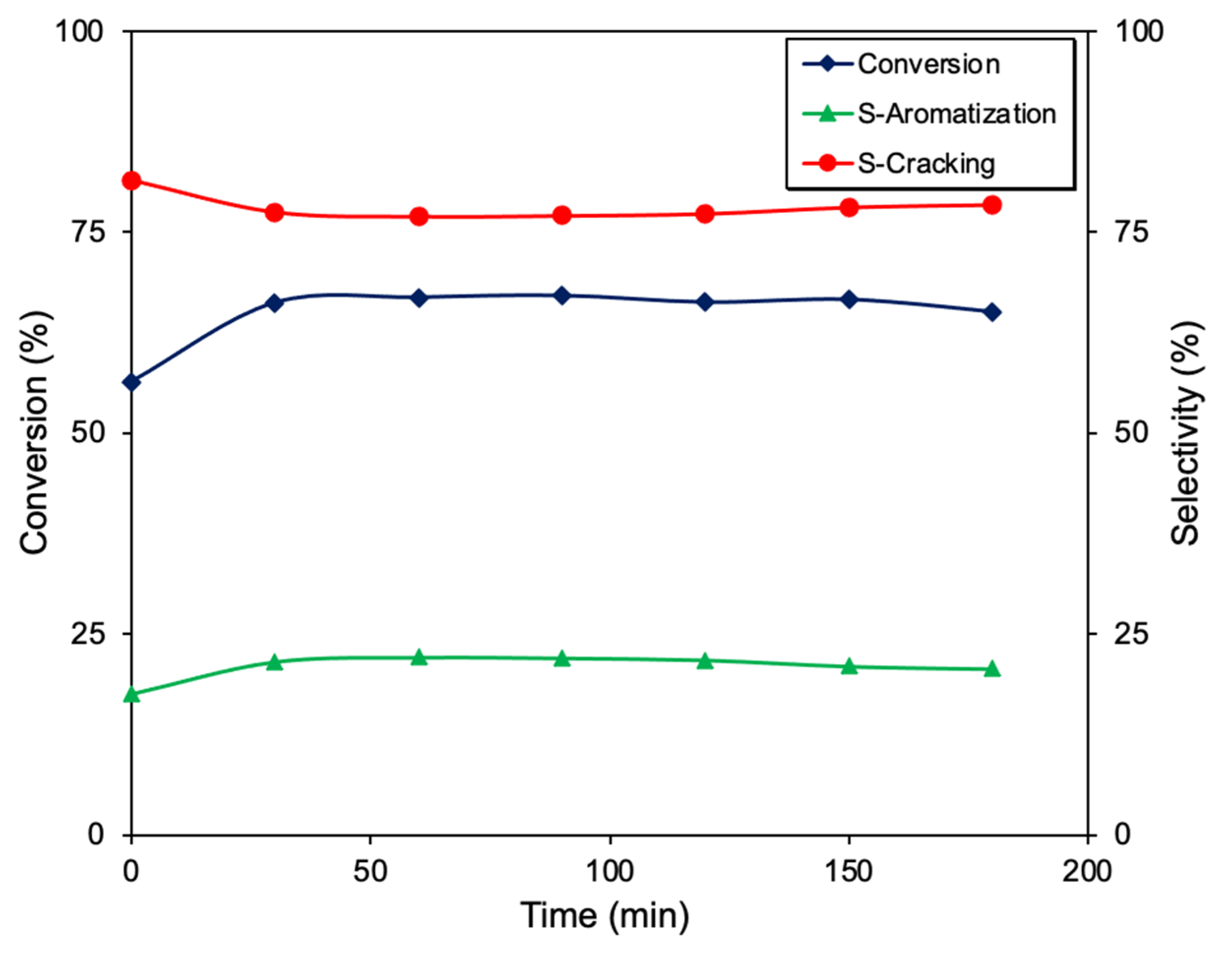
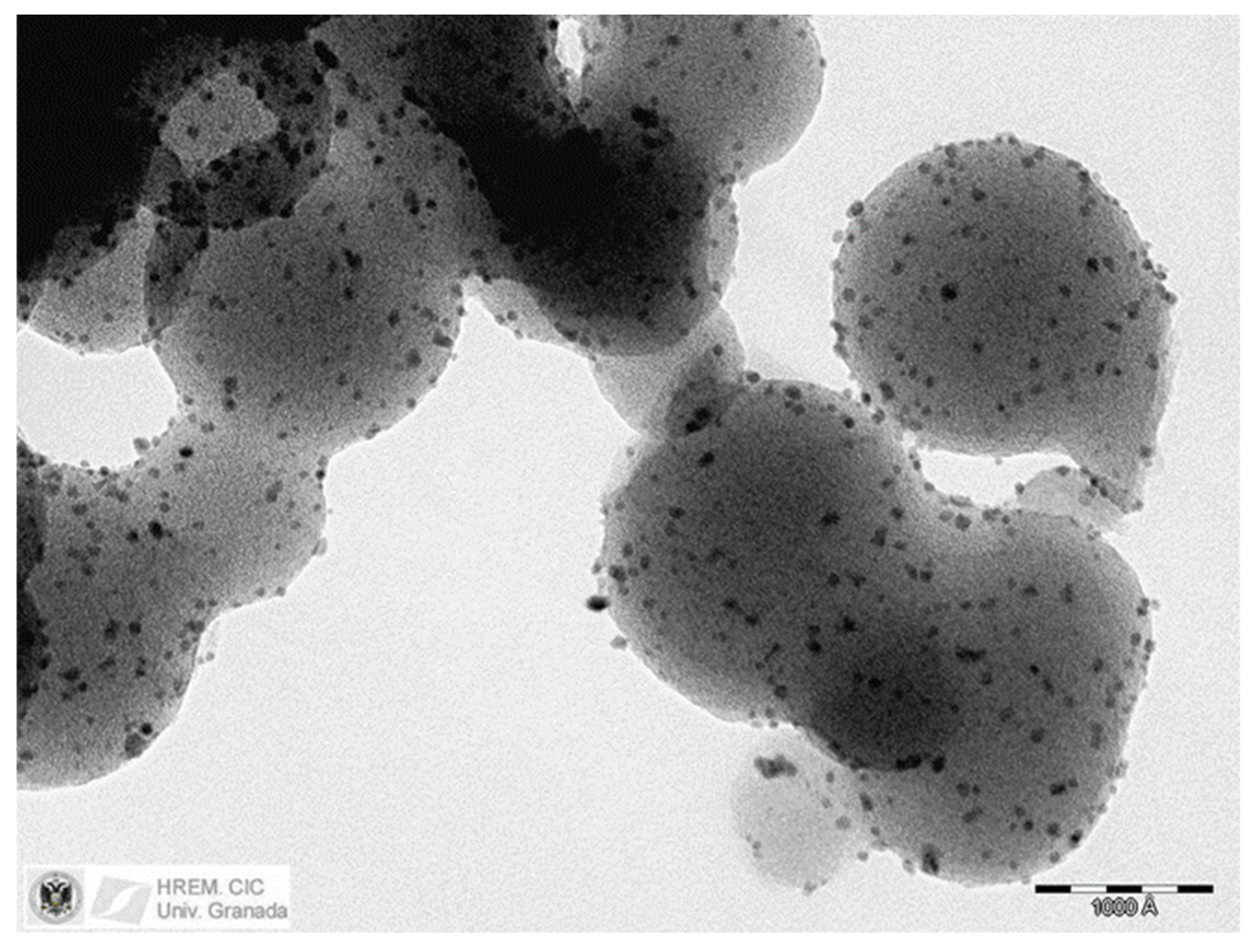
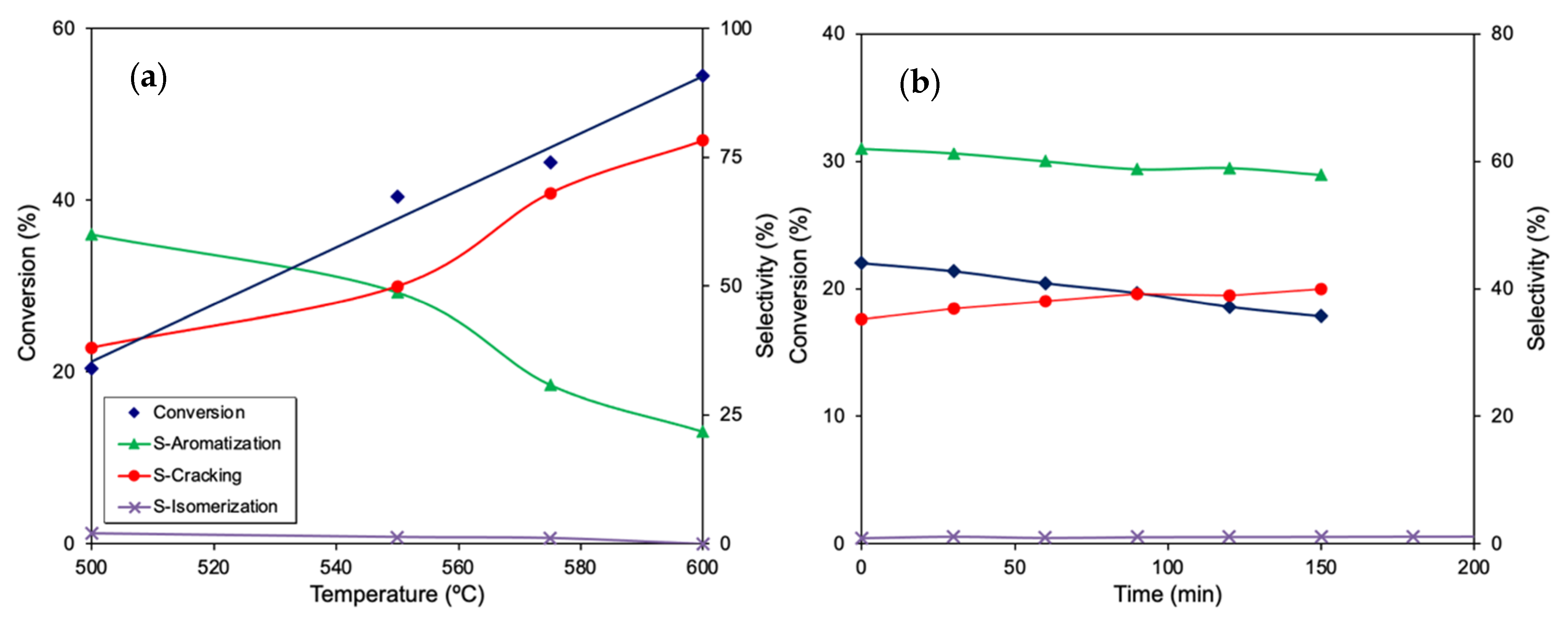


| Sample | Metal (%wt.) | XPS M/C | Active Species | SBET (m2 g−1) | Vmacro (cm3 g−1) | Vmeso (cm3 g−1) |
|---|---|---|---|---|---|---|
| Mo500 | 1.9 | 7.6 | Mo (V) 82% Mo (VI) 18% | 481 | 1.40 | 0.00 |
| W500 | 1.4 | 1.4 | W (VI) 100% | 528 | 1.32 | 0.05 |
| Sample | d Pt | C | S Crack | S Iso | S Arom | Yield Arom |
|---|---|---|---|---|---|---|
| (nm) | (%) | (%) | (%) | (%) | (%) | |
| C900 2Pt | 2.8 | 86.8 | 50.7 | 4.7 | 44.7 | 38.8 |
| C900 5Pt | 9.9 | 60.5 | 32.7 | 2.4 | 64.9 | 39.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. A Comparative Study of Aromatization Catalysts: The Advantage of Hybrid Oxy/Carbides and Platinum-Catalysts Based on Carbon Gels. C 2021, 7, 21. https://doi.org/10.3390/c7010021
Pastrana-Martínez LM, Morales-Torres S, Maldonado-Hódar FJ. A Comparative Study of Aromatization Catalysts: The Advantage of Hybrid Oxy/Carbides and Platinum-Catalysts Based on Carbon Gels. C. 2021; 7(1):21. https://doi.org/10.3390/c7010021
Chicago/Turabian StylePastrana-Martínez, Luisa M., Sergio Morales-Torres, and Francisco J. Maldonado-Hódar. 2021. "A Comparative Study of Aromatization Catalysts: The Advantage of Hybrid Oxy/Carbides and Platinum-Catalysts Based on Carbon Gels" C 7, no. 1: 21. https://doi.org/10.3390/c7010021
APA StylePastrana-Martínez, L. M., Morales-Torres, S., & Maldonado-Hódar, F. J. (2021). A Comparative Study of Aromatization Catalysts: The Advantage of Hybrid Oxy/Carbides and Platinum-Catalysts Based on Carbon Gels. C, 7(1), 21. https://doi.org/10.3390/c7010021







