The Role of Surface Chemistry and Polyethylenimine Grafting in the Removal of Cr (VI) by Activated Carbons from Cashew Nut Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbons from Cashew Nut Shells
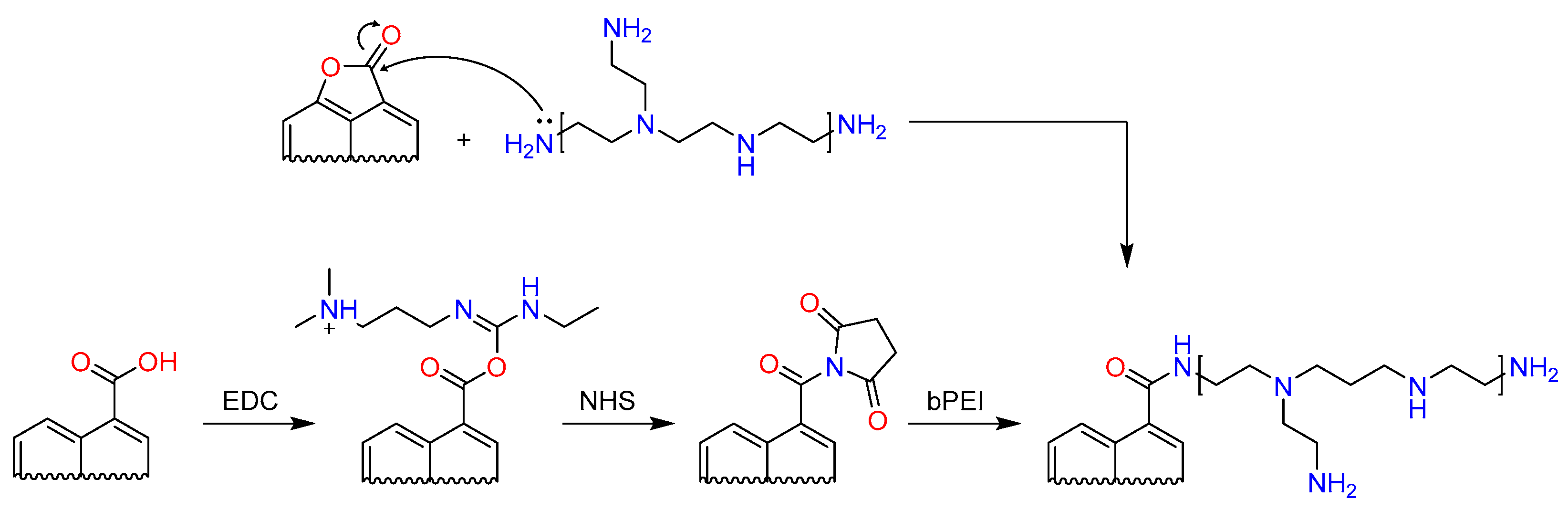
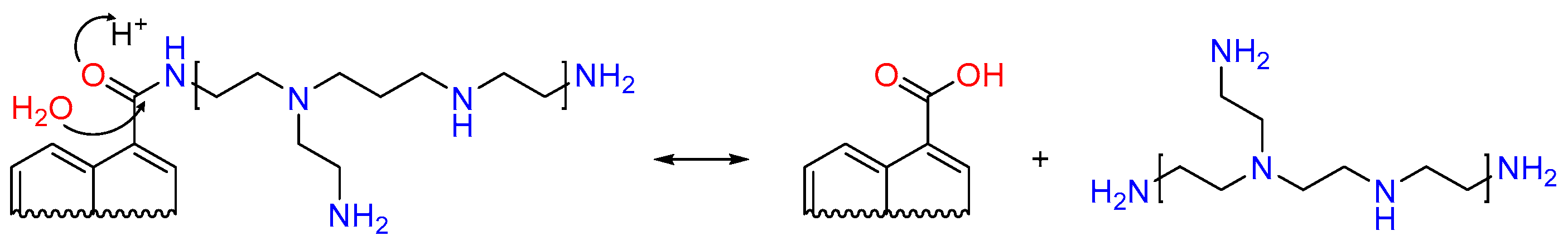
2.2. Modification of Activated Carbons with PEI
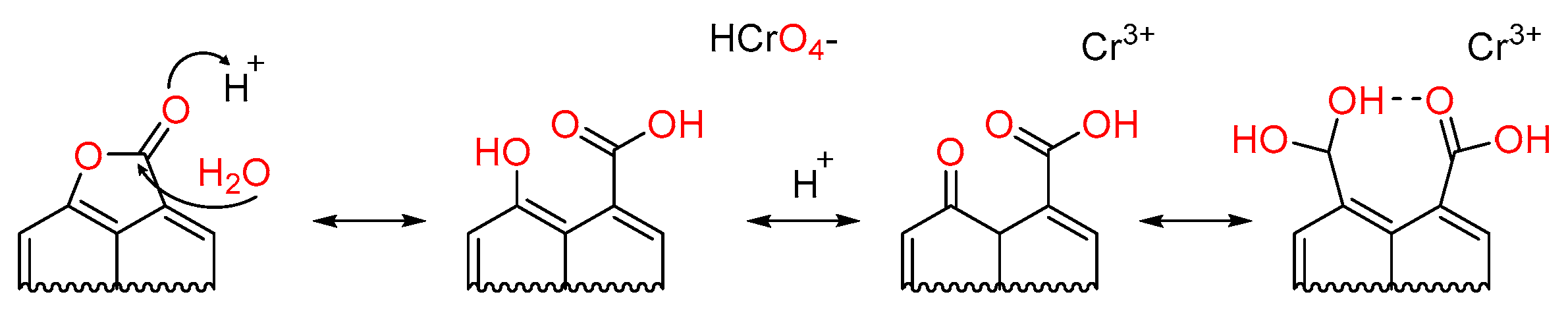
2.3. Cr(VI) Batch Adsorption Studies
2.4. Fourier Transform Infrared (FT–IR) Spectroscopy
2.5. X-Ray Photoelectron Spectroscopy (XPS)
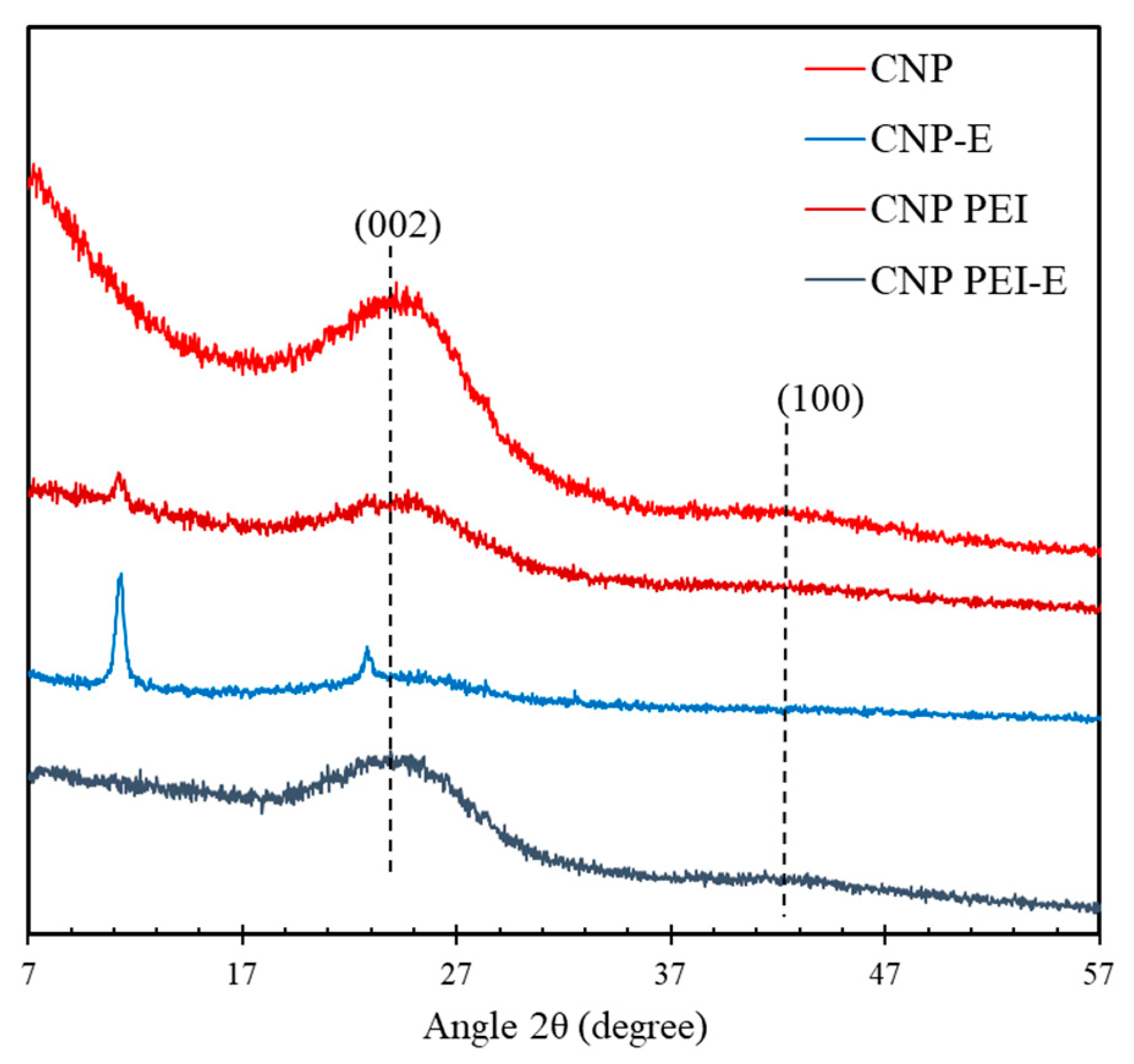
2.6. X-Ray Diffraction (XRD)
2.7. pH of Carbon Surface
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Health Effects of Hexavalent Chromium. OSHA Fact Sheet, (July 2006). Available online: https://www.osha.gov/OshDoc/data_General_Facts/hexavalent_chromium.html (accessed on 20 February 2021).
- EWG (Environmental Working Group). Chromium-6 Is Widespread in US Tap Water. 2009. Available online: https://www.ewg.org/research/chromium6-in-tap-water (accessed on 20 February 2021).
- Cavaco, S.A.; Fernandes, S.; Quina, M.M.; Ferreira, L.M. Removal of chromium from electroplating industry effluents by ion exchange resins. J. Hazard. Mater. 2007, 144, 634–638. [Google Scholar] [CrossRef]
- Ye, Z.; Yin, X.; Chen, L.; He, X.; Lin, Z.; Liu, C.; Ning, S.; Wang, X.; Wei, Y. An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. J. Clean. Prod. 2019, 236, 117631. [Google Scholar] [CrossRef]
- Mousavi Rad, S.A.; Mirbagheri, S.A.; Mohammadi, T. Using reverse osmosis membrane for chromium removal from aque-ous solution. Int. Schol. Sci. Res. Innov. 2009, 3, 505–509. [Google Scholar]
- Modrzejewska, Z.; Kaminski, W. Separation of Cr(VI) on Chitosan Membranes. Ind. Eng. Chem. Res. 1999, 38, 4946–4950. [Google Scholar] [CrossRef]
- Yao, Z.; Du, S.; Zhang, Y.; Zhu, B.; Zhu, L.; John, A.E. Positively charged membrane for removing low concentration Cr(VI) in ultrafiltration process. J. Water Process. Eng. 2015, 8, 99–107. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Wójcik, G.; Wieszczycka, K.; Aksamitowski, P.; Zembrzuska, J. Elimination of carcinogenic chromium(VI) by reduction at two-phase system. Sep. Purif. Technol. 2020, 238, 116410. [Google Scholar] [CrossRef]
- Jin, W.; Du, H.; Zheng, S.; Zhang, Y. Electrochemical processes for the environmental remediation of toxic Cr(VI): A review. Electrochim. Acta 2016, 191, 1044–1055. [Google Scholar] [CrossRef]
- Maitlo, H.A.; Kim, K.-H.; Park, J.Y.; Kim, J.H. Removal mechanism for chromium (VI) in groundwater with cost-effective iron-air fuel cell electrocoagulation. Sep. Purif. Technol. 2019, 213, 378–388. [Google Scholar] [CrossRef]
- Vieira, M.; Oisiovici, R.; Gimenes, M.; Silva, M. Biosorption of chromium(VI) using a Sargassum sp. packed-bed column. Bioresour. Technol. 2008, 99, 3094–3099. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.; Rai, B. Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour. Technol. 2016, 2, S63–S70. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Padilla-Ortega, E.; Robledo-Cabrera, A.; López-Valdivieso, A. Cr(VI) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. J. Environ. Chem. Eng. 2020, 8, 104031. [Google Scholar] [CrossRef]
- Valentín-Reyes, J.; García-Reyes, R.; García-González, A.; Soto-Regalado, E.; Cerino-Córdova, F. Adsorption mechanisms of hexavalent chromium from aqueous solutions on modified activated carbons. J. Environ. Manag. 2019, 236, 815–822. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Das, S.; Mondal, M.K. Exhaustive studies on toxic Cr(VI) removal mechanism from aqueous solution using activated carbon of Aloe vera waste leaves. J. Mol. Liq. 2020, 307, 112956. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.; Sahoo, B.; Mohanty, C.; Meikap, B. Removal of chromium(VI) from wastewater by activated carbon developed from Tamarind wood activated with zinc chloride. Chem. Eng. J. 2009, 150, 25–39. [Google Scholar] [CrossRef]
- Giri, A.K.; Patel, R.; Mandal, S. Removal of Cr (VI) from aqueous solution by Eichhornia crassipes root biomass-derived activated carbon. Chem. Eng. J. 2012, 185–186, 71–81. [Google Scholar] [CrossRef]
- Al-Othman, Z.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Niazi, L.; Lashanizadegan, A.; Sharififard, H. Chestnut oak shells activated carbon: Preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J. Clean. Prod. 2018, 185, 554–561. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Singh, K.; Rastogi, R.; Hasan, S. Removal of Cr(VI) from wastewater using rice bran. J. Colloid Interface Sci. 2005, 290, 61–68. [Google Scholar] [CrossRef]
- Labied, R.; Benturki, O.; Hamitouche, A.Y.E.; Donnot, A. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): Kinetic, equilibrium, and thermodynamic study. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.A.; Khedr, S.A.; Elkholy, S.A. Adsorption of chromium ion (VI) by acid activated carbon. Braz. J. Chem. Eng. 2010, 27, 183–193. [Google Scholar] [CrossRef]
- Solgi, M.; Najib, T.; Ahmadnejad, S.; Nasernejad, B. Synthesis and characterization of novel activated carbon from Medlar seed for chromium removal: Experimental analysis and modeling with artificial neural network and support vector regression. Resour. Technol. 2017, 3, 236–248. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Saha, B.B.; Thu, K.; Kil, H.-S.; Yoon, S.-H.; Miyawaki, J. A benchmark for CO2 uptake onto newly synthesized biomass-derived activated carbons. Appl. Energy 2020, 264, 114720. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Thu, K.; Saha, B.B.; Kil, H.-S.; Yoon, S.-H.; Miyawaki, J. Synthesis of High Grade Activated Carbons From Waste Biomass. In Renewable and Sustainable Materials, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 4, pp. 584–595. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Thu, K.; Saha, B.B.; Kil, H.-S.; Yoon, S.-H.; Miyawaki, J. Thermophysical and Adsorption Characteristics of Waste Biomass-Derived Activated Carbons. In Renewable and Sustainable Materials, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 4, pp. 617–628. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Ji, J.; Dou, M.; Wang, F. Removal of Cr6+ from wastewater via adsorption with high-specific-surface-area nitrogen-doped hierarchical porous carbon derived from silkworm cocoon. Appl. Surf. Sci. 2017, 405, 372–379. [Google Scholar] [CrossRef]
- Valix, M.; Cheung, W.; Zhang, K. Role of heteroatoms in activated carbon for removal of hexavalent chromium from wastewaters. J. Hazard. Mater. 2006, 135, 395–405. [Google Scholar] [CrossRef]
- Fang, J.; Gu, Z.; Gang, D.; Liu, C.; Ilton, E.S.; Deng, B. Cr(VI) Removal from Aqueous Solution by Activated Carbon Coated with Quaternized Poly(4-vinylpyridine). Environ. Sci. Technol. 2007, 41, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, M.; Suo, J.; Xie, Y.; Hu, W.; Wang, X.; Wang, Y.; Zhang, Y. Enhanced Cr(VI) removal by waste biomass derived nitrogen/oxygen co-doped microporous biocarbon. Environ. Sci. Pollut. Res. 2019, 27, 5433–5445. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Ma, L.; Ren, J.; Ma, P.; Li, B.; Wu, N.; Song, Z.; Huang, L. Nitrogen and sulfur codoped micro-mesoporous carbon sheets derived from natural biomass for synergistic removal of chromium(VI): Adsorption behavior and computing mechanism. Sci. Total. Environ. 2020, 730, 138930. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Wu, Z.; Deng, Q.; Tu, W.; Dai, G.; Zeng, Z.; Deng, S. Enhanced Cr(VI) removal by polyethylenimine- and phosphorus-codoped hierarchical porous carbons. J. Colloid Interface Sci. 2018, 523, 110–120. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.M.A.W. Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresour. Technol. 2010, 101, 5098–5103. [Google Scholar] [CrossRef]
- Islam, A.; Angove, M.J.; Morton, D.W. Recent innovative research on chromium (VI) adsorption mechanism. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100267. [Google Scholar] [CrossRef]
- Sanchez-Hachair, A.; Hofmann, A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1,5-diphenylcarbazide colorimetry. Comptes Rendus Chim. 2018, 21, 890–896. [Google Scholar] [CrossRef]
- Sun, X.-F.; Ma, Y.; Liu, X.-W.; Wang, S.-G.; Gao, B.-Y.; Li, X.-M. Sorption and detoxification of chromium(VI) by aerobic granules functionalized with polyethylenimine. Water Res. 2010, 44, 2517–2524. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, W.-J.; Zhang, N.; Li, Y.-S.; Jiang, H.; Sheng, G.-P. Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour. Technol. 2014, 169, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Xing, H.T.; Guo, H.X.; Weng, W.; Hu, S.R.; Li, S.X.; Huang, Y.H.; Sun, X.; Su, Z.B. Investigation on the adsorption properties of Cr(vi) ions on a novel graphene oxide (GO) based composite adsorbent. J. Mater. Chem. A 2014, 2, 12561–12570. [Google Scholar] [CrossRef]
- Geczo, A.; Giannakoudakis, D.A.; Triantafyllidis, K.; Elshaer, M.R.; Rodríguez-Aguado, E.; Bashkova, S. Mechanistic insights into acetaminophen removal on cashew nut shell biomass-derived activated carbons. Environ. Sci. Pollut. Res. 2020, 1–14. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, X.; Wang, X.; Gao, B.; Yue, Q.; Song, W.; Zhang, L.; Wang, H. FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr(VI) removal by amine cross-linking biosorbent. J. Colloid Interface Sci. 2016, 468, 313–323. [Google Scholar] [CrossRef]
- Bandara, P.C.; Peña-Bahamonde, J.; Rodrigues, D.F. Redox mechanisms of conversion of Cr(VI) to Cr(III) by graphene oxide-polymer composite. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of Graphene Oxide-Based Hydrogels as Efficient Dye Adsorbents for Wastewater Treatment. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; López-Ramón, M.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Kehrer, M.; Duchoslav, J.; Hinterreiter, A.; Cobet, M.; Mehic, A.; Stehrer, T.; Stifter, D. XPS investigation on the reactivity of surface imine groups with TFAA. Plasma Process. Polym. 2019, 16, 1800160. [Google Scholar] [CrossRef]
- Manoj, B.; Kunjomana, A.G. Study of stacking structure of amorphous carbon by X-Ray diffraction technique. Int. J. Electro-chem. Sci. 2012, 7, 3127–3134. [Google Scholar]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.S.; Sung, S.J.; Kim, T.; Park, C.R. Highly dispersible edge-selectively oxidized graphene with improved electrical performance. Nanoscale 2016, 9, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
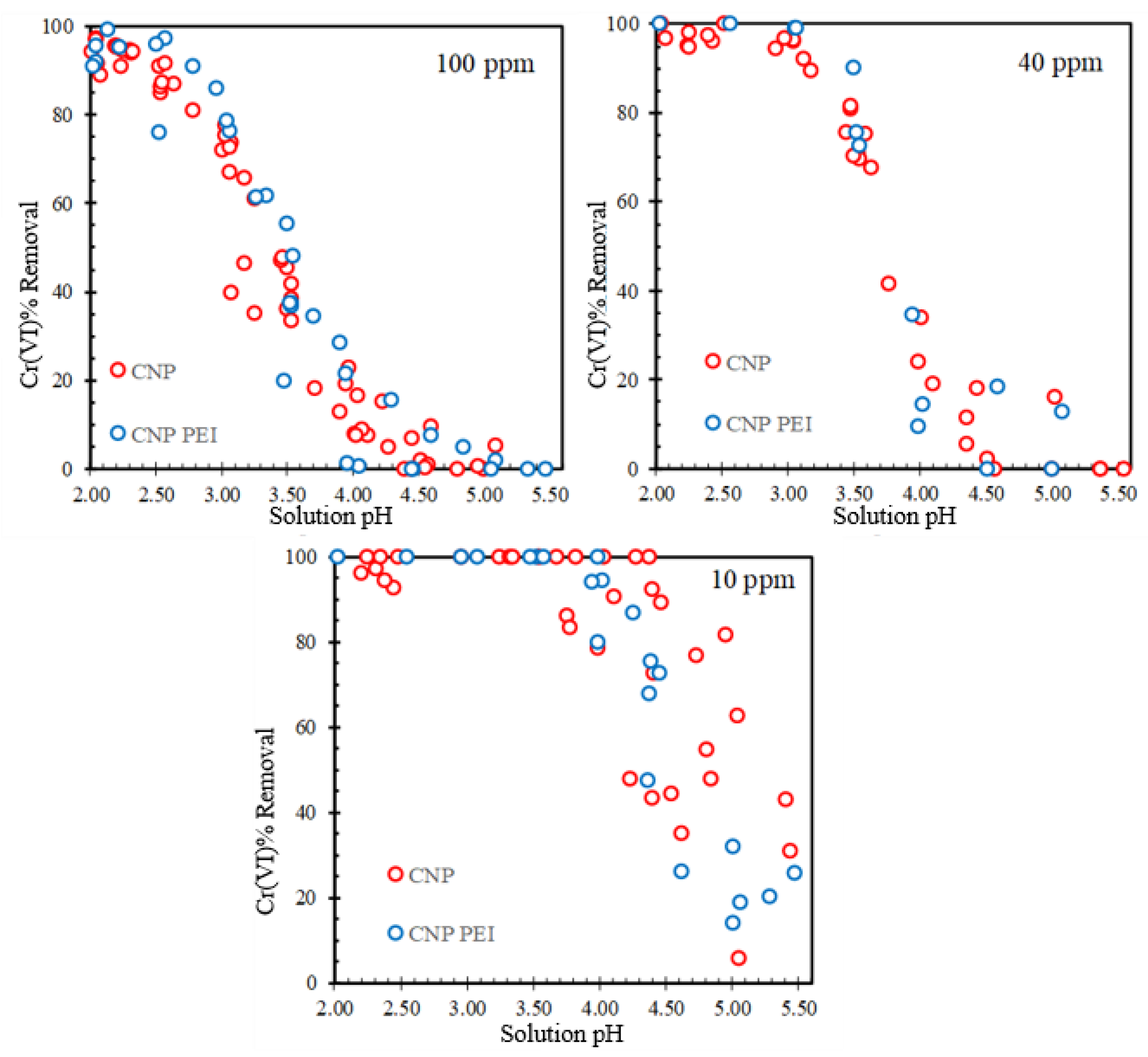
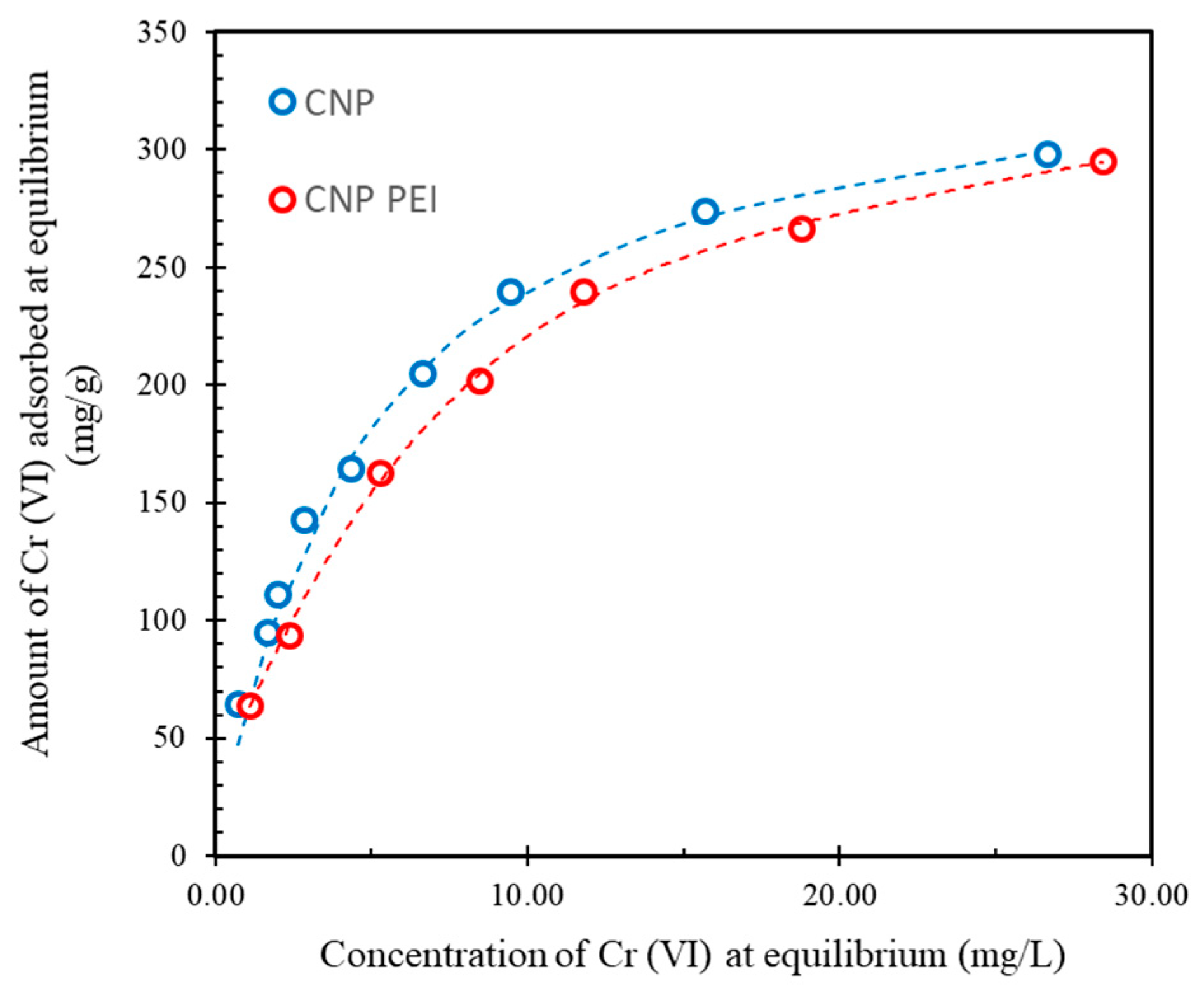
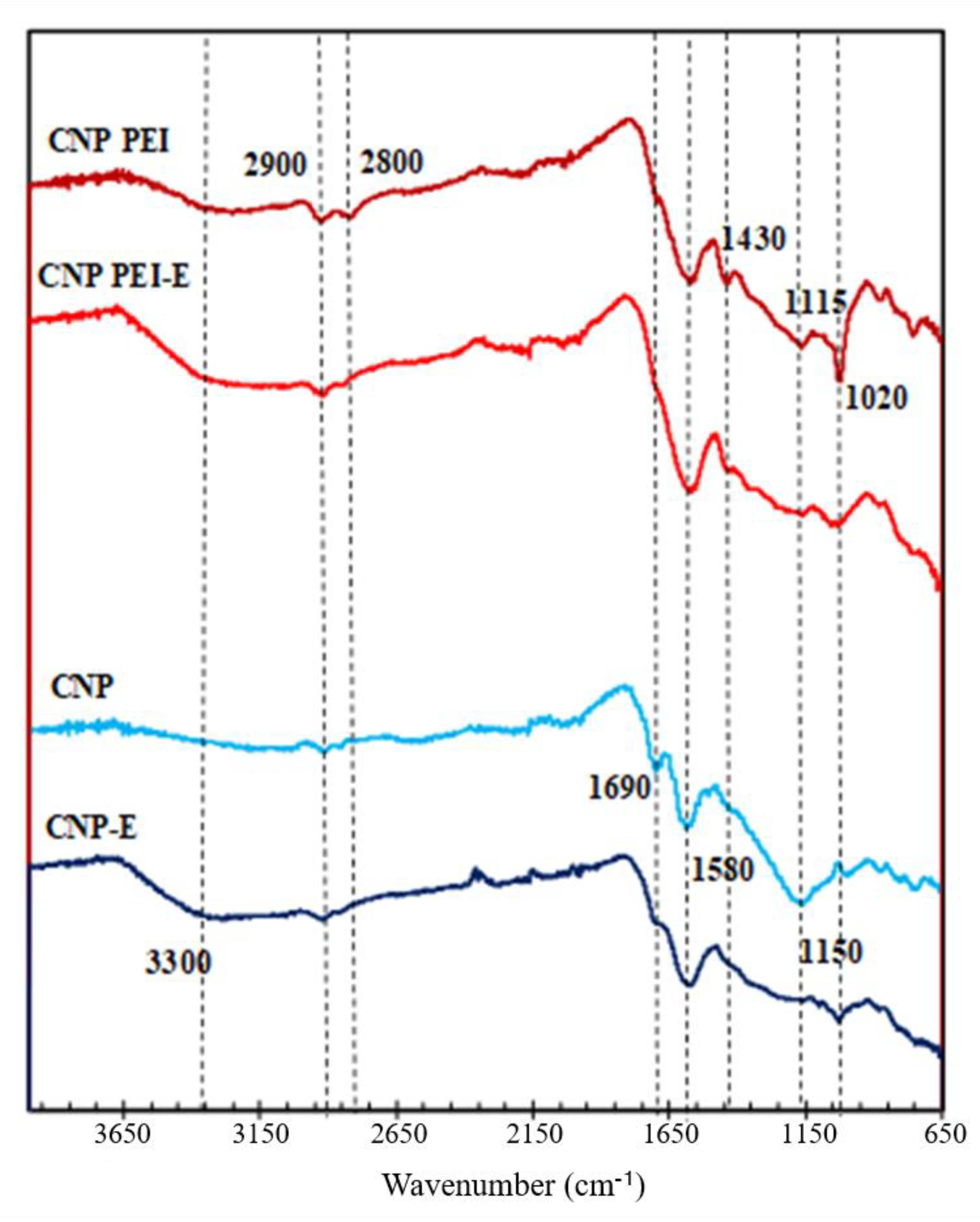
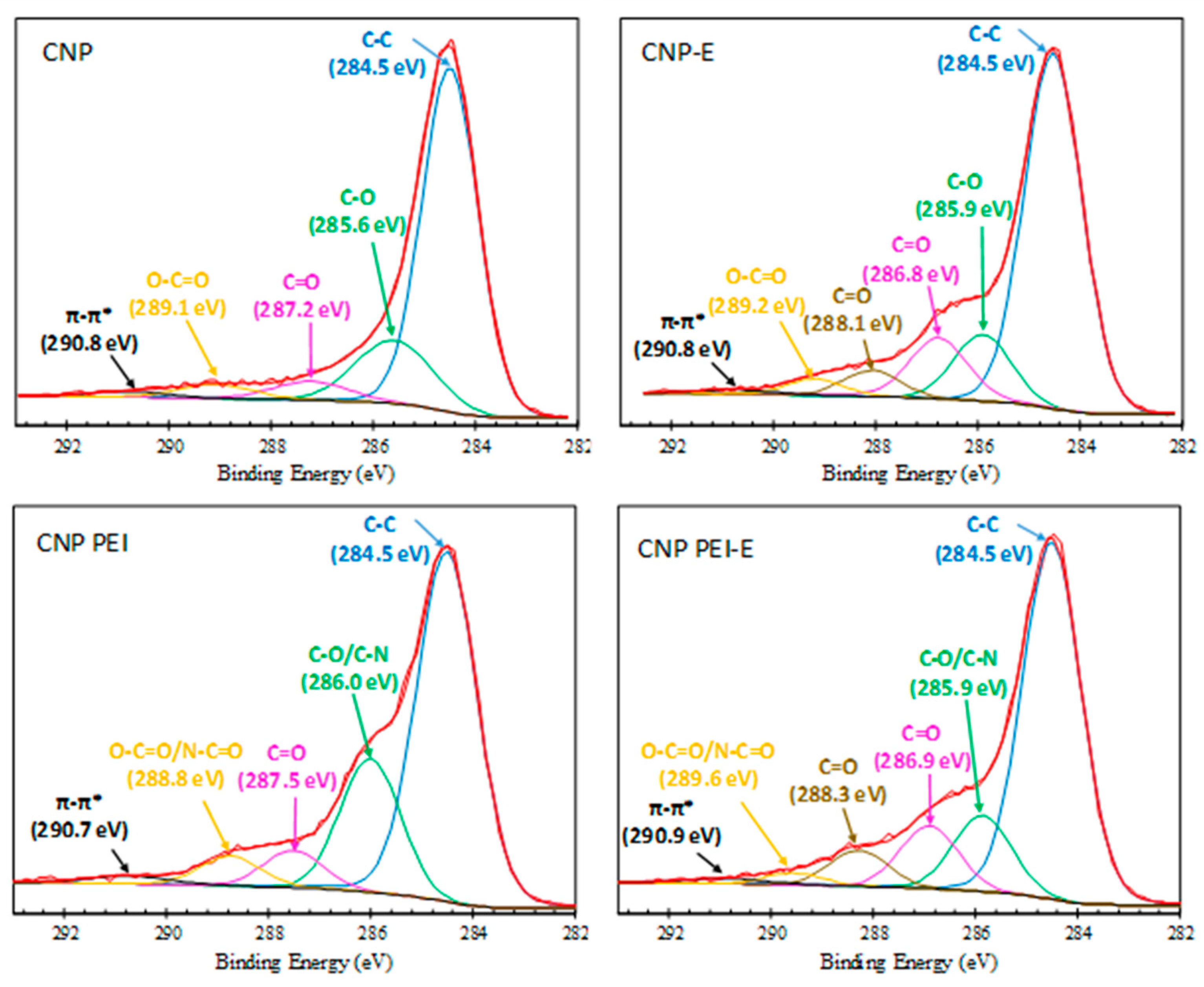
| Sample | pH/pH E 1 | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qm ± CI (mg/g) | KL (L/mg) | R2 | KF (mg/g)(L/mg)1/n | 1/n | R2 | ||
| CNP | 2.9/2.5 | 340 ± 20 | 0.19 | 0.9981 | 97.3 | 0.27 | 0.9481 |
| CNP PEI | 3.5/2.8 | 320 ± 20 | 0.17 | 0.9991 | 98.4 | 0.34 | 0.9810 |
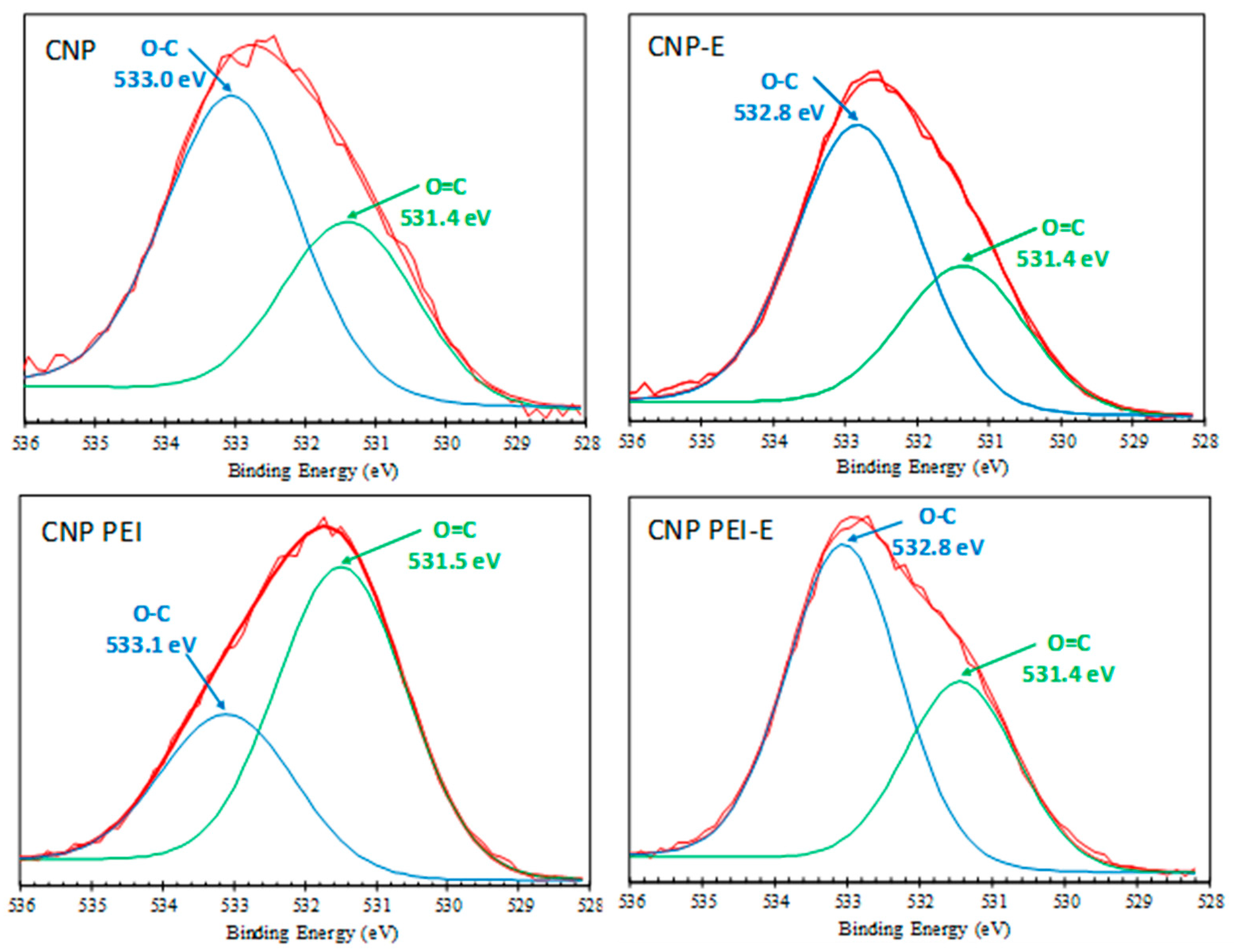
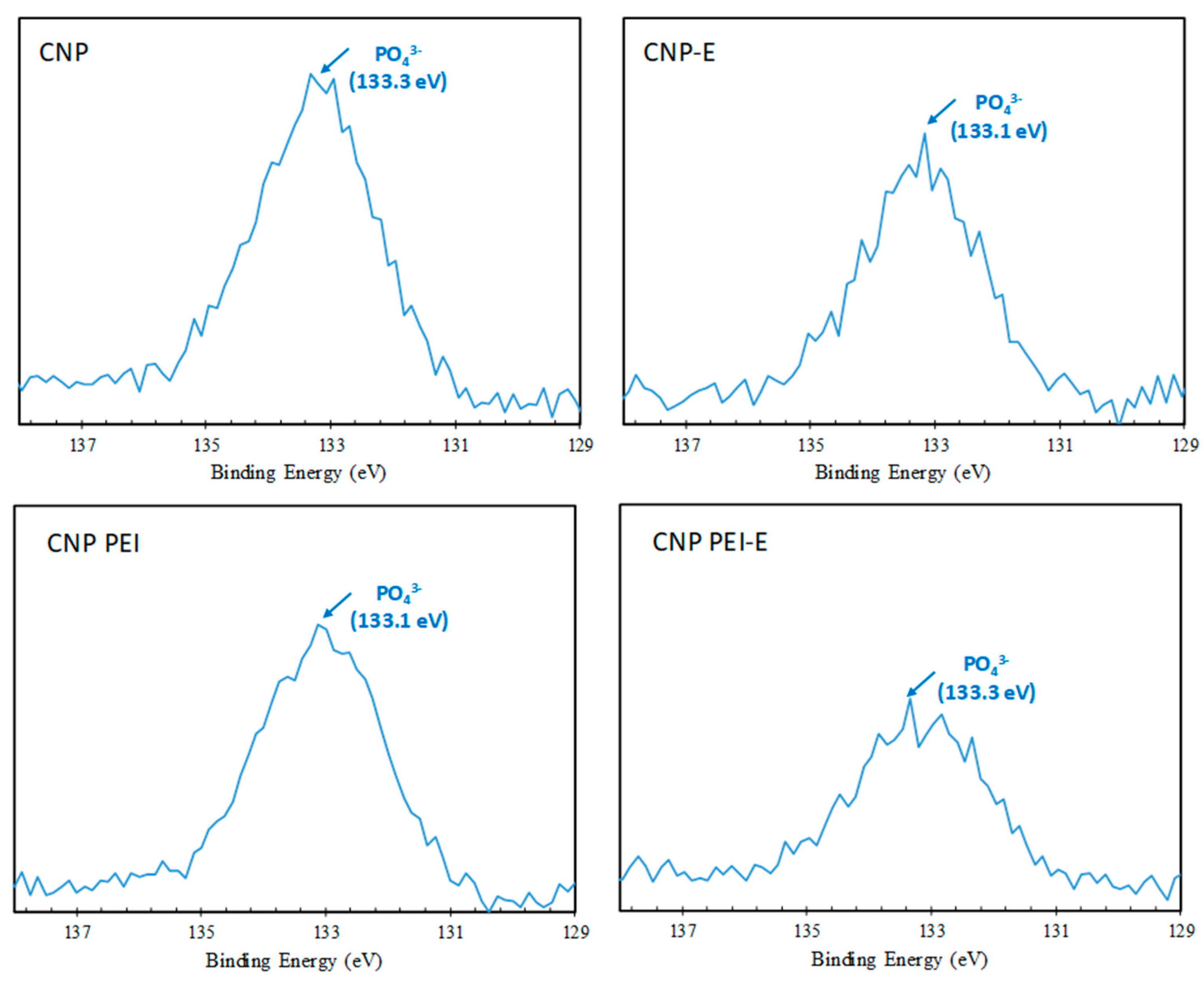
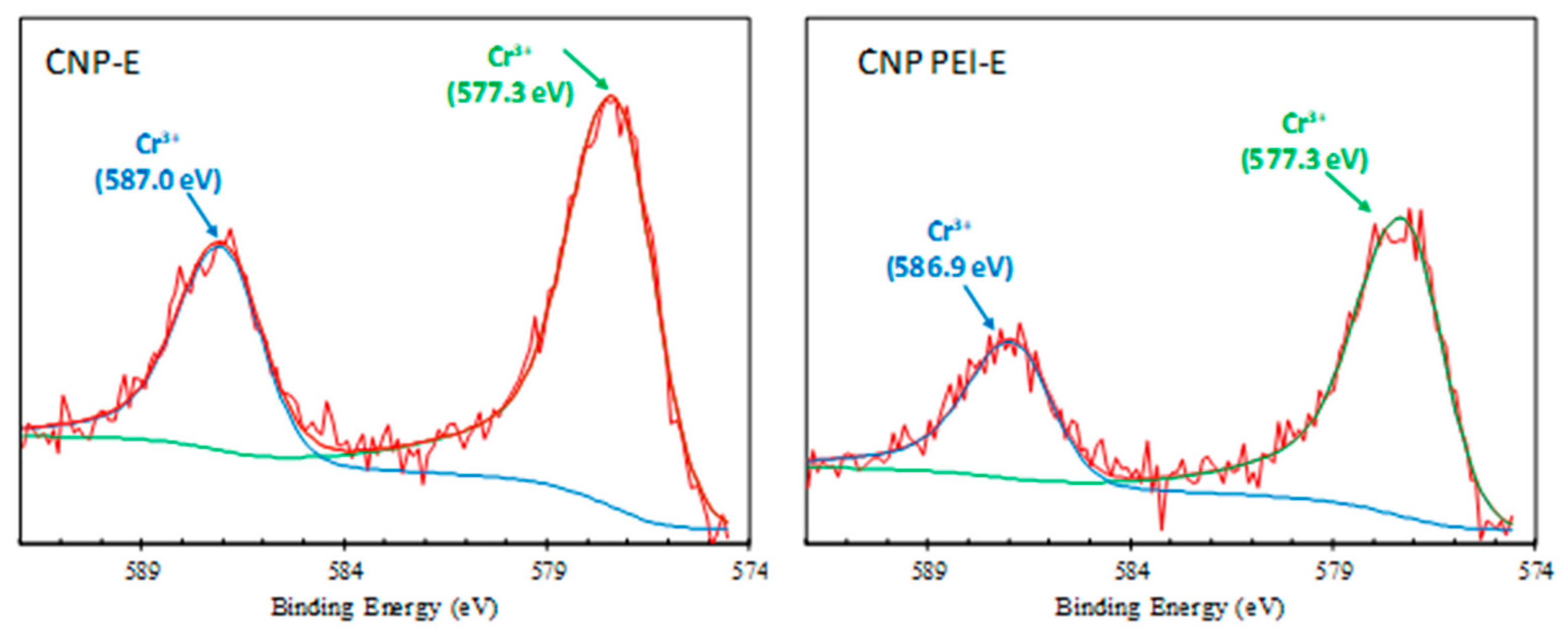
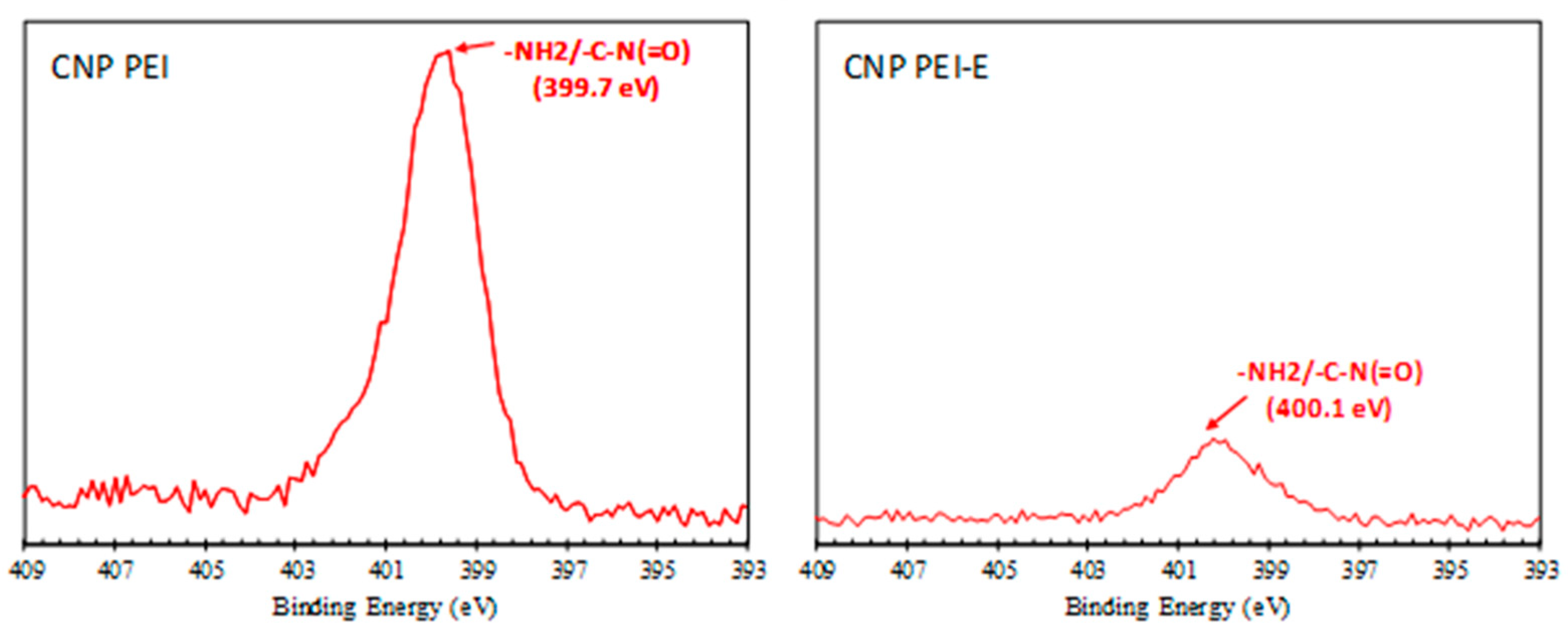
| Binding Energy eV | Bond Assignment | CNP % | CNP-E % | CNP PEI % | CNP PEI-E % |
|---|---|---|---|---|---|
| C 1s | 89.68 | 78.29 | 79.61 | 78.35 | |
| 284.5 | C–C (sp2 carbon) | 71.48 | 66.51 | 63.03 | 65.63 |
| 285.8 ± 0.2 | C–O (phenol, ester, ether)/C–N | 17.78 | 12.25 | 22.95 | 13.43 |
| 287.1 ± 0.4 | C=O (carbonyl) | 5.30 | 12.04 | 6.43 | 11.10 |
| 288.1± 0.1 | C=O (carboxyl/carbonyl) | 4.82 | 6.43 | ||
| 289.1 ± 0.4 | O–C=O (carboxyl, ester)/ N–C=O | 3.89 | 3.22 | 5.56 | 2.09 |
| 290.8 ± 0.1 | π–π* | 1.55 | 1.16 | 2.02 | 1.33 |
| O 1s | 9.59 | 19.91 | 11.68 | 18.50 | |
| 531.4 ± 0.3 | O=C (carbonyl, ester)/O=C–N | 36.09 | 34.39 | 67.41 | 37.25 |
| 532.9 ± 0.1 | O–C (phenol, ether, ester, carboxyl) | 63.91 | 65.61 | 32.59 | 62.75 |
| P 2p | 0.73 | 0.56 | 0.64 | 0.46 | |
| 133.2 ± 0.1 | phosphate | 100 | 100 | 100 | 100 |
| Cr 2p | 1.24 | 0.83 | |||
| 577.3 | Cr (III) | 67.67 | 65.77 | ||
| 586.9 ± 0.1 | Cr (III) | 32.33 | 34.23 | ||
| N 1s | 8.06 | 1.87 | |||
| 399.9 ± 0.2 | amine/amide | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, V.A.; Rivera, J.F.A.; Bello, R.; Rodríguez-Aguado, E.; Elshaer, M.R.; Wodzinski, R.L.; Bashkova, S. The Role of Surface Chemistry and Polyethylenimine Grafting in the Removal of Cr (VI) by Activated Carbons from Cashew Nut Shells. C 2021, 7, 27. https://doi.org/10.3390/c7010027
Smith VA, Rivera JFA, Bello R, Rodríguez-Aguado E, Elshaer MR, Wodzinski RL, Bashkova S. The Role of Surface Chemistry and Polyethylenimine Grafting in the Removal of Cr (VI) by Activated Carbons from Cashew Nut Shells. C. 2021; 7(1):27. https://doi.org/10.3390/c7010027
Chicago/Turabian StyleSmith, Victoria A., Juster F. A. Rivera, Ruby Bello, Elena Rodríguez-Aguado, Mohammed R. Elshaer, Rebecca L. Wodzinski, and Svetlana Bashkova. 2021. "The Role of Surface Chemistry and Polyethylenimine Grafting in the Removal of Cr (VI) by Activated Carbons from Cashew Nut Shells" C 7, no. 1: 27. https://doi.org/10.3390/c7010027
APA StyleSmith, V. A., Rivera, J. F. A., Bello, R., Rodríguez-Aguado, E., Elshaer, M. R., Wodzinski, R. L., & Bashkova, S. (2021). The Role of Surface Chemistry and Polyethylenimine Grafting in the Removal of Cr (VI) by Activated Carbons from Cashew Nut Shells. C, 7(1), 27. https://doi.org/10.3390/c7010027





