Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship
Abstract
1. Introduction
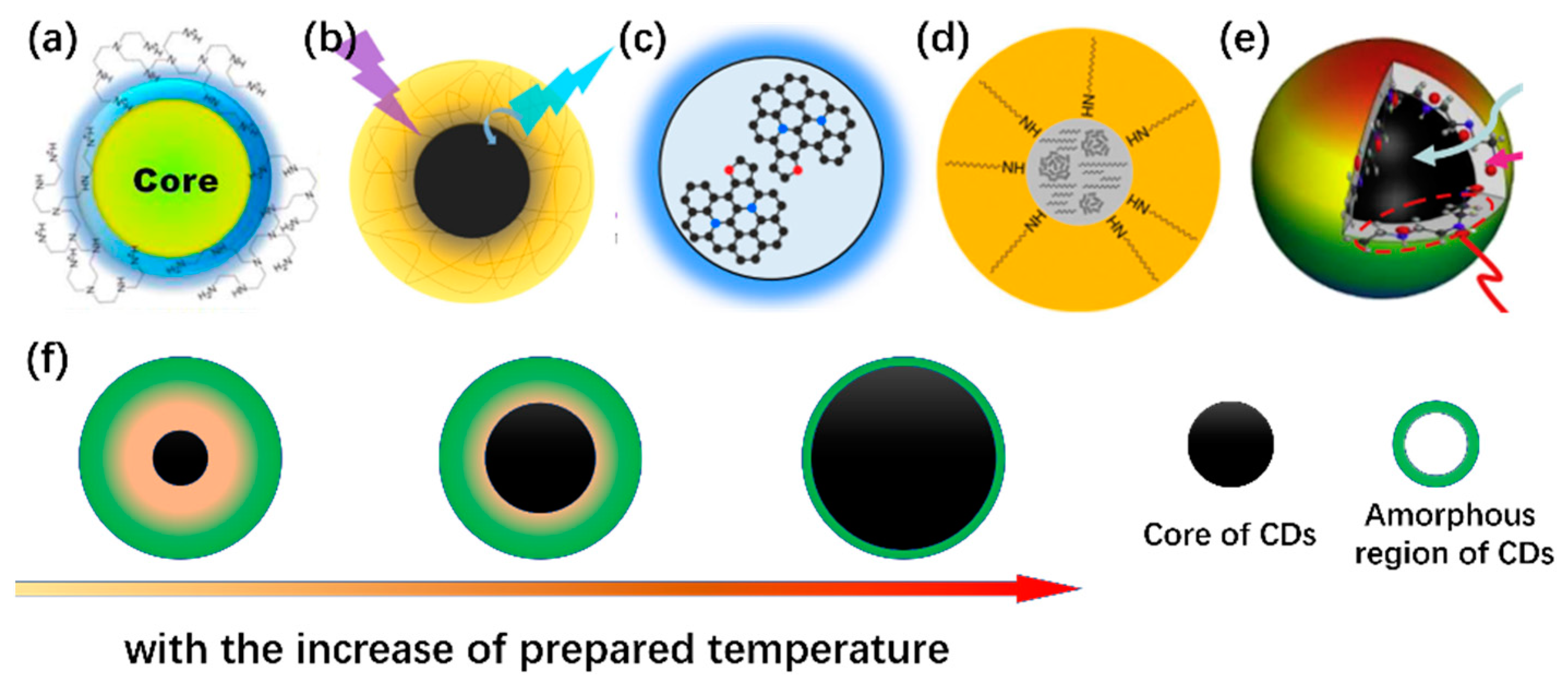
2. Towards a Retro-Engineering of the CD Structure
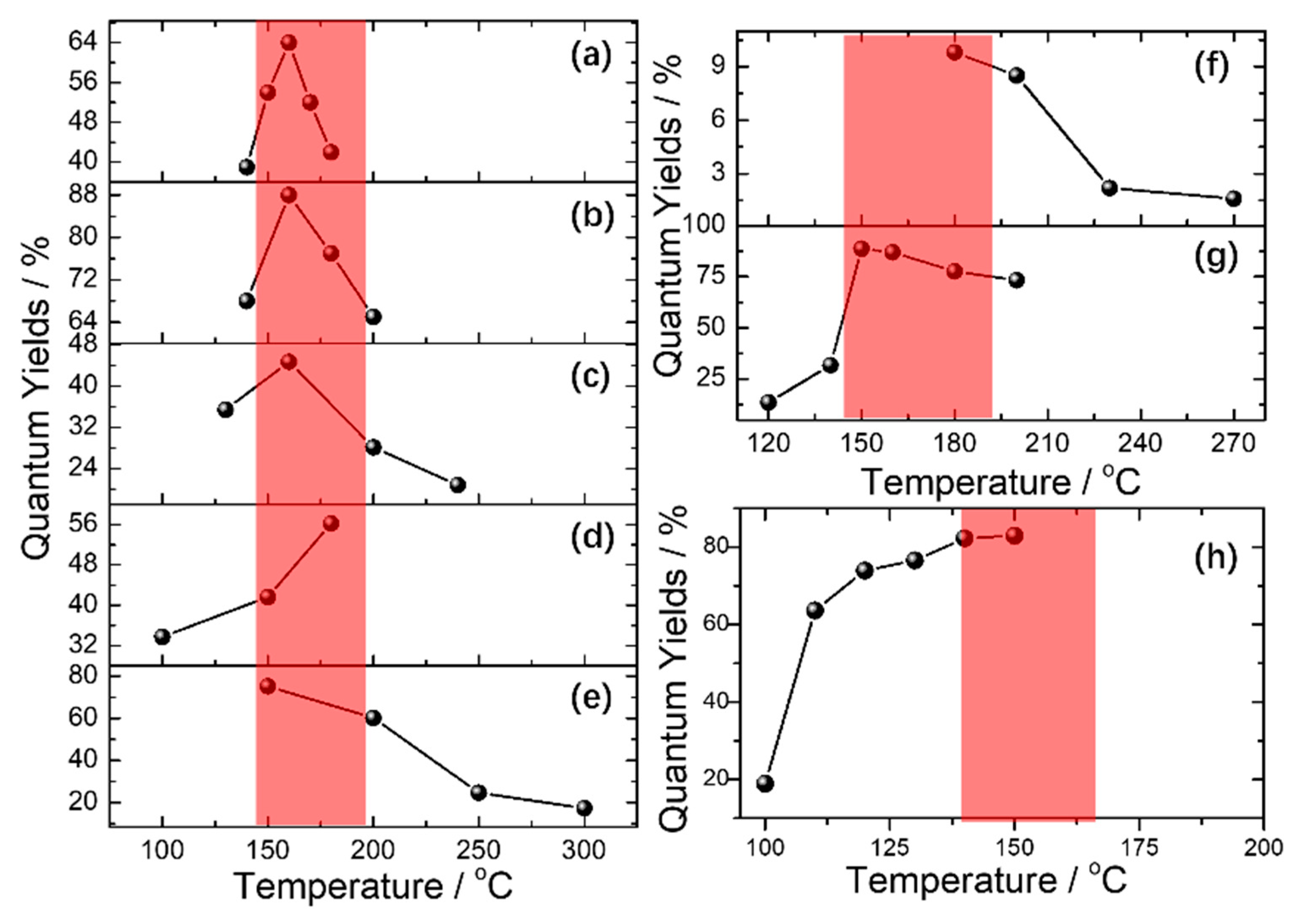
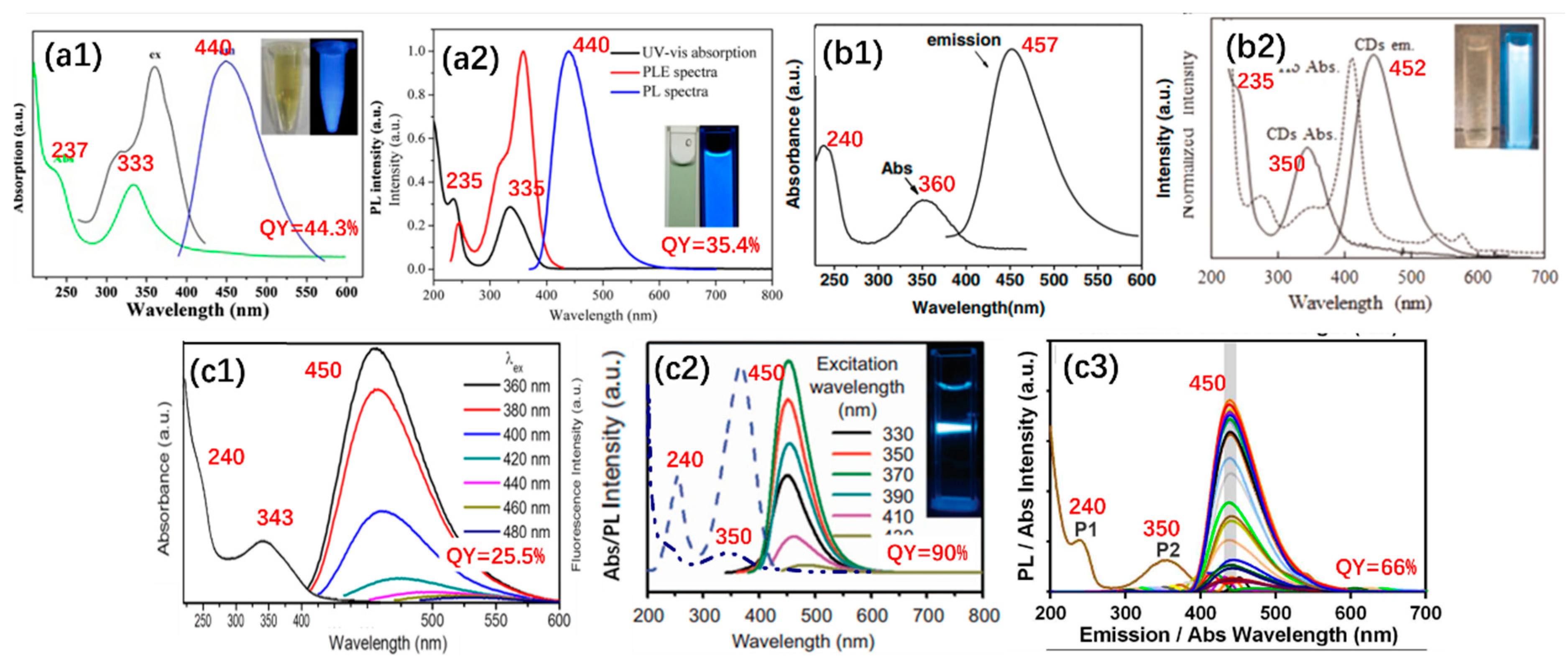
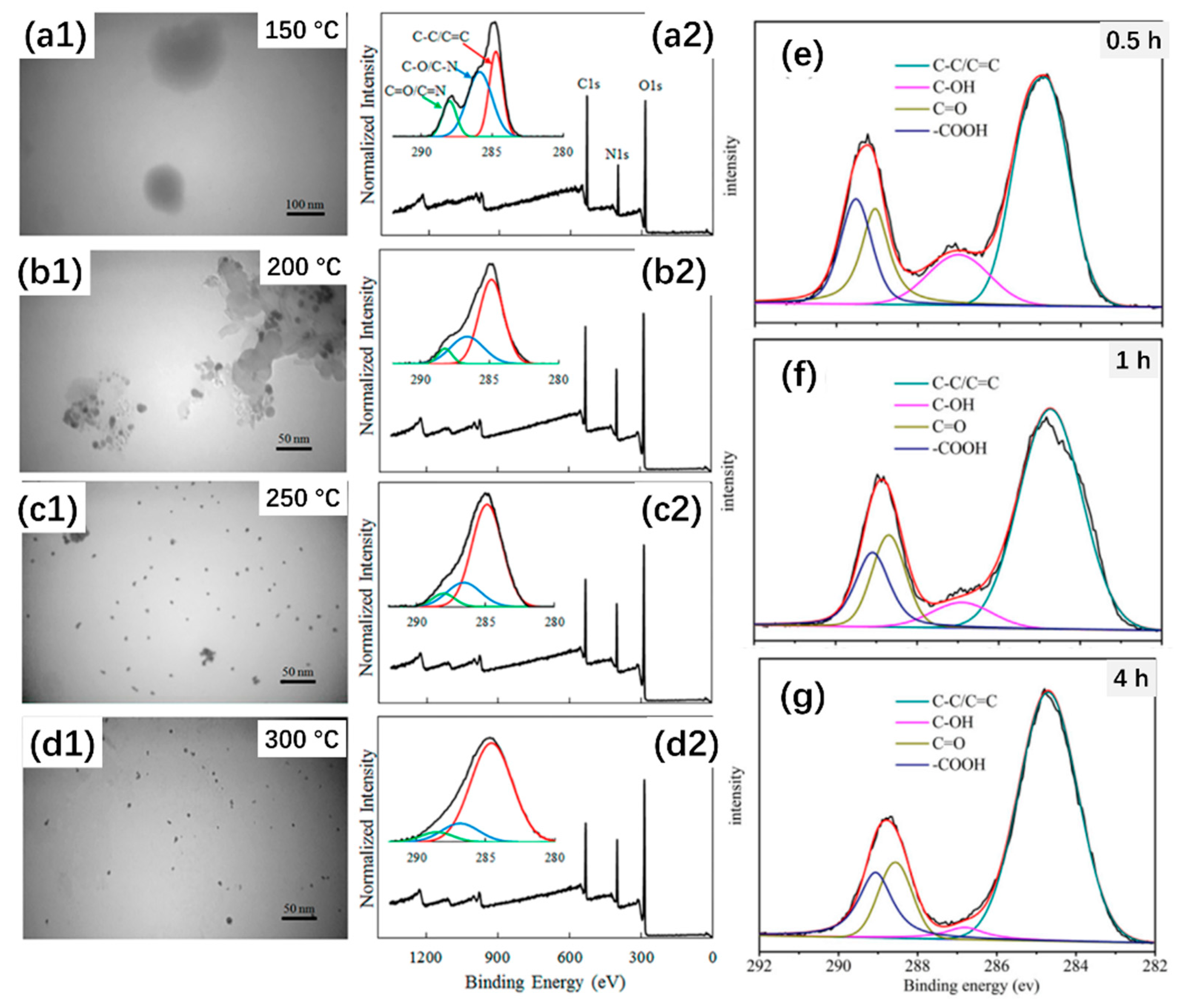
2.1. Carbonization Temperature vs. Optical Properties
2.2. Reaction Time
2.3. Solvothermal vs. Hydrothermal Method
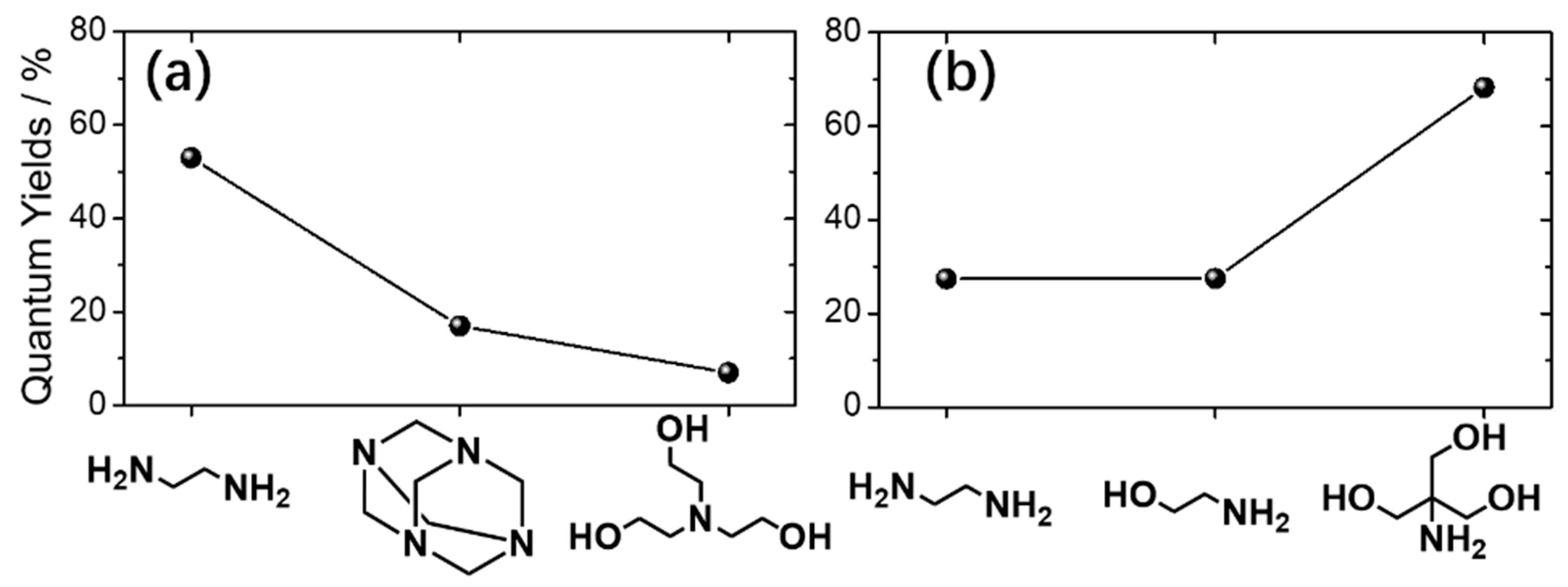
2.4. Nitrogen Sources
3. Optical Properties of CA-Based CDs
3.1. Multicolor Photoluminescence
3.2. Surface Modifications
3.3. External Variables Controlling the Emission
4. Optical Applications
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, L.; Innocenzi, P. Sol-gel chemistry for carbon dots. Chem. Rec. 2018, 18, 1192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ren, J.; Li, J.; Wang, Q.; Wang, Q.; Xie, Z.; Qu, X. Construction of bi-layer biluminophore fast-responding pressure sensitive coating for non-contact unsteady aerodynamic testing. Polym. Test. 2019, 77, 105922. [Google Scholar] [CrossRef]
- Luo, P.G.; Sahu, S.; Yang, S.; Sonkar, S.K.; Wang, J.; Wang, H.; LeCroy, G.E.; Cao, L.; Sun, Y. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. 2013, 1, 2116. [Google Scholar] [CrossRef]
- Hutton, G.A.; Martindale, B.C.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Liu, C.; Ma, D. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502. [Google Scholar] [CrossRef]
- Ren, J.; Sun, J.; Sun, X.; Song, R.; Xie, Z.; Zhou, S. Precisely controlled up/down-conversion liquid and solid state photoluminescence of carbon dots. Adv. Opt. Mater. 2018, 6, 1800115. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Yu, S.; Yang, H. Observation of lasing emission from carbon nanodots in organic solvents. Adv. Mater. 2012, 24, 2263. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sun, X.; Wang, Y.; Song, R.; Xie, Z.; Zhou, S.; Chen, P. Controllable photoluminescent and nonlinear optical properties of polymerizable carbon dots and their arbitrary copolymerized gel glasses. Adv. Opt. Mater. 2018, 6, 1701273. [Google Scholar] [CrossRef]
- Han, Z.; Ni, Y.; Ren, J.; Zhang, W.; Wang, Y.; Xie, Z.; Zhou, S.; Yu, S.F. Highly efficient and ultra-narrow bandwidth orange emissive carbon dots for microcavity lasers. Nanoscale 2019, 11, 11577. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Fluorescent carbon dots in solid-state: From nanostructures to functional devices. Prog. Solid State Chem. 2020, 100295. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.-H.; Sain, M. Future perspectives and review on organic carbon dots in electronic applications. ACS Nano 2019, 13, 6224. [Google Scholar] [CrossRef]
- Hola, K.; Sudolska, M.; Kalytchuk, S.; Nachtigallova, D.; Rogach, A.L.; Otyepka, M.; Zboril, R. Graphitic nitrogen triggers red fluorescence in carbon dots. ACS Nano 2017, 11, 12402. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, F.; Liu, C. Organic-inorganic hybrid functional carbon dot gel glasses. Adv. Mater. 2012, 24, 1716. [Google Scholar] [CrossRef]
- Chahal, S.; Yousefi, N.; Tufenkji, N. Green synthesis of high quantum yield carbon dots from phenylalanine and citric acid: Role of stoichiometry and nitrogen doping. ACS Sustain. Chem. Eng. 2020, 8, 5566–5575. [Google Scholar] [CrossRef]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward efficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering. Adv. Mater. 2016, 28, 3516. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Malfatti, L.; Takahashi, M.; Carboni, D.; Messina, F.; Tokudome, Y.; Takemoto, M.; Innocenzi, P. Design of carbon dots photoluminescence through organo-functional silane grafting for solid-state emitting devices. Sci. Rep. 2017, 7, 5469. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Sun, S.; Zhang, A.; Jiang, K.; Zhang, L.; Dong, C.; Huang, Q.; Wu, A.; Lin, H. Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing. Adv. Mater. 2015, 27, 7782. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, C.M.; Chiriu, D.; Stagi, L.; Casula, M.F.; Thakkar, S.V.; Malfatti, L.; Suzuki, K.; Ricci, P.C.; Corpino, R. Carbon dots in water and mesoporous matrix: Chasing the origin of their photoluminescence. J. Phys. Chem. 2018, 122, 25638. [Google Scholar] [CrossRef]
- Yin, C.; Fan, Y.; Yang, X.; Zhou, X. Highly efficient synthesis of N-doped carbon dots with excellent stability through pyrolysis method. J. Mater. Sci. 2019, 54, 9372. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Bednarz, S.; Żmudzki, P.; Galica, M.; Bogdał, D. Novel efficient fluorophores synthesized from citric acid. RSC Adv. 2015, 5, 34795. [Google Scholar] [CrossRef]
- Mura, S.; Ludmerczki, R.; Stagi, L.; Garroni, S.; Carbonaro, C.M.; Ricci, P.C.; Casula, M.F.; Malfatti, L.; Innocenzi, P. Integrating sol-gel and carbon dots chemistry for the fabrication of fluorescent hybrid organic-inorganic films. Sci. Rep. 2020, 10, 4770. [Google Scholar] [CrossRef]
- Das, A.; Gude, V.; Roy, D.; Chatterjee, T.; De, C.K.; Mandal, P.K. On the molecular origin of photoluminescence of nonblinking carbon dot. J. Phys. Chem. 2017, 121, 9634. [Google Scholar] [CrossRef]
- Liao, S.; Zhao, X.; Zhu, F.; Chen, M.; Wu, Z.; Yang, H.; Chen, X. Novel S, N-doped carbon quantum dot-based “off-on” fluorescent sensor for silver ion and cysteine. Talanta 2018, 180, 300. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Kundu, A.; Lee, J.; Park, B.; Ray, C.; Sankar, K.V.; Kim, W.S.; Lee, S.H.; Cho, I.-J.; Jun, S.C. Facile approach to synthesize highly fluorescent multicolor emissive carbon dots via surface functionalization for cellular imaging. J. Colloid Interface Sci. 2018, 513, 505. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304. [Google Scholar] [CrossRef]
- Xiao, Q.; Liang, Y.; Zhu, F.; Lu, S.; Huang, S. Microwave-assisted one-pot synthesis of highly luminescent N-doped carbon dots for cellular imaging and multi-ion probing. Microchim. Acta 2017, 184, 2429. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J. Novel fluorescence resonance energy transfer optical sensors for vitamin B12 detection using thermally reduced carbon dots. New J. Chem. 2015, 39, 501. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.C.; Suresh, R.; Ramamurthy, P. Unravelling the multiple emissive states in citric-acid-derived carbon dots. J. Phys. Chem. 2016, 120, 1252. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.; Wen, Z.; Zhou, J.; Wang, X.; Wu, Q.; Wang, C. Concentration-dependent color tunability of nitrogen-doped carbon dots and their application for iron (III) detection and multicolor bioimaging. J. Colloid Interface Sci. 2018, 521, 33. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, P.; Zhang, F.; Feng, X.; Wang, Y.; Yang, Y.; Liu, X. Fluorescent probes for “off-on” highly sensitive detection of Hg2+ and L-cysteine based on nitrogen-doped carbon dots. Talanta 2016, 152, 288. [Google Scholar] [CrossRef]
- Du, F.; Zeng, F.; Ming, Y.; Wu, S. Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim. Acta 2013, 180, 453. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Abdollahi, H. Hemoglobin detection using carbon dots as a fluorescence probe. Biosens. Bioelectron. 2015, 71, 470. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 2016, 10, 4410. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zheng, M.; Li, J.; Xie, Z.; Sun, Z. Tailoring color emissions from N-doped graphene quantum dots for bioimaging applications. Light Sci. Appl. 2015, 4, 364. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Huang, C.; Liu, G.; Leung, K.C.F.; Wáng, Y.X.N.J. High performance photoluminescent carbon dots for in vitro and in vivo bioimaging: Effect of nitrogen doping ratios. Langmuir 2015, 31, 8063. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Schneider, J.; Reckmeier, C.J.; Huang, H.; Kasák, P.; Rogach, A.L. Carbonization conditions influence the emission characteristics and the stability against photobleaching of nitrogen doped carbon dots. Nanoscale 2017, 9, 11730. [Google Scholar] [CrossRef] [PubMed]
- Ogi, T.; Aishima, K.; Permatasari, F.A.; Iskandar, F.; Tanabe, E.; Okuyama, K. Kinetics of nitrogen-doped carbon dot formation via hydrothermal synthesis. New J. Chem. 2016, 40, 5555. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738. [Google Scholar] [CrossRef]
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Gel-like carbon dots: Characterization and their potential applications. ChemPhysChem 2017, 18, 890. [Google Scholar] [CrossRef]
- Shamsipur, M.; Barati, A.; Taherpour, A.A.; Jamshidi, M. Resolving the multiple emission centers in carbon dots: From fluorophore molecular states to aromatic domain states and carbon-core states. J. Phys. Chem. Lett. 2018, 9, 4189. [Google Scholar] [CrossRef]
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: All carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribol. Int. 2018, 118, 373. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal. Chem. 2012, 84, 6220. [Google Scholar] [CrossRef]
- Deng, L.; Wang, X.; Kuang, Y.; Wang, C.; Luo, L.; Wang, F.; Sun, X. Development of hydrophilicity gradient ultracentrifugation method for photoluminescence investigation of separated non-sedimental carbon dots. Nano Res. 2015, 8, 2810. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ehrat, F.; Urban, P.; Teves, R.; Wyrwich, R.; Döblinger, M.; Feldmann, J.; Urban, A.S.; Stolarczyk, J.K. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Lee, G.; Do, S.; Joo, T.; Rhee, S.W. Size-controlled soft-template synthesis of carbon nanodots toward versatile photoactive materials. Small 2014, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hu, Y.; Wang, P.; Yang, L.; Al Awak, M.M.; Tang, Y.; Twara, F.K.; Qian, H.; Sun, Y. Modified facile synthesis for quantitatively fluorescent carbon dots. Carbon 2017, 122, 389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, X.; Chen, X.; Shao, H.; Zhai, Y.; Pan, G.; Zhang, H.; Ushakova, E.V.; Zhang, Y.; Song, H. Spectrally tunable solid state fluorescence and room-temperature phosphorescence of carbon dots synthesized via seeded growth method. Adv. Opt. Mater. 2019, 7, 1801599. [Google Scholar] [CrossRef]
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational design of multi-color-emissive carbon dots in a single reaction system by hydrothermal. Adv. Sci. 2020, 2001453. [Google Scholar] [CrossRef]
- Gao, F.; Ma, S.; Li, J.; Dai, K.; Xiao, X.; Zhao, D.; Gong, W. Rational design of high quality citric acid-derived carbon dots by selecting efficient chemical structure motifs. Carbon 2017, 112, 131. [Google Scholar] [CrossRef]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C.Y. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem. Mater. 2010, 22, 4528. [Google Scholar] [CrossRef]
- Ludmerczki, R.; Mura, S.; Carbonaro, C.M.; Mandity, I.M.; Carraro, M.; Senes, N.; Garroni, S.; Granozzi, G.; Calvillo, L.; Marras, S. Carbon dots from citric acid and its intermediates formed by thermal decomposition. Chem. Eur. J. 2019, 25, 11963. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, Z.; Zhu, C.; Ren, J.; Wang, E.; Dong, S. Multifunctional water-soluble luminescent carbon dots for imaging and Hg2+ sensing. J. Mater. Chem. 2014, 2, 6995. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward high-efficient red emissive carbon dots: Facile preparation, unique properties, and applications as multifunctional theranostic agents. Chem. Mater. 2016, 28, 8659. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Cui, P.; Feng, X.; Chen, L.; Yang, Y.; Liu, X. Water-soluble, nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of Hg2+ in aqueous solution. RSC Adv. 2015, 5, 40393. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Hao, L.; Wei, Y.; Cai, X.; Zhu, B. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif. 2018, 51, e12488. [Google Scholar] [CrossRef]
- Wen, Z.; Yin, X. Excitation-independent carbon dots, from photoluminescence mechanism to single-color application. RSC Adv. 2016, 6, 27829. [Google Scholar] [CrossRef]
- Reckmeier, C.J.; Schneider, J.; Xiong, Y.; Häusler, J.; Kasák, P.; Schnick, W.; Rogach, A.L. Aggregated molecular fluorophores in the ammonothermal synthesis of carbon dots. Chem. Mater. 2017, 29, 10352. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of excitation dependent fluorescence in carbon nanodots. J. Phys. Chem. Lett. 2016, 7, 3695. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Sun, K.; Reckmeier, C.; Zhang, T.; Zhang, X.; Zhao, J.; Wu, C.; William, W.Y.; Rogach, A.L. Combination of carbon dot and polymer dot phosphors for white light-emitting diodes. Nanoscale 2015, 7, 12045. [Google Scholar] [CrossRef]
- Wu, Z.L.; Gao, M.X.; Wang, T.T.; Wan, X.Y.; Zheng, L.L.; Huang, C.Z. A general quantitative pH sensor developed with dicyandiamide N-doped high quantum yield graphene quantum dots. Nanoscale 2014, 6, 3868. [Google Scholar] [CrossRef]
- Zuo, P.; Liu, J.; Guo, H.; Wang, C.; Liu, H.; Zhang, Z.; Liu, Q. Multifunctional N, S co-doped carbon dots for sensitive probing of temperature, ferric ion, and methotrexate. Anal. Bioanal. Chem. 2019, 411, 1647. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Q.; Hou, Y.; Liu, Y.; Zhang, Y. High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 2016, 155, 62. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; Von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. 2017, 121, 2014. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Fan, Y.; Guo, X.; Zhou, L.; Lv, Y.; Lin, J. One-step microwave synthesis of N-doped hydroxyl-functionalized carbon dots with ultra-high fluorescence quantum yields. Nanoscale 2016, 8, 15281. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.; Zhong, N.; Gao, Q.; Xiong, H. Highly efficient red-emitting carbon dots with gram-scale yield for bioimaging. Langmuir 2017, 33, 12635. [Google Scholar] [CrossRef]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y. Near-infrared excitation/emission and multiphoton-induced fluorescence of carbon dots. Adv. Mater. 2018, 30, 1705913. [Google Scholar] [CrossRef]
- Anjana, R.; Devi, J.A.; Jayasree, M.; Aparna, R.; Aswathy, B.; Praveen, G.; Lekha, G.; Sony, G. S, N-doped carbon dots as a fluorescent probe for bilirubin. Microchim. Acta 2018, 185, 11. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, L.; Liu, Y.; Meng, X.; Xu, H.; Xu, Y.; Liu, B.; Fang, X.; Li, H.; Ding, T. Supramolecular interactions via hydrogen bonding contributing to citric-acid derived carbon dots with high quantum yield and sensitive photoluminescence. RSC Adv. 2017, 7, 20345. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, N.; Shi, H.; Ji, W.; Guo, X.; Yuan, W.; Hu, Q. One-step microwave synthesis of carbon dots for highly sensitive and selective detection of copper ions in aqueous solution. New J. Chem. 2018, 42, 3097. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, C.; Zhang, Y.; Yang, S.; Chen, S. Zinc ion-doped carbon dots with strong yellow photoluminescence. RSC Adv. 2016, 6, 37189. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, R.; Ling, L.; Li, Q.; Chen, S. Rapid and large-scale production of multi-fluorescence carbon dots via magnetic hyperthermia method. Angew. Chem. Int. Ed. 2019, 59, 3099. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, S.; Guo, J.; Kong, J.; Song, J.; Kang, Z. The design of room-temperature-phosphorescent carbon dots and their application as a security ink. J. Mater. Chem. 2019, 7, 10605. [Google Scholar] [CrossRef]
- Xie, Z.; Zheng, M. Colour-tunable ultralong-lifetime room temperature phosphorescence with external heavy-atom effect in boron-doped carbon dots. Chem. Eng. J. 2020, 127647. [Google Scholar] [CrossRef]
- Liu, E.; Li, D.; Zhou, X.; Zhou, G.; Xiao, H.; Zhou, D.; Tian, P.; Guo, R.; Qu, S. Highly emissive carbon dots in solid state and their applications in light-emitting devices and visible light communication. ACS Sustain. Chem. Eng. 2019, 7, 9301. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.I.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Carbon dots as new generation materials for nanothermometer. Nanoscale Res. Lett. 2020, 15, 182. [Google Scholar] [CrossRef]
- Meierhofer, F.; Dissinger, F.; Weigert, F.; Jungclaus, J.; Müller-Caspary, K.; Waldvogel, S.R.; Resch-Genger, U.; Voss, T. Citric acid based carbon dots with amine type stabilizers: pH-specific luminescence and quantum yield characteristics. J. Phys. Chem. 2020, 124, 8894. [Google Scholar] [CrossRef]
- Stagi, L.; Mura, S.; Malfatti, L.; Carbonaro, C.M.; Ricci, P.C.; Porcu, S.; Secci, F.; Innocenzi, P. Anomalous optical properties of citrazinic acid under extreme pH conditions. ACS Omega 2020. [Google Scholar] [CrossRef]
- Mura, S.; Stagi, L.; Ludmerczki, R.; Malfatti, L.; Innocenzi, P. Reversible aggregation of molecular-like fluorophores driven by extreme pH in carbon dots. Materials 2020, 13, 3654. [Google Scholar] [CrossRef]
- Mura, S.; Stagi, L.; Malfatti, L.; Carbonaro, C.M.; Ludmerczki, R.; Innocenzi, P. Modulating the optical properties of citrazinic acid through the monomer-to-dimer transformation. J. Phys. Chem. 2019, 124, 197. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Du, Q.; Wu, Y.; Hao, X.; Liu, C. Full-band UV shielding and highly daylight luminescent silane-functionalized graphene quantum dot nanofluids and their arbitrary polymerized hybrid gel glasses. J. Mater. Chem. 2016, 4, 9879. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, J.; Xie, Z.; Guan, B.; Wang, J.; Ikeda, T.; Jiang, L. Multi-functional organosilane-polymerized carbon dot inverse opals. Nanoscale 2018, 10, 4642. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Z.; Shang, Y.; Ren, J.; Hu, R.; Guan, B.; Wang, J.; Ikeda, T.; Jiang, L. Lyophilic but nonwettable organosilane-polymerized carbon dots inverse opals with closed-cell structure. ACS Appl. Mater. Interfaces 2018, 10, 6701. [Google Scholar] [CrossRef]
- Zhang, W.; Ni, Y.; Xu, X.; Lu, W.; Ren, P.; Yan, P.; Siu, C.K.; Ruan, S.; Yu, S.F. Realization of multiphoton lasing from carbon nanodot microcavities. Nanoscale 2017, 9, 5957. [Google Scholar] [CrossRef]
- Shangguan, J.; He, D.; He, X.; Wang, K.; Xu, F.; Liu, J.; Tang, J.; Yang, X.; Huang, J. Label-free carbon-dots-based ratiometric fluorescence pH nanoprobes for intracellular pH sensing. Anal. Chem. 2016, 88, 7837. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Wang, X.; Pan, W.; Yu, G.; Wang, J. Carbon dots with red emission for bioimaging of fungal cells and detecting Hg2+ and ziram in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 118230. [Google Scholar] [CrossRef]
- Wang, X.; Yan, P.; Kerns, P.; Suib, S.L.; Loew, L.; Zhao, J. Voltage-dependent photoluminescence of carbon dots. J. Electrochem. Soc. 2020, 167, 147515. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo. J. Mater. Chem. 2016, 4, 5119. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yin, Z.; Jia, Z.; Wei, J. Carbon nanodots derived from urea and citric acid in living cells: Cellular uptake and antioxidation effect. Langmuir 2020, 36, 8632. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Ma, Y.; Wang, B.; Huang, H.; Liu, Y.; Shao, M.; Kang, Z. Carbon dots derived from citric acid and glutathione as a highly efficient intracellular reactive oxygen species scavenger for alleviating the lipopolysaccharide-induced inflammation in macrophages. ACS Appl. Mater. Interfaces 2020, 12, 41088. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, J.; Zhai, Y.; Wang, H.; Bai, X.; Dong, B.; Wang, H.; Song, H. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wei, J.S.; Zhang, P.; Zhou, Z.Y.; Gao, Q.Y.; Xiong, H.M. Solvent-controlled synthesis of highly luminescent carbon dots with a wide color gamut and narrowed emission peak widths. Small 2018, 14, 1800612. [Google Scholar] [CrossRef]





| Methods | Nitrogen Sources | Emission | QYs | Ref. |
|---|---|---|---|---|
| Hydrothermal method (200 °C, 8 h) | Phenylalanine | 413 nm | 65% | [20] |
| Microwave treatment (550 W, 7 min, aqueous solution) | - | 440 nm | 7.2% | [36] |
| Microwave treatment (700 W, 5 min, aqueous solution) | Ethylenediamine | 455 nm | 41.3% | [60] |
| Microwave treatment (700 W, 5 min, aqueous solution) | Ammonia | 450 nm | 44.3% | [37] |
| Hydrothermal method (200 °C, 5 h) | - | 451 nm | 7.2% | [6] |
| Hydrothermal method (180 °C, 4 h) | Urea | 440 nm | 42.2% | [62] |
| Hydrothermal method (200 °C, 5 h) | Ethylenediamine | 470 nm | 60% | [68] |
| Hydrothermal method (180 °C, 3 h) | Dicyandiamide | 452 nm | 36.5% | [69] |
| Hydrothermal method (200 °C, 4 h) | L-cysteine | 432 nm | 75% | [70] |
| Hydrothermal method (200 °C, 6 h) | Tri(hydroxymethyl) aminomethane | 408 nm | 75% | [71] |
| Hydrothermal method (180 °C, 4 h) | Thiourea | 448 nm | 73.1% | [72] |
| Methods | Precursors (Including CA) | Emission | QYs | Ref. |
|---|---|---|---|---|
| Microwave treatment (700 W, 40 s, aqueous solution) | L-cysteine | 425 nm | 78% | [77] |
| Hydrothermal method (180 °C, 8 h) | Diethylenetriamine | 433 nm | 98% | [78] |
| Hydrothermal method (200 °C, 5 h) | Ethylenediamine | 445 nm | 80.6% | [6] |
| Microwave treatment (400 W, 80 min, 150 °C, aqueous solution) | Ethylenediamine | 445 nm | 82.99% | [34] |
| Hydrothermal method (160 °C, 4 h) | Ethylenediamine | 450 nm | 94% | [30] |
| Microwave treatment (800 W, 4 min, aqueous solution) | L-cysteine, dextrin | 495 nm | 22% | [79] |
| Hydrothermal method (190 °C, 2 h) | Urea | 455 nm | 29% | [27] |
| 510 nm | 30% | |||
| Thermal decomposition (170 °C, 70 min) | Dicyandiamide | 528 nm | 73.12% | [80] |
| Hydrothermal method (200 °C, 4 h) | Ethylenediamine | 530 nm | 63.9% | [65] |
| Hydrothermal method (200 °C, 12 h) | Urea, ZnCl2 | 580 nm | 51.2% | [81] |
| Solvothermal method (180 °C, 4 h, formamide solution) | Ethanediamine | 627 nm | 53% | [75] |
| Microwave treatment (400 W, formamide solution) | - | 640 nm | 22.9% | [61] |
| Solvothermal method (160 °C, 6 h, DMF solution) | Urea | 760 nm | 10% | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Malfatti, L.; Innocenzi, P. Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship. C 2021, 7, 2. https://doi.org/10.3390/c7010002
Ren J, Malfatti L, Innocenzi P. Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship. C. 2021; 7(1):2. https://doi.org/10.3390/c7010002
Chicago/Turabian StyleRen, Junkai, Luca Malfatti, and Plinio Innocenzi. 2021. "Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship" C 7, no. 1: 2. https://doi.org/10.3390/c7010002
APA StyleRen, J., Malfatti, L., & Innocenzi, P. (2021). Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship. C, 7(1), 2. https://doi.org/10.3390/c7010002






