Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
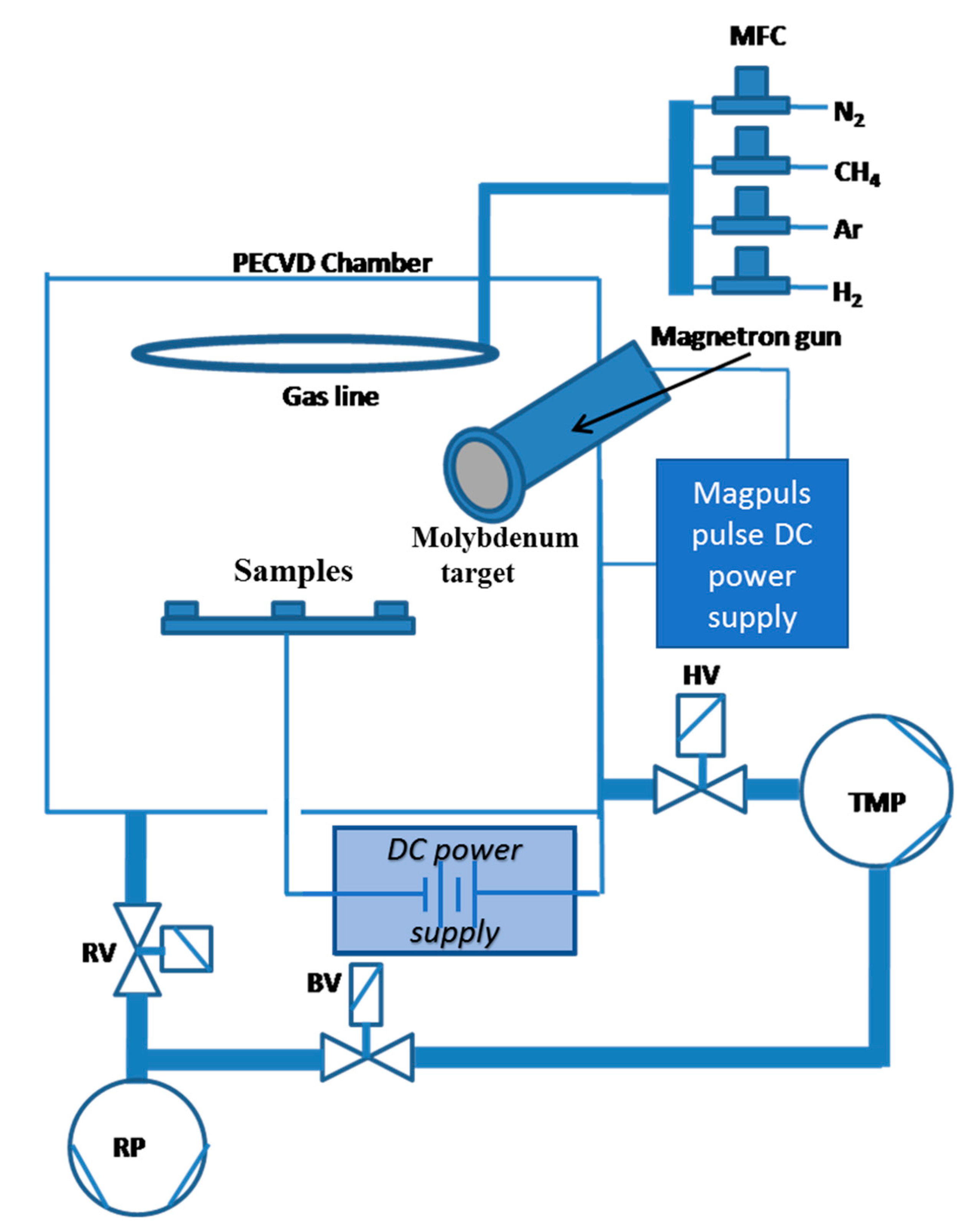
2.2. Deposition
2.3. Characterization
2.4. Properties
3. Results and Discussion
3.1. XPS Studies and Compositions
3.2. XRD Studies
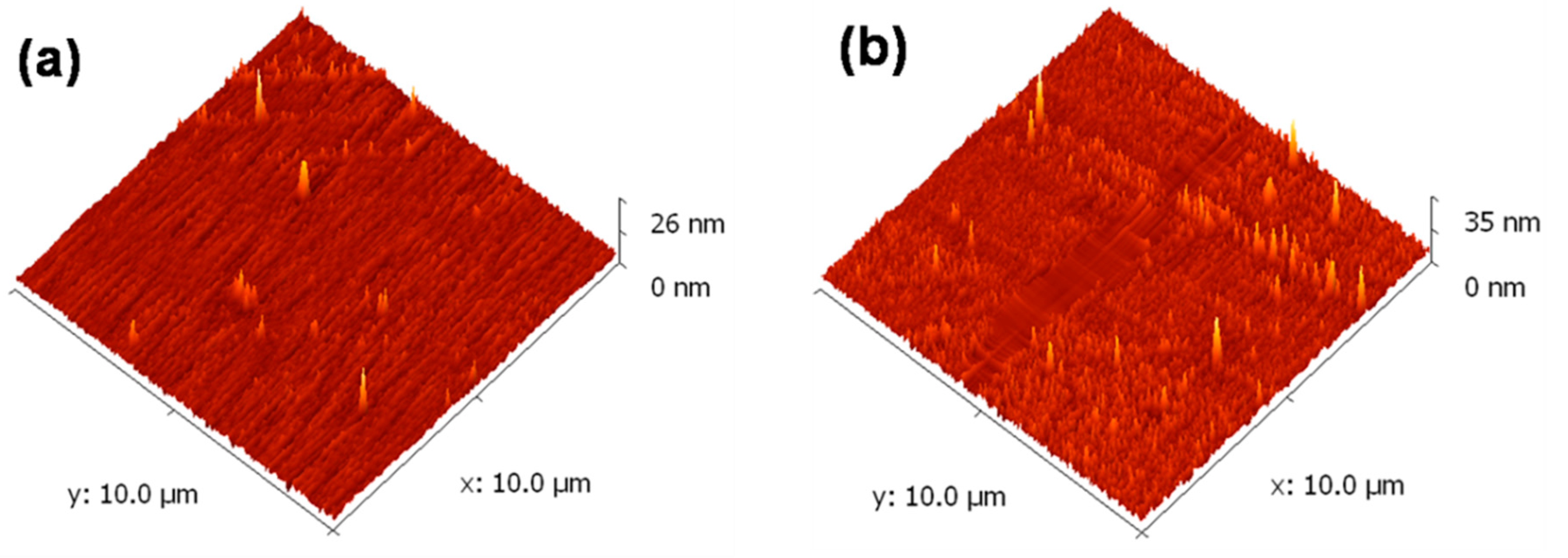
3.3. FESEM and AFM Studies
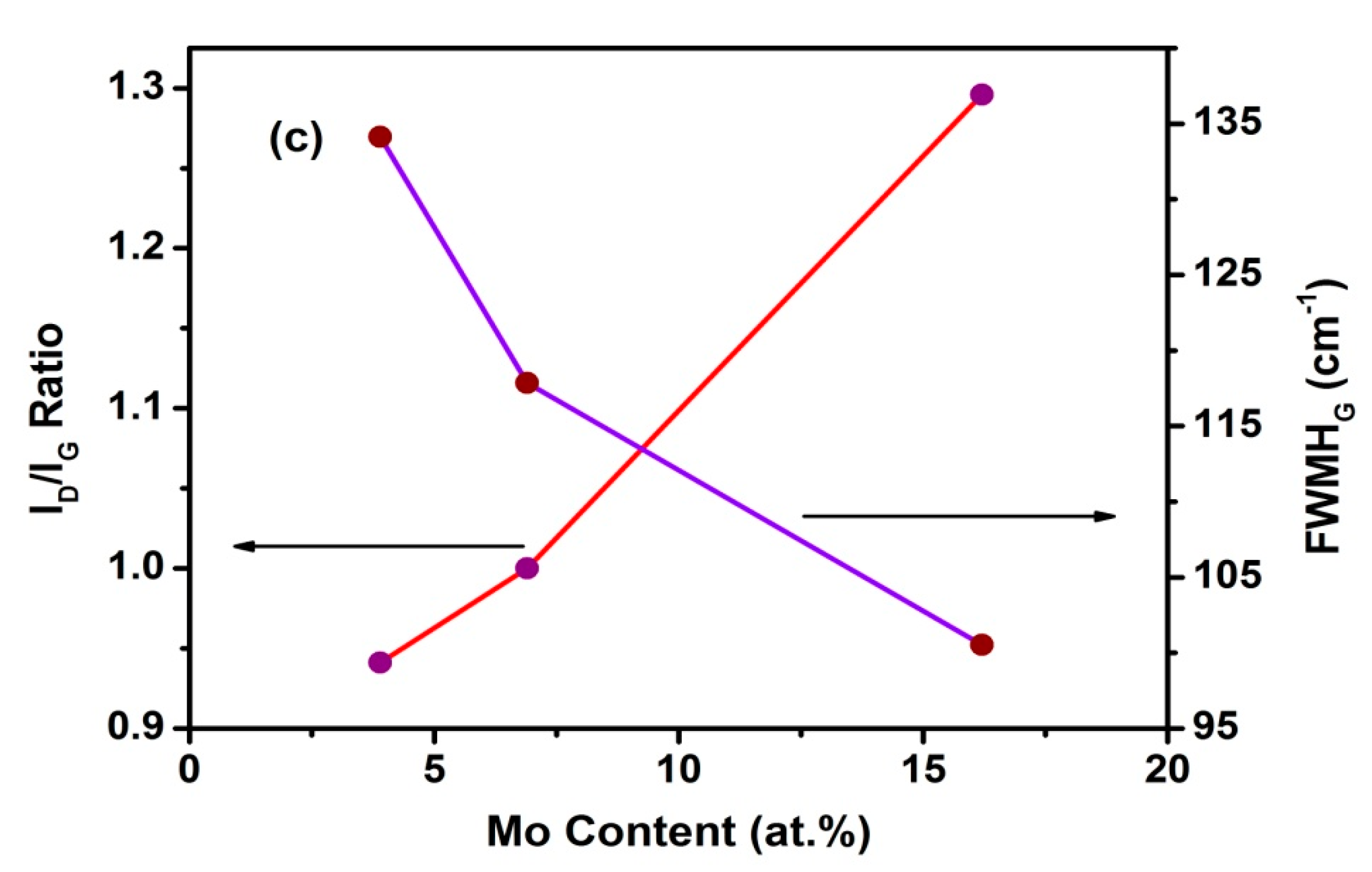
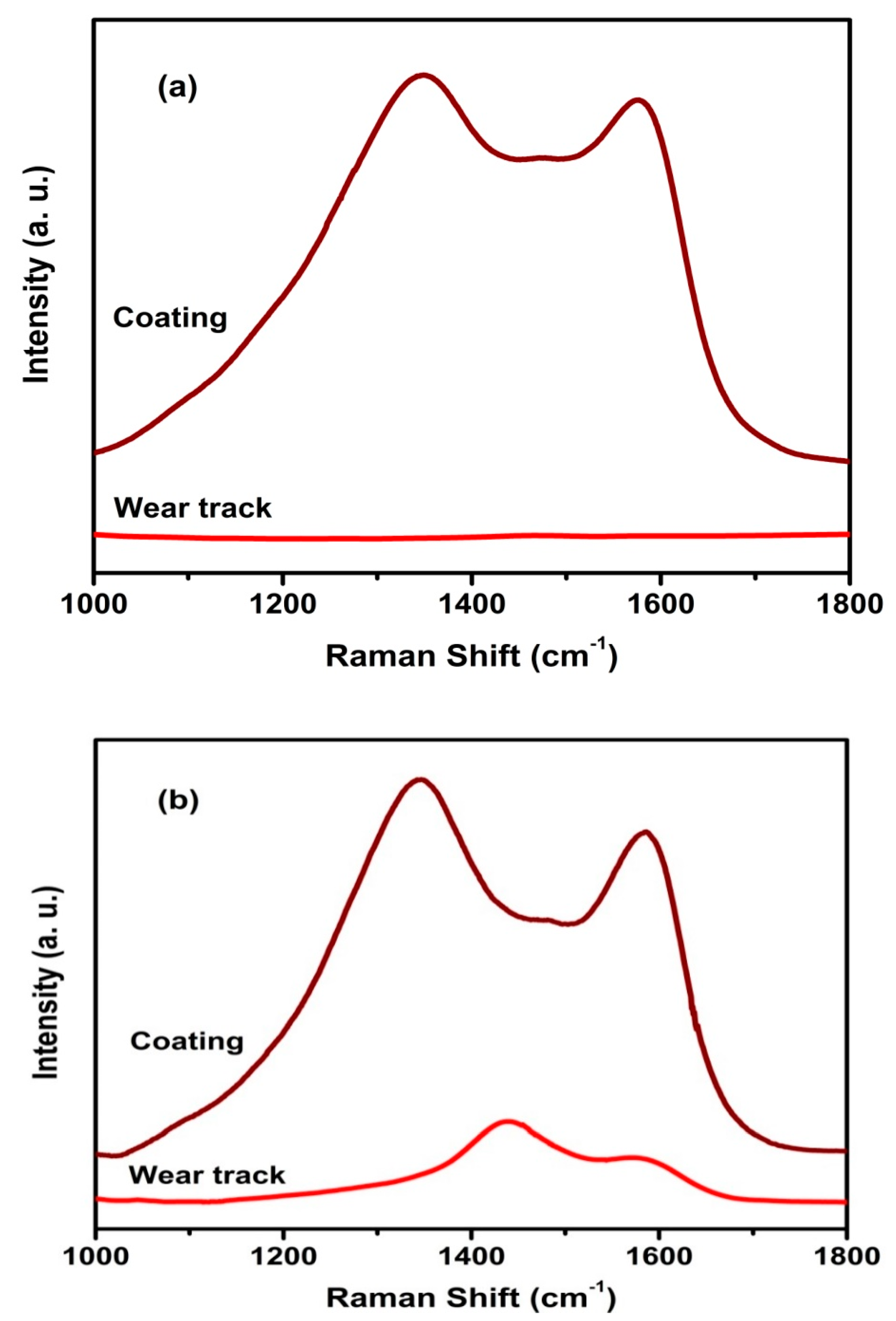
3.4. Raman Spectroscopy Studies
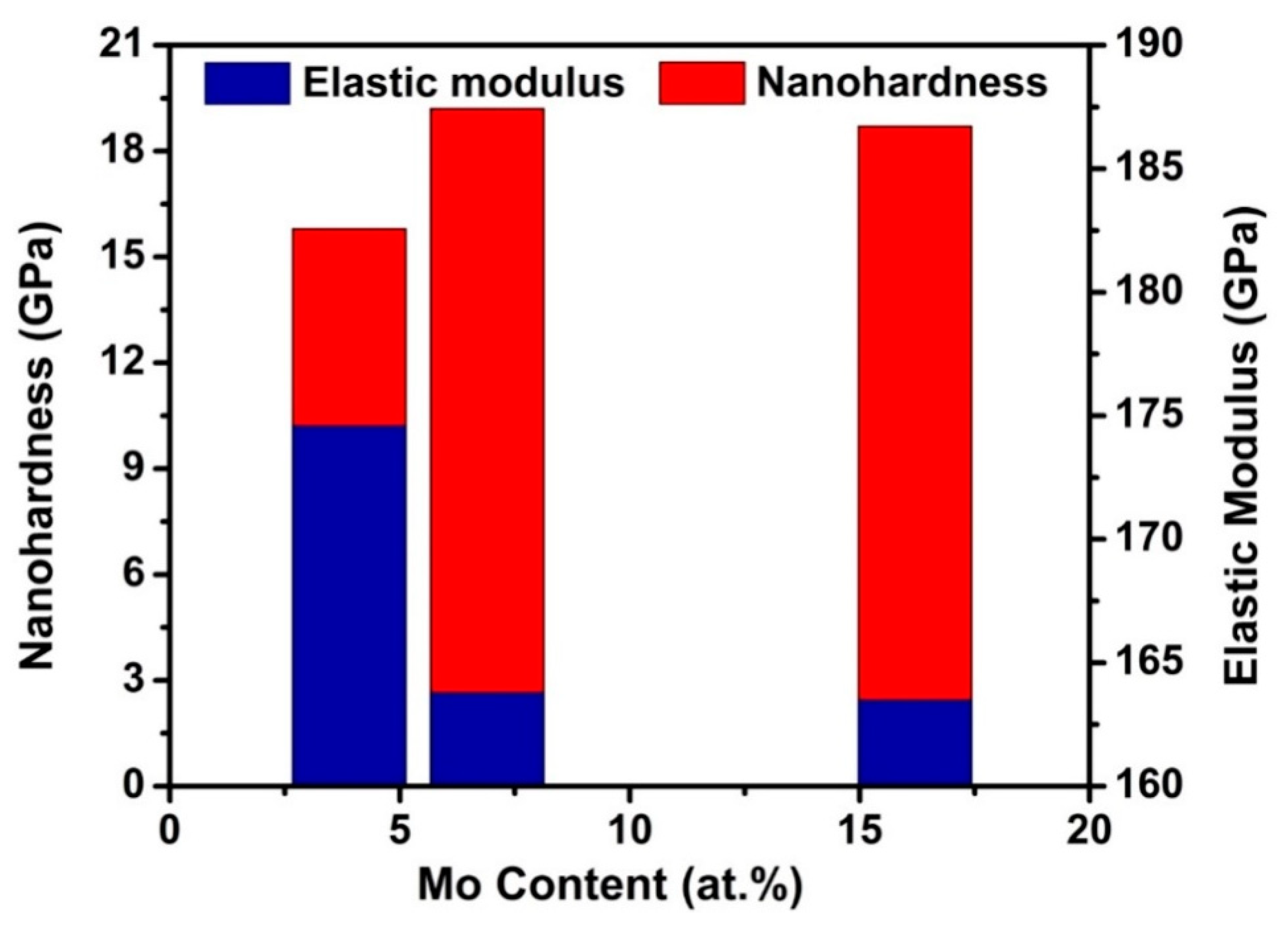
3.5. Mechanical Properties
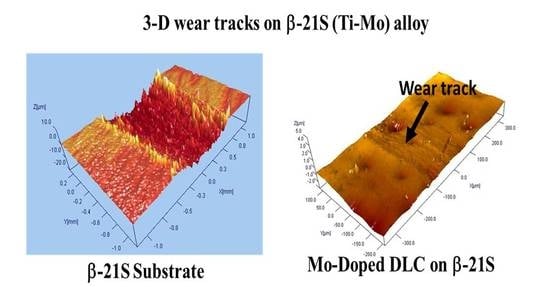
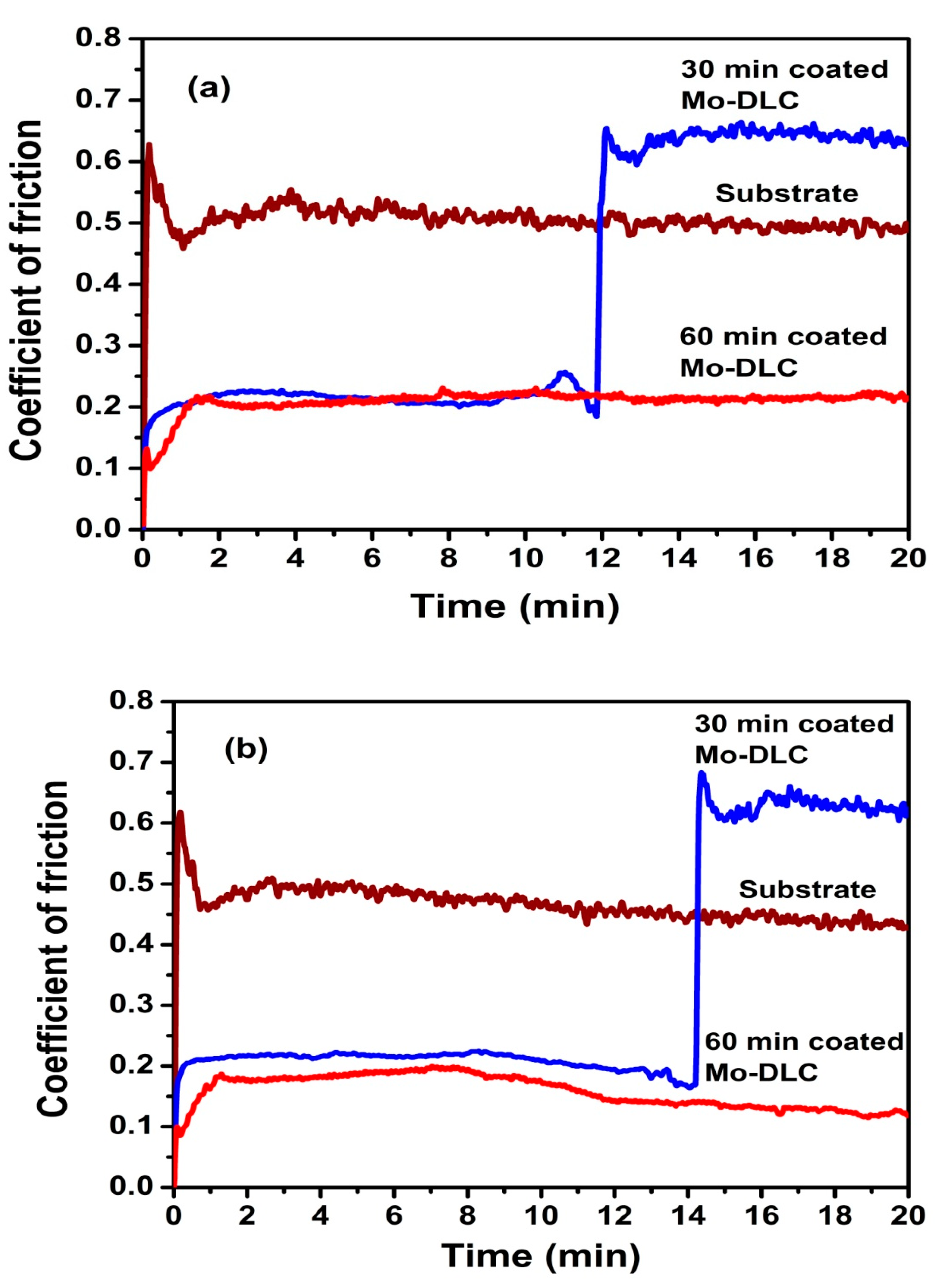
3.6. Tribological Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viswanathan, S.; Mohan, L.; Bera, P.; Shanthiswaroop, S.; Muniprakash, M.; Barshilia, H.C.; Anandan, C. Corrosion and wear resistance properties of multilayered diamond-like carbon nanocomposite coating. Surf. Interface Anal. 2018, 50, 265–276. [Google Scholar] [CrossRef]
- Viswanathan, S.; Mohan, L.; Bera, P.; Kumar, V.P.; Barshilia, H.C.; Anandan, C. Corrosion and wear behaviors of Cr-doped diamond-like carbon coatings. J. Mater. Eng. Perform. 2017, 26, 3633–3647. [Google Scholar] [CrossRef]
- Viswanathan, S.; Mohan, L.; Chakraborty, M.; Mandal, C.; Bera, P.; Aruna, S.T.; Anandan, C. Carbon plasma immersion ion implantation and DLC deposition on Ni−Ti alloy. Mater. Manuf. Process. 2018, 33, 1121–1127. [Google Scholar] [CrossRef]
- Viswanathan, S.; Reddy, M.M.; Mohan, L.; Bera, P.; Barshilia, H.C.; Anandan, C. Corrosion and wear properties of Ti/Tetrahedral amorphous carbon multilayered coating. J. Bio Tribo Corros. 2017, 3, 39. [Google Scholar] [CrossRef]
- Maerten, T.; Jaoul, C.; Oltra, R.; Duport, P.; Le Niniven, C.; Tristant, P.; Meunier, F.; Jarry, O. Micrometric growth defects of DLC thin films. J. Carbon Res. 2019, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Anandan, C.; Mohan, L.; Babu, P.D. Electrochemical studies and growth of apatite on molybdenum doped DLC coatings on titanium alloy β-21S. Appl. Surf. Sci. 2014, 296, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Gnanavel, S.; Ponnusamy, S.; Mohan, L.; Radhika, R.; Muthamizhchelvan, C.; Ramasubramanian, K. Electrochemical behavior of biomedical titanium alloys coated with diamond carbon in Hanks’ solution. J. Mater. Eng. Perform. 2018, 27, 1635–1641. [Google Scholar] [CrossRef]
- Mohan, L.; Babu, P.D.; Kumar, P.; Anandan, C. Influence of zirconium doping on the growth of apatite and corrosion behavior of DLC-coated titanium alloy Ti-13Nb-13Zr. Surf. Interface Anal. 2013, 45, 1785–1791. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Grips, V.K.W. Corrosion behavior of titanium alloy beta-21S coated with diamond like carbon in Hank’s solution. Appl. Surf. Sci. 2012, 258, 6331–6340. [Google Scholar] [CrossRef]
- Gachon, Y.; Héau, C. Study of mechanical behavior of diamond-like carbon coatings by several instrumented tribometers. Thin Solid Films 2000, 377–378, 360–365. [Google Scholar] [CrossRef]
- Wang, D.; Chang, C.; Ho, W. Characterization of hydrogen-free diamond-like carbon flms deposited by pulsed plasma technology. Thin Solid Films 1999, 355–356, 246–251. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon: State of the art. Diam. Relat. Mater. 1999, 8, 428–434. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K. Biomedical applications of diamond-like carbon coatings: A Review. J. Biomed. Mater. Res. B 2007, 1, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.A.; Choudhury, D.; Nine, M.J.; Osman, N.A.A. Effects of surface coating on reducing friction and wear of orthopaedic implants. Sci. Technol. Adv. Mater. 2014, 15, 014402. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Sharma, A.; Sankar, J.; Narayan, J. Mechanical properties of diamond-like carbon composite thin films prepared by pulsed laser deposition. Compos. B Eng. 1999, 30, 675–684. [Google Scholar] [CrossRef]
- Aizawa, T.; Wasa, K.; Nogami, Y. Plasma oxidation printing into DLC and graphite for surface functionalization. J. Carbon Res. 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Racine, B.; Benlahsen, M.; Zellama, K.; Goudeau, P.; Zarrabian, M.; Turban, G. Effect of the hydrogen on the intrinsic stress in hydrogenated amorphous carbon films deposited from an electron cyclotron resonance plasma. Appl. Phys. Lett. 1998, 73, 3226–3228. [Google Scholar] [CrossRef]
- Chang, L.C.; Wang, Y.D. Microstructure and adhesion characteristics of diamond-like carbon films deposited on steel substrates. Diam. Relat. Mater. 2001, 10, 1528–1534. [Google Scholar] [CrossRef]
- Choy, K.; Felix, E. Functionally graded diamond-like carbon coatings on metallic substrates. Mater. Sci. Eng. A 2000, 278, 162–169. [Google Scholar] [CrossRef]
- Khamseh, S.; Alibakhshi, E.; Mahdavian, M.; Saeb, M.R.; Vahabi, H.; Kokanyan, N.; Laheurte, P. Magnetron-sputtered copper/diamond-like carbon composite thin films with super anti-corrosion properties. Surf. Coat. Technol. 2018, 333, 148–157. [Google Scholar] [CrossRef]
- Jing, P.P.; Gong, Y.L.; Deng, Q.Y.; Zhang, Y.Z.; Huang, N.; Leng, Y.X. The formation of the “rod-like wear debris” and tribological properties of Ag-doped diamond-like carbon films fabricated by a high-power pulsed plasma vapor deposition technique. Vacuum 2020, 173, 109125. [Google Scholar] [CrossRef]
- Ray, S.C.; Pong, W.F.; Papakonstantinou, P. Iron, nitrogen and silicon doped diamond like carbon (DLC) thin films: A comparative study. Thin Solid Films 2016, 610, 42–47. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Chen, Y.; Zhang, G.; Liu, W.; Zhang, J. The effect of applied negative bias voltage on the structure of Ti-doped a-C:H films deposited by FCVA. Appl. Surf. Sci. 2007, 253, 3722–3726. [Google Scholar] [CrossRef]
- Singh, V.; Palshin, V.; Tittsworth, R.C.; Meletis, E.I. Local structure of composite Cr-containing diamond-like carbon thin films. Carbon 2006, 44, 1280–1286. [Google Scholar] [CrossRef]
- Podgornik, B.; Hren, D.; Vižintin, J. Low-friction behaviour of boundary-lubricated diamond-like carbon coatings containing tungsten. Thin Solid Films 2005, 476, 92–100. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Z.; Fu, Z.; Wu, S.; Yue, W.; Wang, C. Microstructural, mechanical and tribological properties of tungsten-gradually doped diamond-like carbon films with functionally graded interlayers. Surf. Coat. Technol. 2011, 205, 3631–3638. [Google Scholar] [CrossRef]
- Fu, R.K.Y.; Mei, Y.F.; Fu, M.Y.; Liu, X.Y.; Chu, P.K. Thermal stability of metal-doped diamond-like carbon fabricated by dual plasma deposition. Diam. Relat. Mater. 2005, 14, 1489–1493. [Google Scholar] [CrossRef]
- Wei, Q.; Narayan, R.J.; Narayan, J.; Sankar, J.; Sharma, A.K. Improvement of wear resistance of pulsed laser deposited diamond-like carbon films through incorporation of metals. Mat. Sci. Eng. B 1998, 53, 262–266. [Google Scholar] [CrossRef]
- Khan, M.I.; Rasheed, I.; Naeem, M.A.; Majeed, S.; Mustafa, G.M.; Fatima, M.; Mahmood, A.; Al-Masry, W.; Iqbal, M.; Kattan, N.A.; et al. Structural, electrical and mechanical behavior evaluation of palladium doped diamond like carbon thin films. J. Mater. Res. Technol. 2020, 9, 8289–8295. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, T.; Qian, X.; Zhu, Y.; Liu, X. Mechanical properties and biocompatibility of Ti-doped diamond-like carbon films. ACS Omega 2020, 5, 22772–22777. [Google Scholar] [CrossRef]
- Chaus, A.S.; Fedosenko, T.N.; Rogachev, A.V.; Čaplovič, Ľ. Surface, microstructure and optical properties of copper-doped diamond-like carbon coating deposited in pulsed cathodic arc plasma. Diam. Relat. Mater. 2014, 42, 64–70. [Google Scholar] [CrossRef]
- Santana, J.A.C.; Skomski, R.; Singh, V.; Palshin, V.; Petukhov, A.; Losovyj, Y.B.; Sokolov, A.; Dowben, P.A.; Ketsman, I. Magnetism of Cr-doped diamond-like carbon. J. Appl. Phys. 2009, 105, 07A930. [Google Scholar] [CrossRef] [Green Version]
- Gnanavel, S.; Ponnusamy, S.; Mohan, L. Biocompatible response of hydroxyapatite coated on near-β titanium alloys by E-beam evaporation method. Biocatal. Agric. Biotechnol. 2018, 15, 364–369. [Google Scholar] [CrossRef]
- Gnanavel, S.; Ponnusamy, S.; Mohan, L.; Muthamizhchelvan, C. In vitro corrosion behaviour of Ti–6Al–4V and 316L stainless steel alloys for biomedical implant applications. J. Bio Tribo Corros. 2018, 4, 1. [Google Scholar] [CrossRef]
- Mohan, L.; Chakraborty, M.; Viswanathan, S.; Mandal, C.; Bera, P.; Aruna, S.T.; Anandan, C. Corrosion, wear, and cell culture studies of oxygen ion implanted Ni-Ti alloy. Surf. Interface Anal. 2017, 49, 828–836. [Google Scholar] [CrossRef]
- Anandan, C.; Mohan, L. Effect of postnitride annealing on wear and corrosion behavior of titanium alloy Ti-6Al-4V. J. Mater. Eng. Perform. 2016, 25, 4416–4424. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C. Wear and corrosion behavior of oxygen implanted biomedical titanium alloy Ti-13Nb-13Zr. Appl. Surf. Sci. 2013, 282, 281–290. [Google Scholar] [CrossRef]
- Mohan, L.; Durgalakshmi, D.; Geetha, M.; Narayanan, T.S.N.S.; Asokamani, R. Electrophoretic deposition of nanocomposite (HAp + TiO2) on titanium alloy for biomedical applications. Ceram. Int. 2012, 38, 3435–3443. [Google Scholar] [CrossRef]
- Anandan, C.; Babu, P.D.; Mohan, L. Effect of gas composition on nitriding and wear behavior of nitrided titanium alloy Ti-15V-3Cr-3Al-3Sn. J. Mater. Eng. Perform. 2013, 22, 2623–2633. [Google Scholar] [CrossRef]
- Mohan, L.; Dennis, C.; Padmapriya, N.; Anandan, C.; Rajendran, N. Effect of electrolyte temperature and anodization time on formation of TiO2 nanotubes for biomedical applications. Mater. Today Commun. 2020, 23, 101103. [Google Scholar] [CrossRef]
- Niinomi, M.; Kuroda, D.; Fukunaga, K.; Morinaga, M.; Kato, Y.; Yashiro, T.; Suzuki, A. Corrosion wear fracture of new β type biomedical titanium alloys. Mater. Sci. Eng. A 1999, 263, 193–199. [Google Scholar] [CrossRef]
- Mohan, L.; Raja, M.D.; Uma, T.S.; Rajendran, N.; Anandan, C. In-vitro biocompatibility studies of plasma-nitrided titanium alloy β-21S using fibroblast cells. J. Mater. Eng. Perform. 2016, 25, 1508–1514. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Aleixo, G.; Caram, R.; Guastaldi, A.C. Development of Ti–Mo alloys for biomedical applications: Microstructure and electrochemical characterization. Mater. Sci. Eng. A 2007, 452–453, 727–731. [Google Scholar] [CrossRef]
- Sung, B.-S.; Park, T.-E.; Yun, Y.-H. Microstructures and electrochemical behavior of Ti-Mo alloys for biomaterials. Adv. Mater. Sci. Eng. 2015, 2015, 872730. [Google Scholar] [CrossRef] [Green Version]
- Mohan, L.; Anandan, C. Effect of gas composition on corrosion behavior and growth of apatite on plasma nitrided titanium alloy beta-21S. Appl. Surf. Sci. 2013, 268, 288–296. [Google Scholar] [CrossRef]
- Anandan, C.; Mohan, L. In vitro corrosion behavior and apatite growth of oxygen plasma ion implanted titanium alloy β-21S. J. Mater. Eng. Perform. 2013, 22, 3507–3516. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Grips, V.K.W. Investigation of electrochemical behavior of nitrogen implanted Ti-15Mo-3Nb-3Al alloy in Hank’s solution. J. Mater. Sci. Mater. Med. 2013, 24, 623–633. [Google Scholar] [CrossRef]
- Luo, Y.; Ge, S.; Jin, Z.; Fisher, J. Effect of surface modification on surface properties and tribological behaviours of titanium alloys. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 311–316. [Google Scholar] [CrossRef]
- Uwais, Z.A.; Hussein, M.A.; Samad, M.A.; Al-Aqeeli, N. Surface modification of metallic biomaterials for better tribological properties: A review. Arab. J. Sci. Eng. 2017, 42, 4493–4512. [Google Scholar] [CrossRef]
- Tallant, D.R.; Parmeter, J.E.; Siegal, M.P.; Simpson, R.L. The thermal stability of diamond-like carbon. Diam. Relat. Mater. 1995, 4, 191–199. [Google Scholar] [CrossRef]
- Ji, L.; Li, H.; Zhao, F.; Chen, J.; Zhou, H. Microstructure and mechanical properties of Mo/DLC nanocomposite films. Diam. Relat. Mater. 2008, 17, 1949–1954. [Google Scholar] [CrossRef]
- Rusli, H.; Yoon, S.F.; Huang, Q.F.; Ahn, J.; Zhang, Q.; Yang, H.; Wu, Y.S.; Teo, E.J.; Osipowicz, T.; Watt, F. Metal-containing amorphous carbon film development using electron cyclotron resonance CVD. Diam. Relat. Mater. 2001, 10, 132–138. [Google Scholar] [CrossRef]
- Gwo, J.; Chu, C.L.; Tsai, M.J.; Lee, S. Enhancement of Ti-containing hydrogenated carbon (TiC:H) films by high-power plasma-sputtering. Appl. Surf. Sci. 2012, 258, 3433–3437. [Google Scholar] [CrossRef]
- Kumar, P.; Babu, P.D.; Mohan, L.; Anandan, C.; Grips, V.K.W. Wear and corrosion behavior of Zr-Doped DLC on Ti-13Zr-13Nb biomedical alloy. J. Mater. Eng. Perform. 2013, 22, 283–293. [Google Scholar] [CrossRef]
- Panda, M.; Krishnan, R.; Krishna, N.G.; Amirthapandian, S.; Magudapathy, P.; Kamruddin, M. Tuning the tribological property of PLD deposited DLC-Au nanocomposite thin films. Cearm. Int. 2019, 45, 8847–8855. [Google Scholar] [CrossRef]
- Qi, F.; Leng, Y.X.; Sun, H.; Huang, N. Mechanical properties of DLC/Ti-O bilayer films. IEEE Trans. Plasma Sci. 2009, 37, 1136–1139. [Google Scholar] [CrossRef]
- Küttel, O.M.; Martinu, L.; Poitras, D.; Klemberg-Sapieha, J.E.; Wertheimer, M.R. Diamond-like carbon films deposited in a dual microwave-radio-frequency plasma. Mater. Sci. Eng. B 1992, 11, 321–324. [Google Scholar] [CrossRef]
- Mazare, A.; Anghel, A.; Surdu-Bob, C.; Totea, G.; Demetrescu, I.; Ionita, D. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Films 2018, 657, 16–23. [Google Scholar] [CrossRef]
- Swiatek, L.; Olejnik, A.; Grabarczyk, J.; Jedrzejczak, A.; Sobczyk-Guzenda, A.; Kaminska, M.; Jakubowski, W.; Szymanski, W.; Bociaga, D. Multi-doped diamond like-carbon coatings (DLC-Si/Ag) for biomedical applications fabricated using the modified chemical vapour deposition method. Diam. Relat. Mater. 2016, 67, 54–62. [Google Scholar] [CrossRef]
- Bai, W.Q.; Li, L.L.; Xie, Y.J.; Liu, D.G.; Wang, X.L.; Jin, G.; Tu, J.P. Corrosion and tribocorrosion performance of M (M = Ta, Ti) doped amorphous carbon multilayers in Hank’s solution. Surf. Coat. Technol. 2016, 305, 11–22. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Guo, C.; Liu, W.; Yang, Z.; Sun, X.; Zhang, Z.; Jiang, F. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Tang, X.S.; Wang, H.J.; Feng, L.; Shao, L.X.; Zou, C.W. Mo doped DLC nanocomposite coatings with improved mechanical and blood compatibility properties. Appl. Surf. Sci. 2014, 311, 758–762. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Flores-Sahagun, T.H.S.; Paredes, R.C. A perspective on molybdenum biocompatibility and antimicrobial activity for applications in implants. J. Mater. Sci. 2016, 51, 2806–2816. [Google Scholar] [CrossRef]
- Fu, R.K.Y.; Mei, Y.; Shen, L.; Siu, G.; Chu, P.K.; Cheung, W.; Wong, S. Molybdenum–carbon film fabricated using metal cathodic arc and acetylene dual plasma deposition. Surf. Coat. Technol. 2004, 186, 112–117. [Google Scholar] [CrossRef]
- Byon, E.; Kim, J.-K.; Rha, J.-J.; Kwon, S.-C.; Mu, Z.; Liu, C.; Li, G. Effect of metal ion implantation on thermal instability of diamond-like carbon films. Surf. Coat. Technol. 2007, 201, 6670–6673. [Google Scholar] [CrossRef]
- Kolawole, F.O.; Ramirez, M.A.; Kolawole, S.K.; Varela, L.B.; Tschiptschin, A.P. Deposition and characterization of molybdenum oxide (MoO3) nanoparticles incorporated diamond-like carbon coatings using pulsed-DC PECVD. Mater. Lett. 2020, 278, 128420. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, R.Y.; Yan, S.J.; Zhang, R.; Yang, B.; Zhang, Z.D.; Huang, Z.H.; Fu, D.J. Structure and properties of Mo containing diamond-like carbon films produced by ion source assisted cathodic arc ion-plating. Appl. Surf. Sci. 2013, 286, 109–114. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Mérel, P.; Tabbal, M.; Chaker, M.; Moisa, S.; Margot, J. Direct evaluation of the sp3 content in diamond-like-carbon films by XPS. Appl. Surf. Sci. 1998, 136, 105–110. [Google Scholar] [CrossRef]
- Haerle, R.; Riedo, E.; Pasquarello, A.; Baldereschi, A. sp2/sp3 hybridization ratio in amorphous carbon from C 1s core-level shifts: X-ray photoelectron spectroscopy and first-principles calculation. Phys. Rev. B 2001, 65, 045101. [Google Scholar] [CrossRef]
- Zhang, L.; Ball, M.R.; Liu, Y.; Kuech, T.F.; Huber, G.W.; Mavrikakis, M.; Hermans, I.; Dumesic, J.A. Synthesis gas conversion over Rh/Mo catalysts prepared by atomic layer deposition. ACS Catal. 2019, 9, 1810–1819. [Google Scholar] [CrossRef]
- Alhajri, N.S.; Anjum, D.H.; Takanabe, K. Molybdenum carbide–carbon nanocomposites synthesized from a reactive template for electrochemical hydrogen evolution. J. Mater. Chem. A 2014, 2, 10548–10556. [Google Scholar] [CrossRef]
- Tai, F.C.; Lee, S.C.; Chen, J.; Wei, C.; Chang, S.H. Multipeak fitting analysis of Raman spectra on DLCH film. J. Raman Spectrosc. 2009, 40, 1055–1059. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Irmer, G.; Dorner-Reisel, A. Mirco-Raman studies on DLC coatings. Adv. Eng. Mater. 2005, 7, 694–705. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Phil. Trans. R. Soc. Lond. A 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Origin of the 1150−cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 2001, 63, 121405. [Google Scholar] [CrossRef]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Veirs, D.K.; Ager, J.W.; Rubin, M.D.; Hopper, C.B.; Bogy, D.B. Effects of substrate temperature on chemical structure of amorphous carbon films. J. Appl. Phys. 1992, 71, 2243–2248. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, S.; Kumar, N.; Krishnan, R.; Ravindran, T.R.; Amirthapandian, S.; Dash, S.; Tyagi, A.K.; Sridharan, M. Influence of transition metal doping on the tribological properties of pulsed laser deposited DLC films. Ceram. Int. 2015, 41, 1797–1805. [Google Scholar] [CrossRef]
- Khun, N.W.; Liu, E.; Yang, G.C.; Ma, W.G.; Jiang, S.P. Structure and corrosion behavior of platinum/ruthenium/nitrogen doped diamondlike carbon thin films. J. Appl. Phys. 2009, 106, 013506. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Wu, G.; Wang, A. Preparation, characterization and properties of Cr-incorporated DLC films on magnesium alloy. Diam. Relat. Mater. 2010, 19, 1307–1315. [Google Scholar] [CrossRef]
- Veres, M.; Tóth, S.; Koós, M. Grain boundary fine structure of ultrananocrystalline diamond thin films measured by Raman scattering. Appl. Phys. Lett. 2007, 91, 031913. [Google Scholar] [CrossRef]
- Voevodin, A.A.; O’Neill, J.P.; Zabinski, J.S. Tribological performance and tribochemistry of nanocrystalline WC/amorphous diamond-like carbon composites. Thin Solid Films 1999, 342, 194–200. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Ji, L.; Wang, Y.; Liu, X.; Zhou, H.; Chen, J. Effect of microstructural evolution on mechanical and tribological properties of Ti-doped DLC films: How was an ultralow friction obtained? J. Vac. Sci. Technol. A 2016, 34, 031504. [Google Scholar] [CrossRef]
- Vitu, T.; Escudeiro, A.; Polcar, T.; Cavaleiro, A. Sliding properties of Zr-DLC coatings: The effect of tribolayer formation. Surf. Coat. Technol. 2014, 258, 734–745. [Google Scholar] [CrossRef]
- Müller, I.C.; Sharp, J.; Rainforth, W.M.; Hovsepian, P.; Ehiasarian, A. Tribological response and characterization of Mo–W doped DLC coating. Wear 2017, 376–377, 1622–1629. [Google Scholar] [CrossRef]

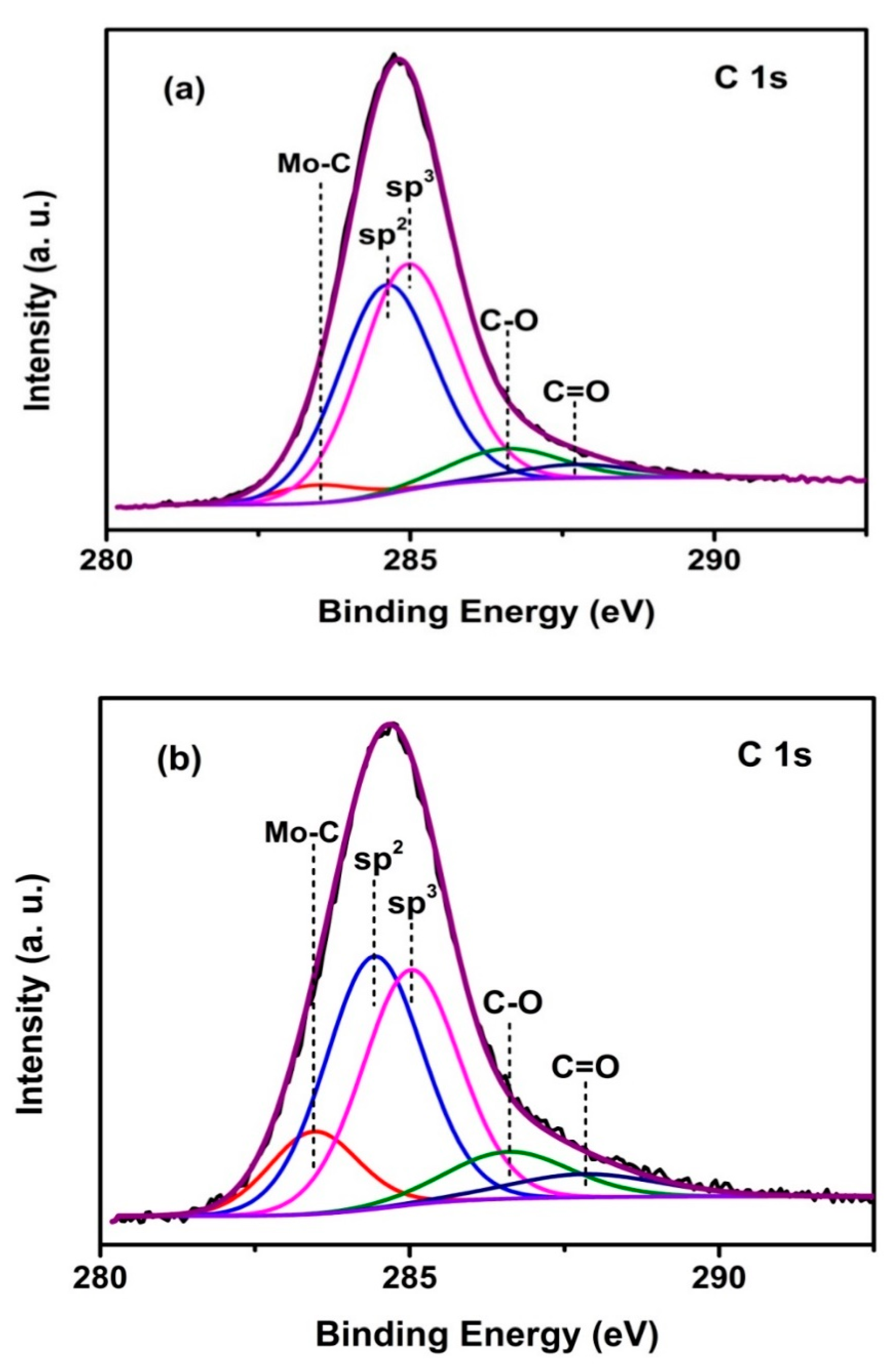
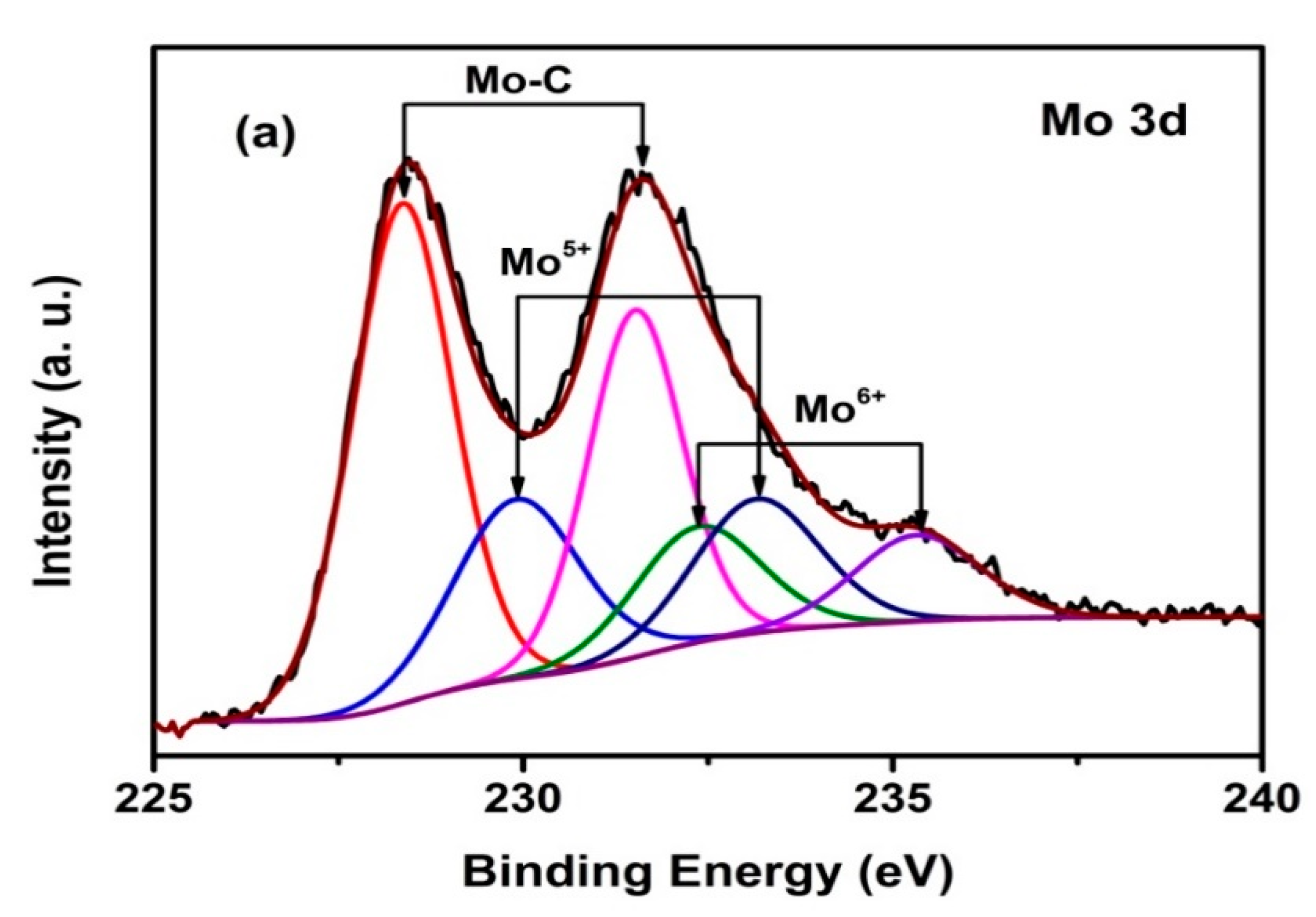
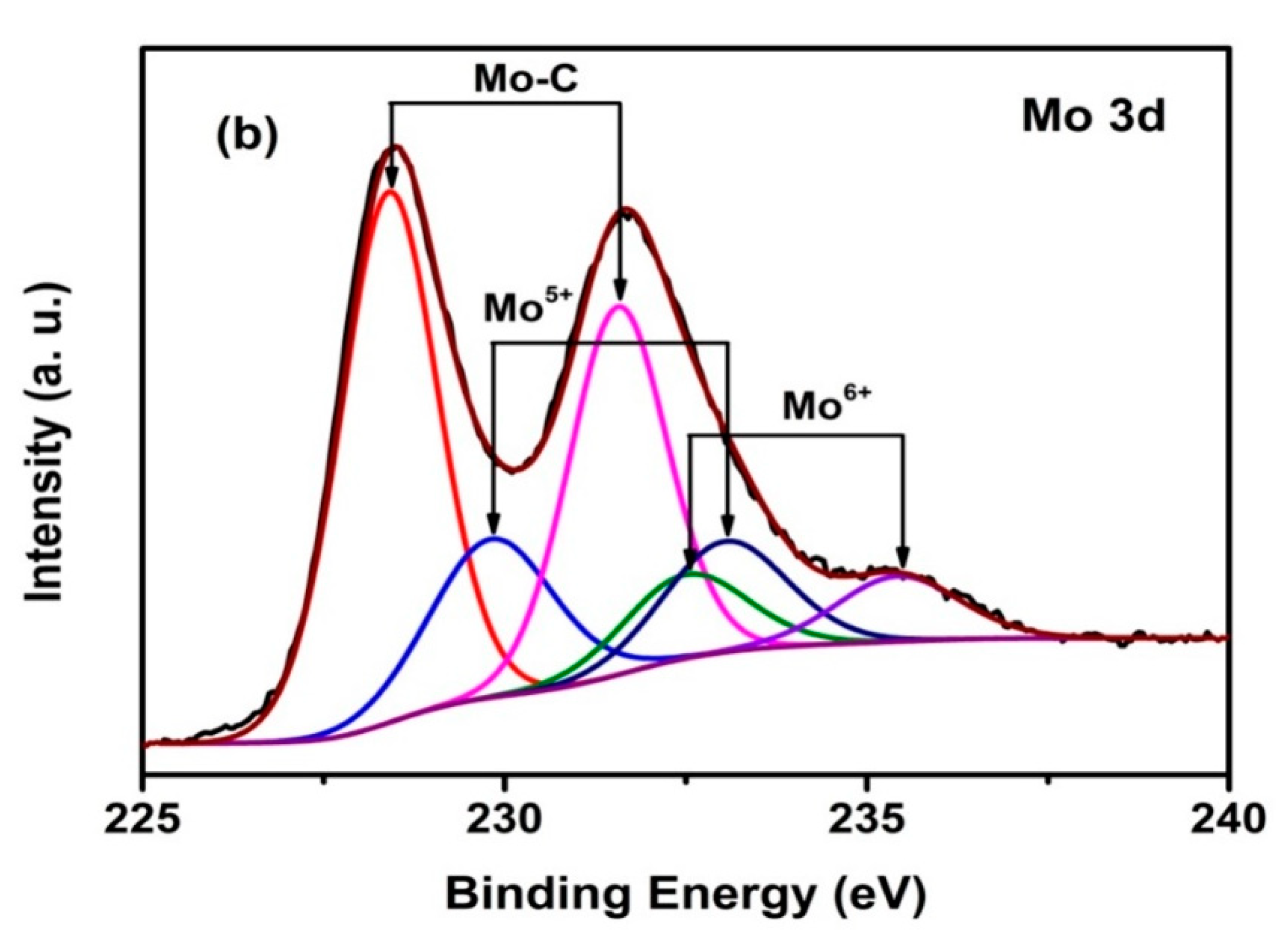
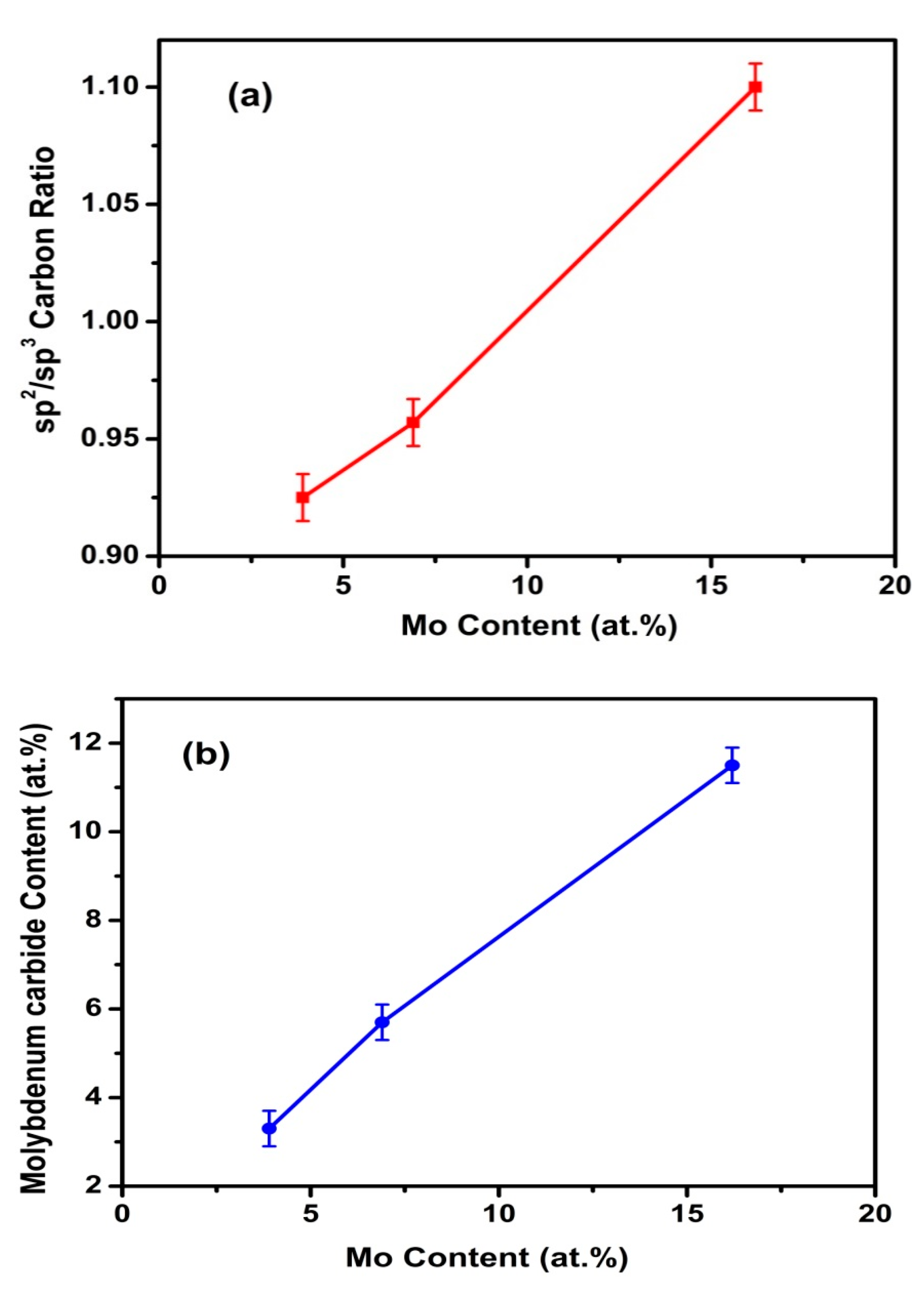







| Duty Cycle (%) | Coating | C Species | Binding Energy of C 1s (eV) | Relative Peak Area (%) | Mo Species | Binding Energy of Mo 3d5/2 (eV) | Relative Peak Area (%) |
|---|---|---|---|---|---|---|---|
| 10 | 3.9 at.% Mo-DLC | Mo–C | 283.4 | 3.3 | Mo–C | 228.4 | 56.2 |
| sp2 | 284.6 | 41.5 | Mo4+ | 229.9 | 26.7 | ||
| sp3 | 285 | 44.7 | Mo6+ | 232.4 | 17.1 | ||
| C–O | 286.6 | 7.2 | |||||
| C=O | 287.7 | 3.3 | |||||
| 40 | 6.9 at.% Mo-DLC | Mo–C | 283.4 | 5.7 | Mo–C | 228.4 | 63.3 |
| sp2 | 284.5 | 40.8 | Mo4+ | 229.8 | 21.5 | ||
| sp3 | 285 | 42.5 | Mo6+ | 232.4 | 15.2 | ||
| C–O | 286.7 | 7.5 | |||||
| C=O | 287.8 | 3.5 | |||||
| 85 | 16.2 at.% Mo-DLC | Mo–C | 283.5 | 11.5 | Mo–C | 228.4 | 63.5 |
| sp2 | 284.4 | 38.3 | Mo4+ | 229.8 | 23.3 | ||
| sp3 | 285 | 35.6 | Mo6+ | 232.4 | 13.2 | ||
| C–O | 286.6 | 9.1 | |||||
| C=O | 287.8 | 5.5 |
| Sample | Coefficient of Friction | |
|---|---|---|
| 3 N | 5 N | |
| β-21S Ti-Mo | 0.62 | 0.43 |
| Mo-DLC (30 min) | 0.2 | 0.17 |
| Mo-DLC (60 min) | 0.17 | 0.15 |
| Sample | Wear Loss (mm3) | |
|---|---|---|
| 3 N | 5 N | |
| β-21S Ti-Mo | 0.00475 | 0.015 |
| Mo-DLC (30 min) | 0.0025 | 0.0095 |
| Mo-DLC (60 min) | Negligible | 0.00008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padmanaban, D.B.; Mohan, L.; Giri, P.; Bera, P.; Anandan, C.; Barshilia, H.C. Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy. C 2021, 7, 1. https://doi.org/10.3390/c7010001
Padmanaban DB, Mohan L, Giri P, Bera P, Anandan C, Barshilia HC. Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy. C. 2021; 7(1):1. https://doi.org/10.3390/c7010001
Chicago/Turabian StylePadmanaban, Dilli Babu, Loganathan Mohan, Preetam Giri, Parthasarathi Bera, Chinnasamy Anandan, and Harish C. Barshilia. 2021. "Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy" C 7, no. 1: 1. https://doi.org/10.3390/c7010001
APA StylePadmanaban, D. B., Mohan, L., Giri, P., Bera, P., Anandan, C., & Barshilia, H. C. (2021). Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy. C, 7(1), 1. https://doi.org/10.3390/c7010001