Improving the Performance of Printable Carbon Electrodes by Femtosecond Laser Treatment
Abstract
1. Introduction
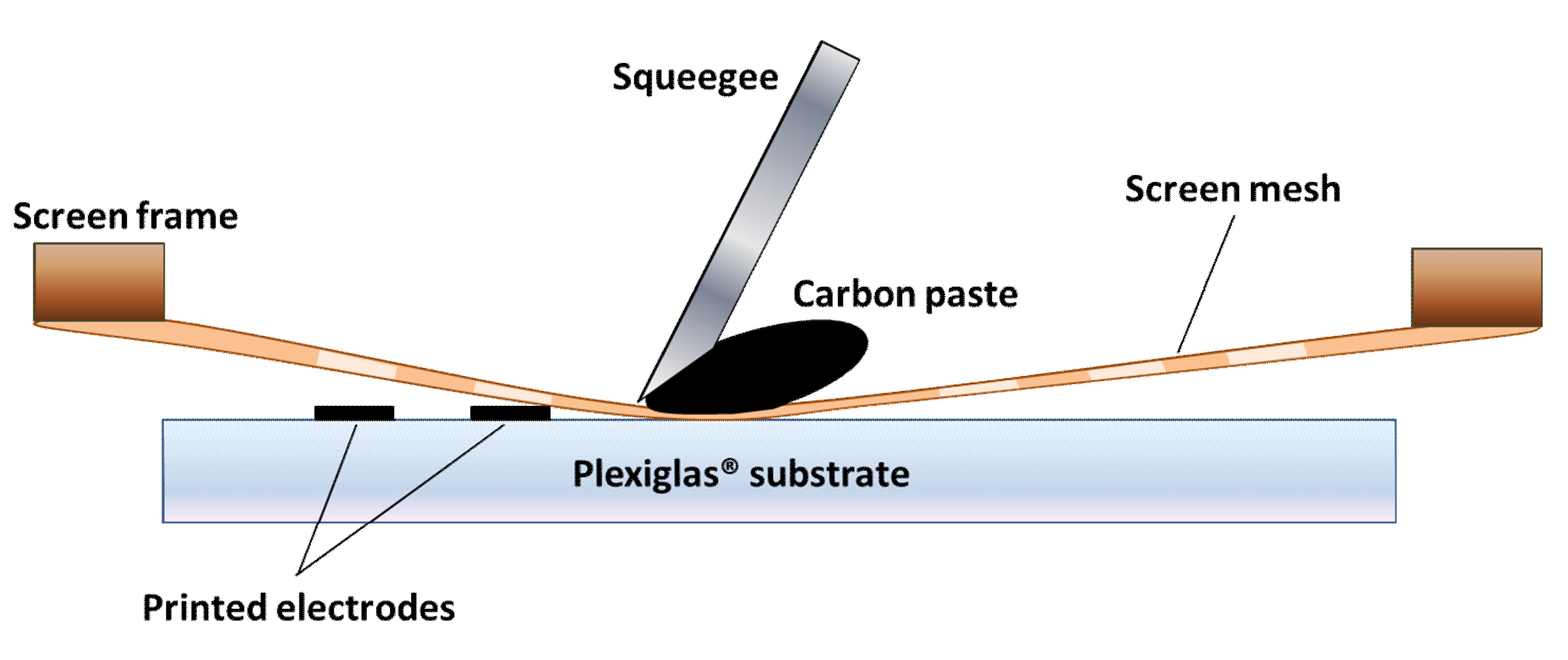
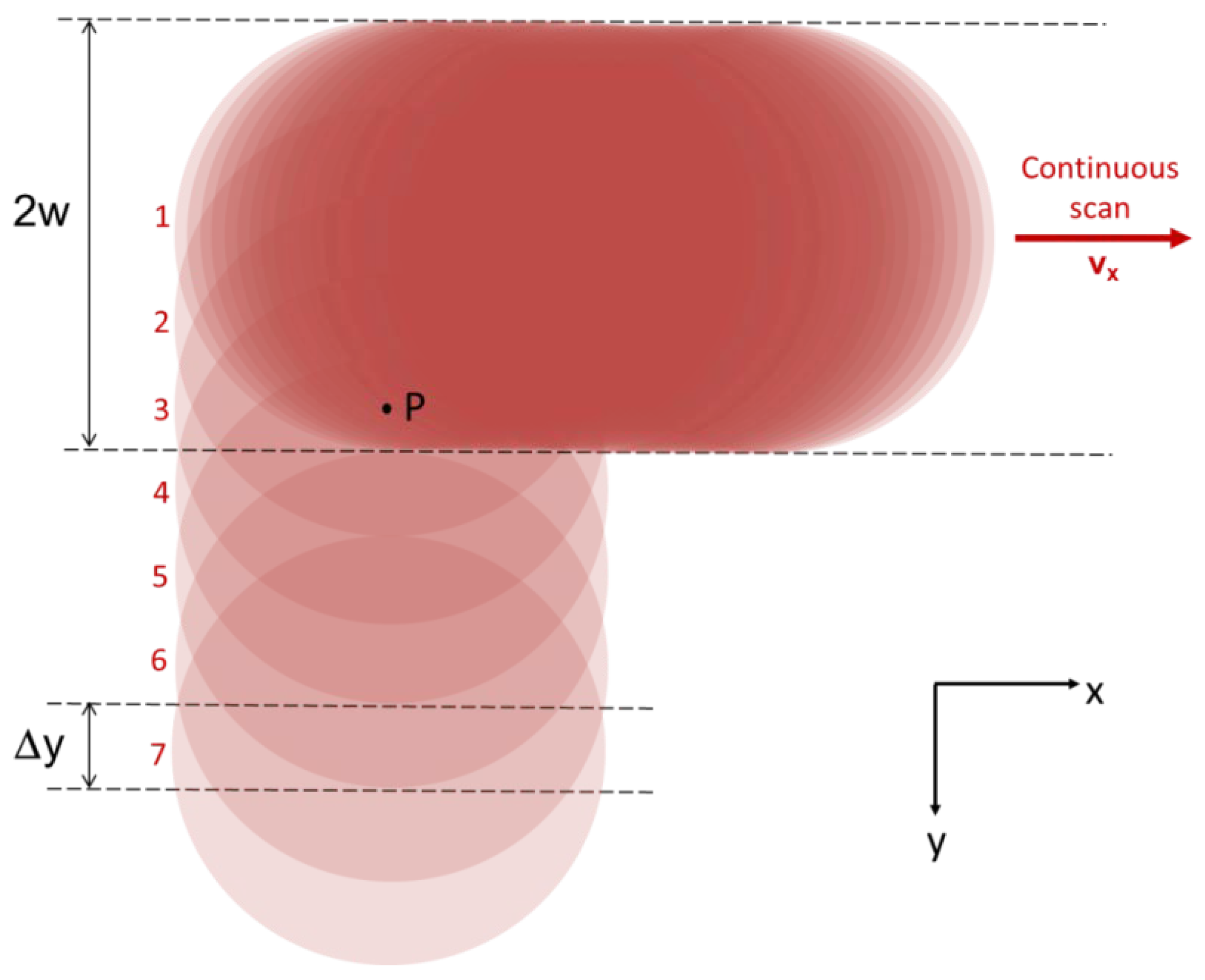
2. Materials and Methods
3. Results and Discussion
3.1. Visual Inspection
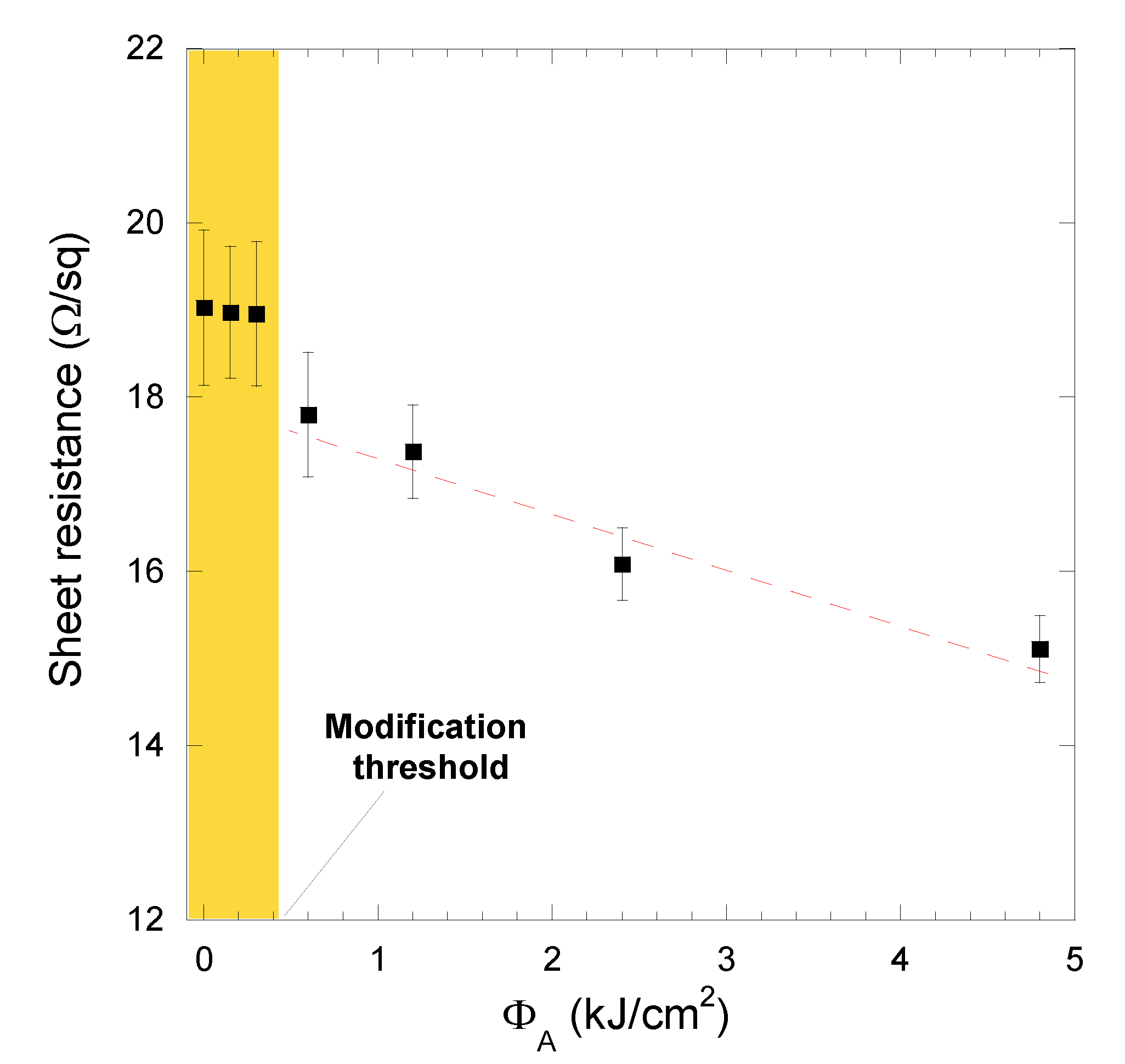
3.2. Four-Point Probe Resistivity Measurements
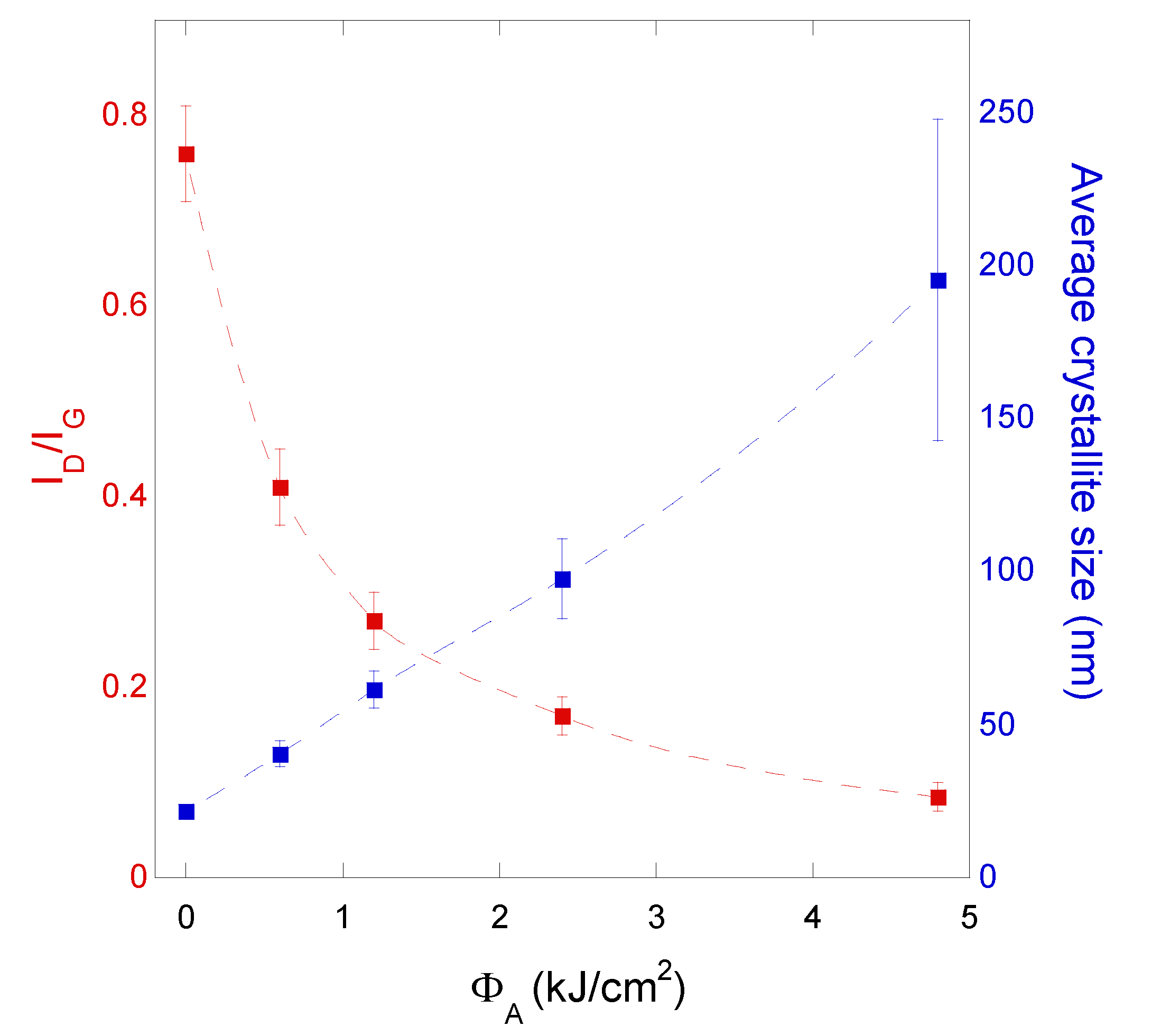
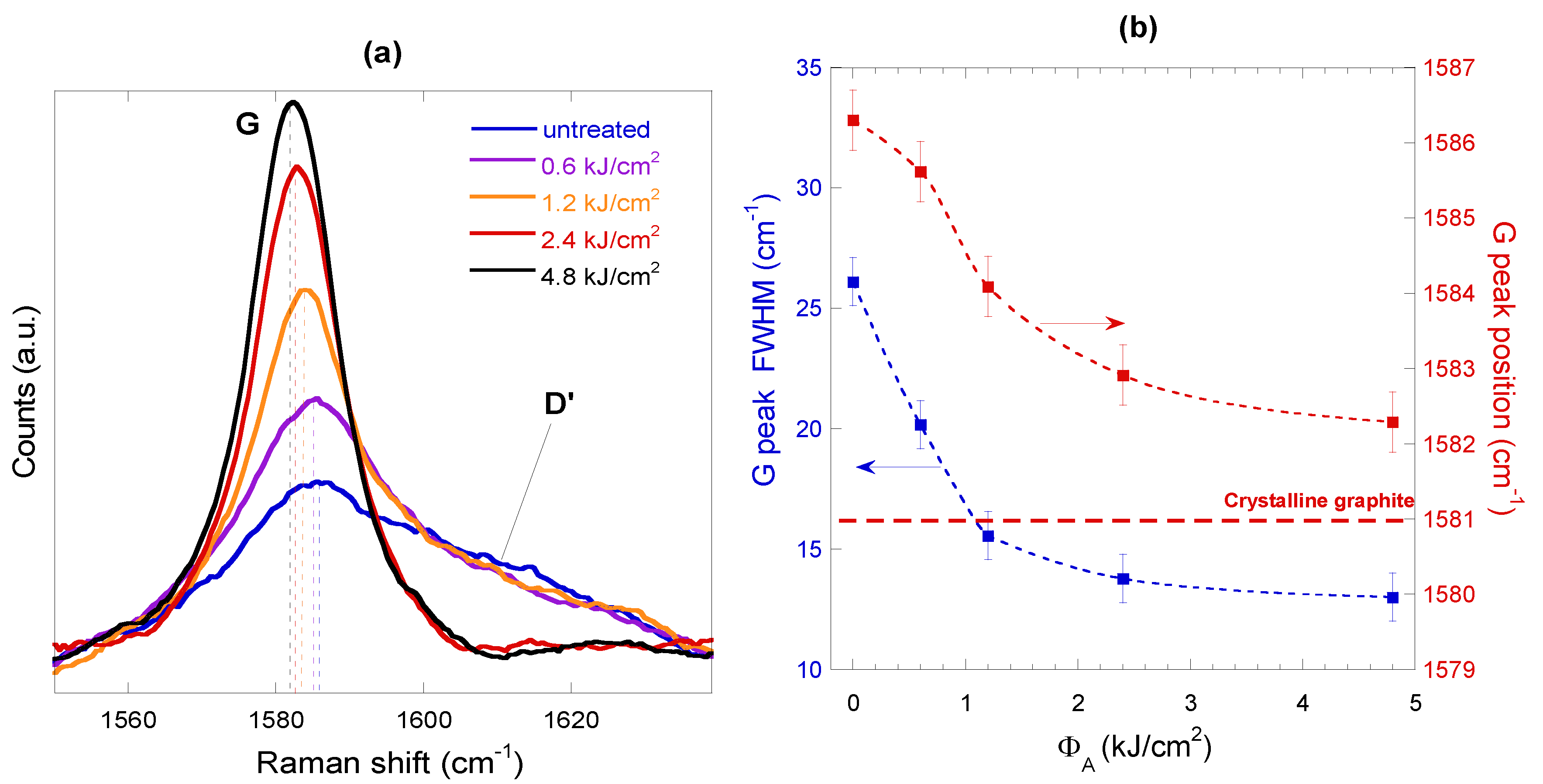
3.3. Raman Characterization
- 1.
- G band is due to the longitudinal optical (LO) and transverse optical (TO) modes of the graphite honeycomb lattice vibration, which degenerate at the center (i.e., the Γ point) of the Brillouin zone, and is characteristic of sp2-hybridized C-C bonds; therefore, it is a fingerprint of graphitic materials.
- 2.
- D and 2D bands are related to double resonance processes. In both cases, an electron–hole pair, generated by the incident laser light, recombines after two electron-phonon scattering events involving phonons with opposite momenta. When a defect is present, one of the scattering events occurs elastically, leading to the D band; conversely, if both the events occur inelastically, the 2D band is generated. That is the reason why D band is the most significant Raman feature related to the amount of disorder in a carbon-based material, whereas 2D band (sometimes indicated in the literature as G’ band) is Raman-active also in a defect-free material.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhu, X.; Yang, Z.; Zhang, X.; Niu, J.; Wang, Z.; Zuo, S.; Priya, S.; Liu, S.; Yang, D. Record Efficiency Stable Flexible Perovskite Solar Cell Using Effective Additive Assistant Strategy. Adv. Mater. 2018, 30, 1801418. [Google Scholar] [CrossRef]
- Chapa, M.; Alexandre, M.F.; Mendes, M.J.; Águas, H.; Fortunato, E.; Martins, R. All-Thin-Film Perovskite/C–Si Four-Terminal Tandems: Interlayer and Intermediate Contacts Optimization. ACS Appl. Energy Mater. 2019, 2, 3979–3985. [Google Scholar] [CrossRef]
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Di Carlo, A.; Ducati, C. In situ observation of heat-induced degradation of perovskite solar cells. Nat. Energy 2016, 1, 15012. [Google Scholar] [CrossRef]
- O’Keeffe, P.; Catone, D.; Paladini, A.; Toschi, F.; Turchini, S.; Avaldi, L.; Martelli, F.; Agresti, A.; Pescetelli, S.; Del Rio Castillo, A.E.; et al. Graphene-Induced Improvements of Perovskite Solar Cell Stability: Effects on Hot-Carriers. Nano Lett. 2019, 19, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Edvinsson, T. How to Make a Most Stable Perovskite Solar Cell. Matter 2019, 1, 562–564. [Google Scholar] [CrossRef]
- Domanski, K.; Correa-Baena, J.P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Grätzel, M. Not all that glitters is gold: Metal-migration-induced degradation in perovskite solar cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef]
- Besleaga, C.; Abramiuc, L.E.; Stancu, V.; Tomulescu, A.G.; Sima, M.; Trinca, L.; Plugaru, N.; Pintilie, L.; Nemnes, G.A.; Iliescu, M.; et al. Iodine migration and degradation of perovskite solar cells enhanced by metallic electrodes. J. Phys. Chem. Lett. 2016, 7, 5168–5175. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, L.; Gao, P. Promise of Commercialization: Carbon Materials for Low-Cost Perovskite Solar Cells. Chin. Phys. B 2018, 27, 018805. [Google Scholar] [CrossRef]
- Jiang, P.; Jones, T.W.; Duffy, N.W.; Anderson, K.F.; Bennett, R.; Grigore, M.; Marvig, P.; Xiong, Y.; Liu, T.; Sheng, Y.; et al. Fully printable perovskite solar cells with highly-conductive, low-temperature, perovskite-compatible carbon electrode. Carbon 2018, 129, 830–836. [Google Scholar] [CrossRef]
- Liang, L.; Cai, Y.; Li, X.; Nazeeruddin, M.K.; Gao, P. All that glitters is not gold: Recent progress of alternative counter electrodes for perovskite solar cells. Nano Energy 2018, 52, 211–238. [Google Scholar] [CrossRef]
- Georgakilas, V.; Koutsioukis, A.; Petr, M.; Tucek, J.; Zboril, R. Remarkable enhancement of the electrical conductivity of carbon nanostructured thin films after compression. Nanoscale 2016, 8, 11413–11417. [Google Scholar] [CrossRef] [PubMed]
- Kumanek, B.; Wasiak, K.; Stando, G.; Stando, P.; Łukowiec, D.; Janas, D. Simple Method to Improve Electrical Conductivity of Films Made from Single-Walled Carbon Nanotubes. Nanomaterials 2019, 9, 1113. [Google Scholar] [CrossRef]
- Jang, W.S.; Chae, S.S.; Lee, S.J.; Song, K.M.; Baik, H.K. Improved electrical conductivity of a non-covalently dispersed graphene–carbon nanotube film by chemical p-type doping. Carbon 2012, 50, 943–951. [Google Scholar] [CrossRef]
- Im, J.S.; Kim., J.G.; Lee, Y.S. Fluorination effects of carbon black additives for electrical properties and EMI shielding efficiency by improved dispersion and adhesion. Carbon 2009, 47, 2640–2647. [Google Scholar] [CrossRef]
- Kohlmeyer, R.R.; Lor, M.; Deng, J.; Liu, H.; Chen, J. Preparation of stable carbon nanotube aerogels with high electrical conductivity and porosity. Carbon 2011, 49, 2352–2361. [Google Scholar] [CrossRef]
- Savi, P.; Yasir, M.; Bartoli, M.; Giorcelli, M.; Longo, M. Electrical and Microwave Characterization of Thermal Annealed Sewage Sludge Derived Biochar Composites. Appl. Sci. 2020, 10, 1334. [Google Scholar] [CrossRef]
- Wu, J.; Agrawal, M.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic Light-Emitting Diodes on Solution-Processed Graphene Transparent Electrodes. ACS Nano 2010, 4, 43–48. [Google Scholar] [CrossRef]
- Lim, Y.; Chu, J.W.; Lee, D.H.; Kwon, S.H.; Shin, H. Increase in graphitization and electrical conductivity of glassy carbon nanowires by rapid thermal annealing. J. Alloys Compd. 2017, 702, 465–471. [Google Scholar] [CrossRef]
- Mishra, A.; Ahmad, Z.; Zimmermann, I.; Martineau, D.; Shakoor, R.A.; Touati, F.; Riaz, K.; Al-Muhtaseb, S.A.; Nazeeruddin, M.K. Effect of annealing temperature on the performance of printable carbon electrodes for perovskite solar cells. Org. Electron. 2019, 65, 375–380. [Google Scholar] [CrossRef]
- Bonse, J.; Hertwig, A.; Koter, R.; Weise, M.; Beck, U.; Reinstädt, P.; Griepentrog, M.; Krüger, J.; Picquart, M.; Haro-Poniatowski, E. Femtosecond laser pulse irradiation effects on thin hydrogenated amorphous carbon layers. Appl. Phys. A Mater. Sci. Process. 2013, 112, 9–14. [Google Scholar] [CrossRef]
- Kononenko, T.V.; Kononenko, V.V.; Pimenov, S.M.; Zavedeev, E.V.; Konov, V.I.; Romano, V.; Dumitru, G. Effects of pulse duration in laser processing of diamond-like carbon films. Diamond Relat. Mater 2005, 14, 1368–1376. [Google Scholar] [CrossRef]
- Girolami, M.; Bellucci, A.; Calvani, P.; Orlando, S.; Valentini, V.; Trucchi, D.M. Raman investigation of femtosecond laser-induced graphitic columns in single-crystal diamond. Appl. Phys. A Mater. Sci. Process. 2014, 117, 143–147. [Google Scholar] [CrossRef]
- Girolami, M.; Criante, L.; Di Fonzo, F.; Lo Turco, S.; Mezzetti, A.; Notargiacomo, A.; Pea, M.; Bellucci, A.; Calvani, P.; Valentini, V.; et al. Graphite distributed electrodes for diamond-based photon-enhanced thermionic emission solar cells. Carbon 2017, 111, 48–53. [Google Scholar] [CrossRef]
- Komlenok, M.; Bolshakov, A.; Ralchenko, V.; Konov, V.; Conte, G.; Girolami, M.; Oliva, P.; Salvatori, S. Diamond detectors with laser induced surface graphite electrodes. Nucl. Instrum. Methods Phys. Res. A 2016, 837, 136–142. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, D.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Lespade, P.; Marchand, A.; Couzi, M.; Cruege, F. Caracterisation de materiaux carbones par microspectrometrie Raman. Carbon 1984, 22, 375–385. [Google Scholar] [CrossRef]
- Wilhelm, H.; Lelaurain, M.; McRae, E.; Humbert, B. Raman spectroscopic studies on well-defined carbonaceous materials of strong two-dimensional character. J. Appl. Phys. 1998, 84, 6552–6558. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.L.; Campos, J.L.E.; Fernandes, T.F.D.; Rocha, J.N.; Machado, L.R.P.; Soares, E.M.; Miquita, R.D.; Miranda, H.; Rabelo, C.; Vilela Neto, O.P.; et al. Raman spectroscopy analysis of number of layers in mass-produced graphene flakes. Carbon 2020, 161, 181–189. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Speziali, N.L.; Jorio, A.; Pimenta, M.A. Measuring the degree of stacking order in graphite by Raman spectroscopy. Carbon 2008, 46, 272–275. [Google Scholar] [CrossRef]
- Wang, B.; Cunning, B.V.; Kim, N.Y.; Kargar, F.; Park, S.Y.; Li, Z.; Joshi, S.R.; Peng, L.; Modepalli, V.; Chen, X.; et al. Ultrastiff, Strong, and Highly Thermally Conductive Crystalline Graphitic Films with Mixed Stacking Order. Adv. Mater. 2019, 31, 1903039. [Google Scholar] [CrossRef]



| Square | Φp (J/cm2) | vx (mm/s) | Nx | Φx (J/cm2) | Δy (μm) | ΦA (kJ/cm2) |
|---|---|---|---|---|---|---|
| #1 | 0.24 | 4 | 125 | 30 | 100 | 0.15 |
| #2 | 0.24 | 2 | 250 | 60 | 100 | 0.3 |
| #3 | 0.24 | 1 | 500 | 120 | 100 | 0.6 |
| #4 | 0.24 | 0.5 | 1000 | 240 | 100 | 1.2 |
| #5 | 0.24 | 0.25 | 2000 | 480 | 100 | 2.4 |
| #6 | 0.24 | 0.125 | 4000 | 960 | 100 | 4.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolami, M.; Bellucci, A.; Mastellone, M.; Serpente, V.; Orlando, S.; Valentini, V.; Palma, A.L.; Di Carlo, A.; Trucchi, D.M. Improving the Performance of Printable Carbon Electrodes by Femtosecond Laser Treatment. C 2020, 6, 48. https://doi.org/10.3390/c6030048
Girolami M, Bellucci A, Mastellone M, Serpente V, Orlando S, Valentini V, Palma AL, Di Carlo A, Trucchi DM. Improving the Performance of Printable Carbon Electrodes by Femtosecond Laser Treatment. C. 2020; 6(3):48. https://doi.org/10.3390/c6030048
Chicago/Turabian StyleGirolami, Marco, Alessandro Bellucci, Matteo Mastellone, Valerio Serpente, Stefano Orlando, Veronica Valentini, Alessandro L. Palma, Aldo Di Carlo, and Daniele M. Trucchi. 2020. "Improving the Performance of Printable Carbon Electrodes by Femtosecond Laser Treatment" C 6, no. 3: 48. https://doi.org/10.3390/c6030048
APA StyleGirolami, M., Bellucci, A., Mastellone, M., Serpente, V., Orlando, S., Valentini, V., Palma, A. L., Di Carlo, A., & Trucchi, D. M. (2020). Improving the Performance of Printable Carbon Electrodes by Femtosecond Laser Treatment. C, 6(3), 48. https://doi.org/10.3390/c6030048









