Silicon/Biogas-Derived Carbon Nanofibers Composites for Anodes of Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
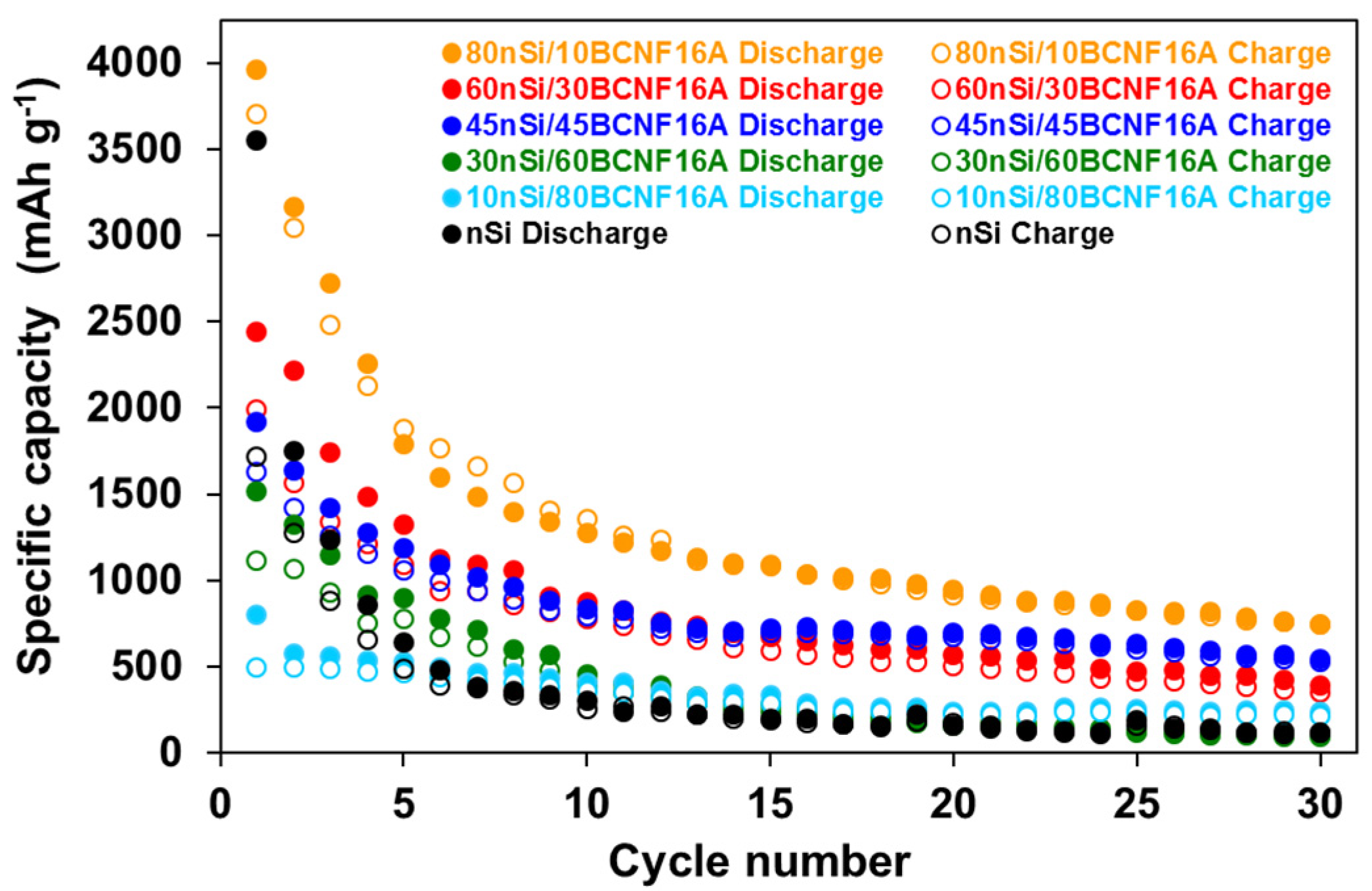
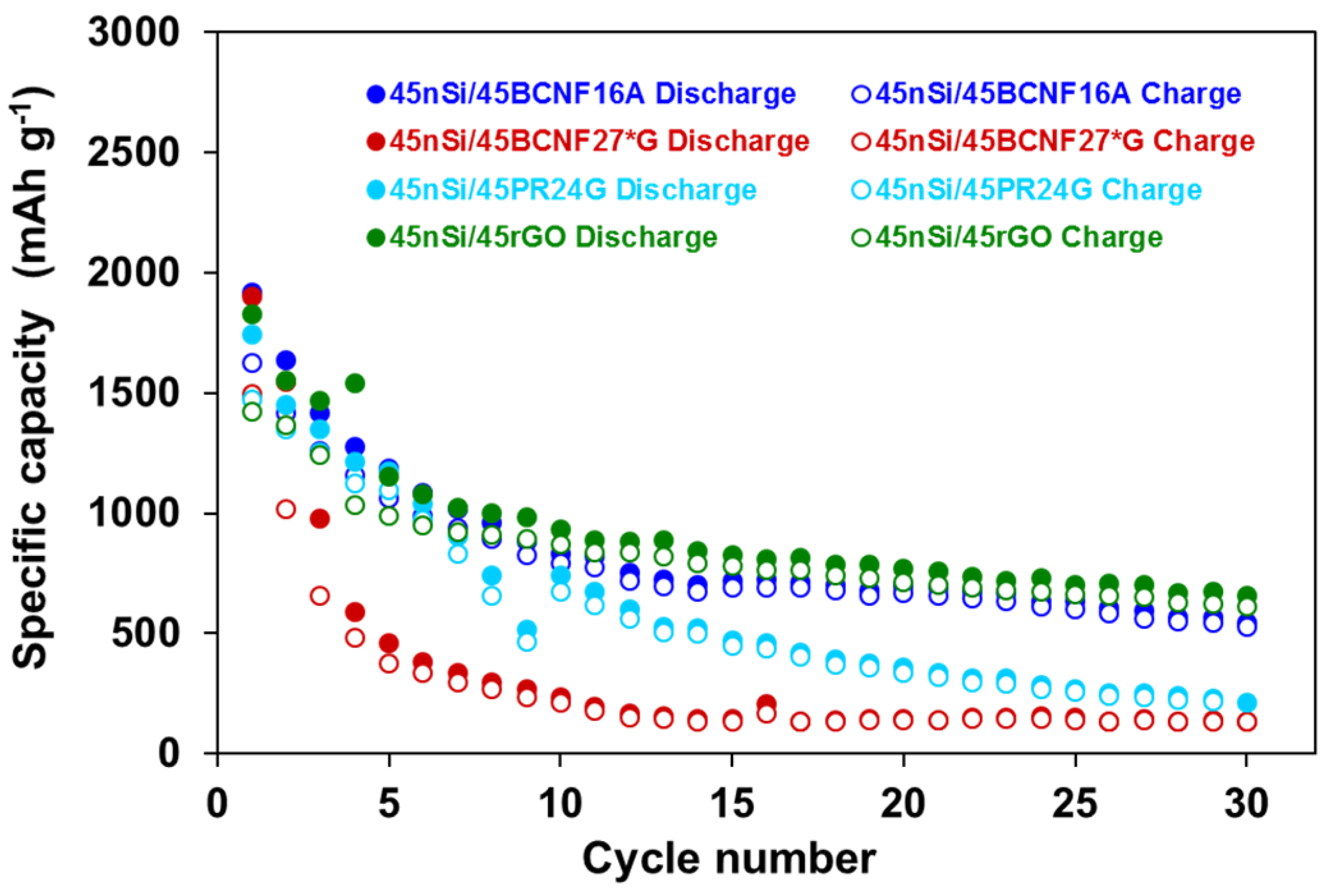
3.1. Influence of nSi Proportion and BCNFs Carbon Matrices on the Electrochemical Performance of nSi/BCNFs Anodes
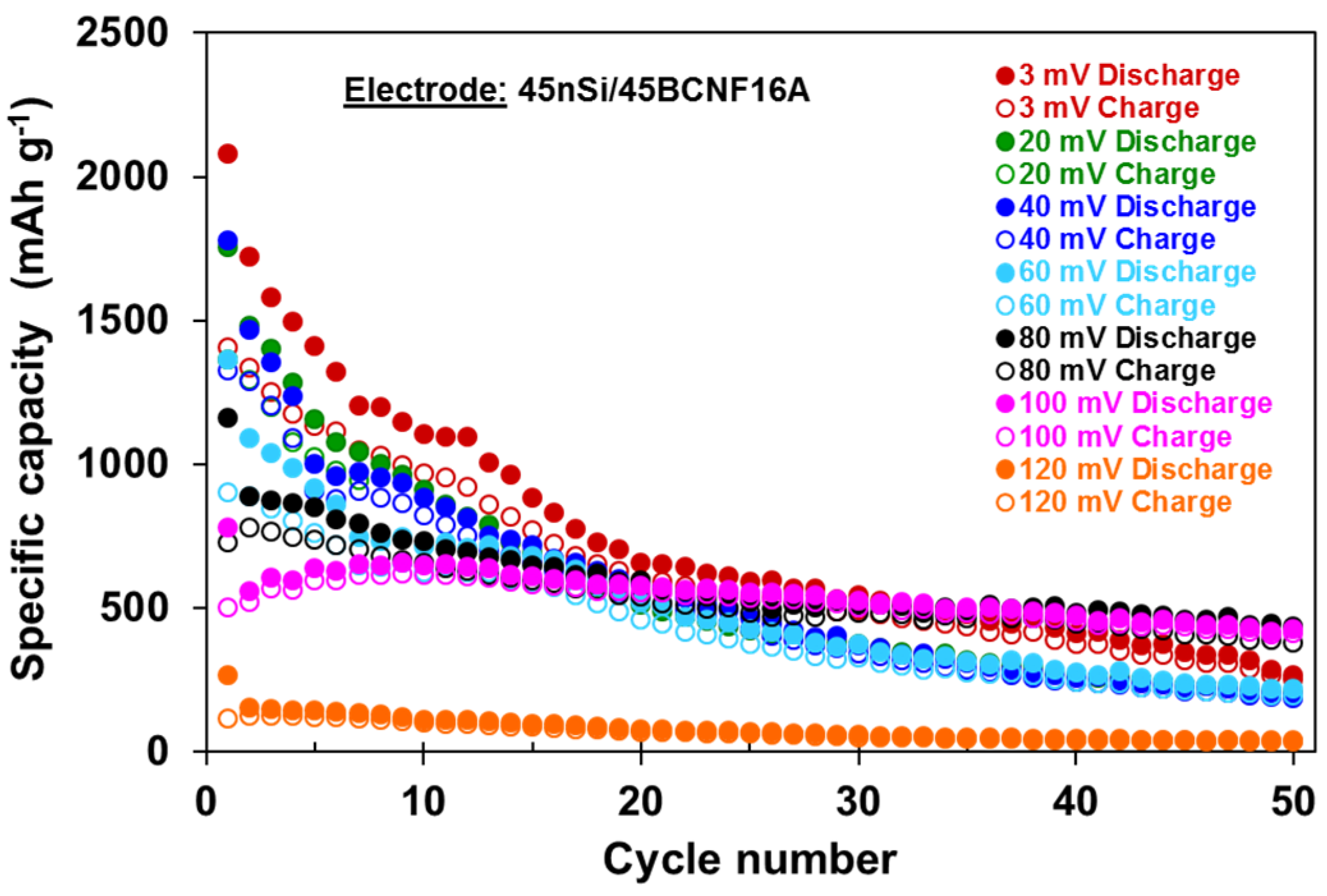
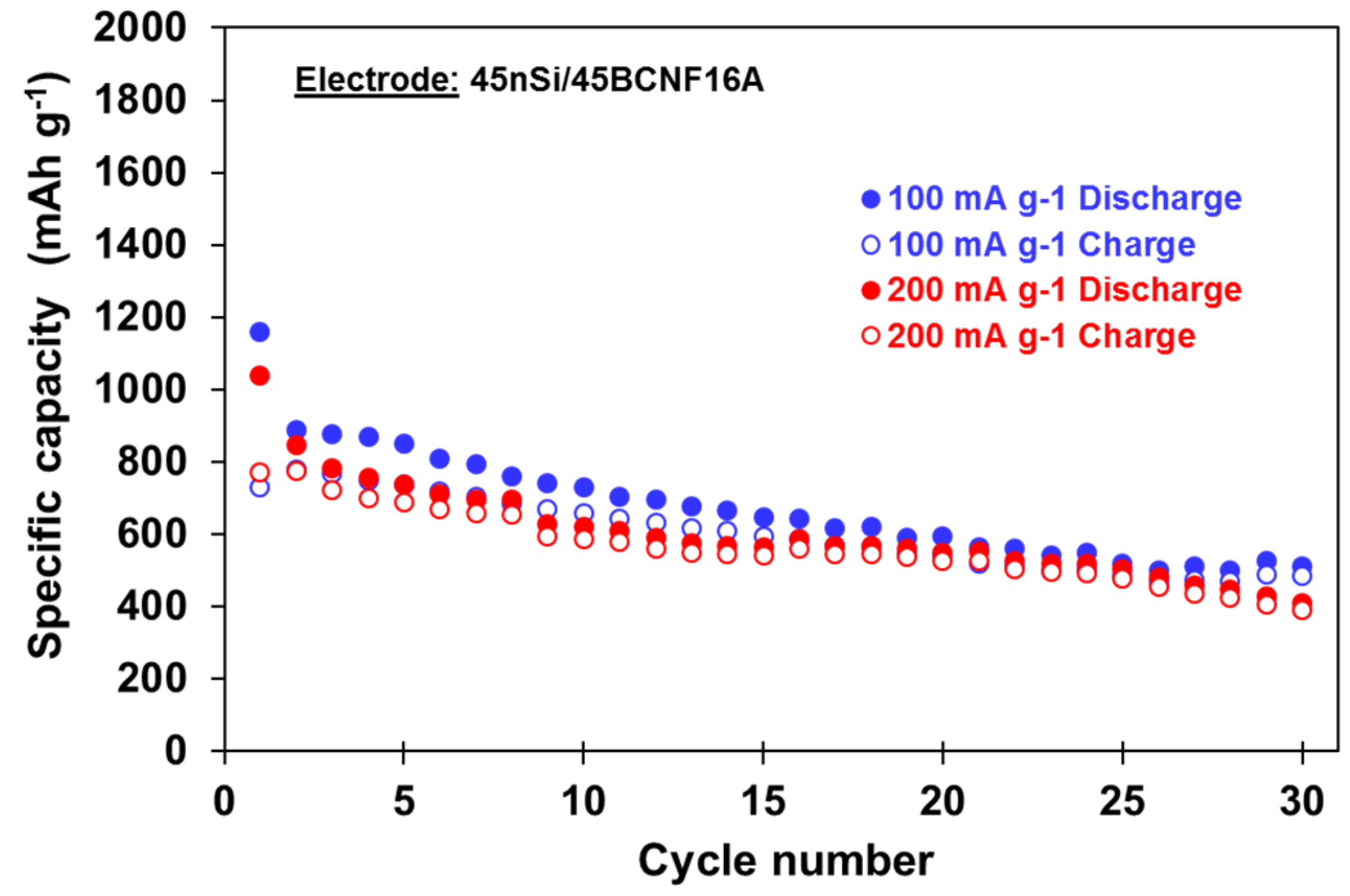
3.2. Influence of Cycling Conditions on the Electrochemical Performance of 45nSi/45BCNF16A Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Malode, S.J.; Shetti, N.P.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Electrode materials for lithium-ion batteries. Mater. Sci. Energy Technol. 2018, 1, 182–187. [Google Scholar] [CrossRef]
- Ozanam, F.; Rosso, M. Silicon as anode material for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 2–11. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.; Zheng, X.; Xu, X.; Yao, C.; Sun, W.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Obrovac, M.N. Si-alloy negative electrodes for Li-ion batteries. Curr. Opin. Electrochem. 2018, 9, 8–17. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, J.W.; Sung, Y.-E.; Oh, S.M. Failure modes of silicon powder negative electrode in lithium secondary batteries. Electrochem. Solid State Lett. 2004, 7, A306–A309. [Google Scholar] [CrossRef]
- Song, M.-K.; Park, S.; Alamgir, F.M.; Cho, J.; Liu, M. Nanostructured electrodes for lithium-ion and lithium-air batteries: The latest developments, challenges, and perspectives. Mater. Sci. Eng. R Rep. 2011, 72, 203–252. [Google Scholar] [CrossRef]
- Xu, W.; Vegunta, S.S.S.; Flake, J.C. Surface-modified silicon nanowire anodes for lithium-ion batteries. J. Power Sources 2011, 196, 8583–8589. [Google Scholar] [CrossRef]
- Shen, X.; Tian, Z.; Fan, R.; Shao, L.; Zhang, D.; Cao, G.; Kou, L.; Bai, Y. Research progress on silicon/carbon composite anode materials for lithium-ion battery. J. Energy Chem. 2018, 27, 1067–1090. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Shao, D.; Tang, D.; Yang, J.; Li, Y.; Zhang, L. Nano-structured composite of Si/(S-doped-carbon nanowire network) as anode material for lithium-ion batteries. J. Power Sources 2015, 297, 344–350. [Google Scholar] [CrossRef]
- Morita, T.; Takami, N. Nano Si cluster-SiOx-C composite material as high-capacity anode material for rechargeable lithium batteries. J. Electrochem. Soc. 2006, 153, A425–A430. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Komaba, S.; Yabuuchi, N.; Ozeki, T.; Han, Z.-J.; Shimomura, K.; Yui, H.; Katayama, Y.; Miura, T. Comparative study of sodium polyacrylate and poly(vinylidene fluoride) as binders for high capacity Si–graphite composite negative electrodes in Li-ion batteries. J. Phys. Chem. C 2012, 116, 1380–1389. [Google Scholar] [CrossRef]
- Liu, W.-R.; Yang, M.-H.; Wu, H.-C.; Chiao, S.M.; Wu, N.-L. Enhanced cycle life of Si anode for Li-ion batteries by using modified elastomeric binder. Electrochem. Solid State Lett. 2005, 8, A100–A103. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Mazouzi, D.; Lestriez, B.; Roué, L.; Guyomard, D. Silicon composite electrode with high capacity and long cycle life. Electrochem. Solid State Lett. 2009, 12, A215–A218. [Google Scholar] [CrossRef]
- Li, J.; Lewis, R.; Dahn, J. Sodium carboxymethyl cellulose a potential binder for Si negative electrodes for Li-ion batteries. Electrochem. Solid State Lett. 2007, 10, A17–A20. [Google Scholar] [CrossRef]
- Beattie, S.D.; Larcher, D.; Morcrette, M.; Simon, B.; Tarascon, J.-M. Si Electrodes for Li-Ion Batteries—A New Way to Look at an Old Problem. J. Electrochem. Soc. 2008, 155, A158–A163. [Google Scholar] [CrossRef]
- Hochgatterer, N.; Schweiger, M.; Koller, S.; Raimann, P.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/graphite composite electrodes for high-capacity anodes: Influence of binder chemistry on cycling stability. Electrochem. Solid State Lett. 2008, 11, A76–A80. [Google Scholar] [CrossRef]
- Bridel, J.S.; Azaïs, T.; Morcrette, M.; Tarascon, J.M.; Larcher, D. Key parameters governing the reversibility of Si/carbon/CMC electrodes for Li-ion batteries. Chem. Mater. 2010, 22, 1229–1241. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C. A polymer scaffold binder structure for high capacity silicon anode of lithium-ion battery. Chem. Commun. 2010, 46, 1428–1430. [Google Scholar] [CrossRef]
- Lux, S.F.; Schmuck, M.; Jeong, S.; Passerini, S.; Winter, M.A.; Balducci, A. Li-ion anodes in air-stable and hydrophobic ionic liquid-based electrolyte for safer and greener batteries. Int. J. Energy Res. 2010, 34, 97–106. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, L.; Zhong, H. Carboxymethyl chitosan: A new water soluble binder for Si anode of Li-ion batteries. J. Power Sources 2014, 247, 327–331. [Google Scholar] [CrossRef]
- Kuruba, R.; Datta, M.K.; Damodaran, K.; Jampani, P.H.; Gattu, B.; Patel, P.P.; Shanthi, P.M.; Damle, S.; Kumta, P.N. Guar gum: Structural and electrochemical characterization of natural polymer based binder for silicon–carbon composite rechargeable Li-ion battery anodes. J. Power Sources 2015, 298, 331–340. [Google Scholar] [CrossRef]
- Ling, M.; Xu, Y.; Zhao, H.; Gu, X.; Qiu, J.; Li, S.; Wu, M.; Song, X.; Yan, C.; Liu, G.; et al. Dual-functional gum arabic binder for silicon anodes in lithium ion batteries. Nano Energy 2015, 12, 178–185. [Google Scholar] [CrossRef]
- Cuesta, N.; Cameán, I.; Ramos, A.; de Llobet, S.; García, A.B. Graphitic nanomaterials from biogas-derived carbon nanofibers. Fuel Process. Technol. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Leveau, L.; Laïk, B.; Pereira-Ramos, J.-P.; Gohier, A.; Tran-Van, P.; Cojocaru, C.-S. Cycling strategies for optimizing silicon nanowires performance as negative electrode for lithium battery. Electrochim. Acta 2015, 157, 218–224. [Google Scholar] [CrossRef]
- Cui, L.-F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core−shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nature Nanotechol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wang, J.W.; Huang, S.; Fan, F.; Huang, X.; Liu, Y.; Krylyuk, S.; Yoo, J.; Dayeh, S.A.; Davydov, A.V. In situ atomic-scale imaging of electrochemical lithiation in silicon. Nature Nanotechol. 2012, 7, 749–756. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible cycling of crystalline silicon powder. J. Electrochem. Soc. 2007, 154, A103–A108. [Google Scholar] [CrossRef]
- Ramos, A.; Cameán, I.; Cuesta, N.; García, A.B. Graphitized stacked-cup carbon nanofibers as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 146, 769–775. [Google Scholar] [CrossRef]
- Li, J.; Dahn, J.R. An in situ X-Ray diffraction study of the reaction of Li with crystalline Si. J. Electrochem. Soc. 2007, 154, A156–A161. [Google Scholar] [CrossRef]
- Cuesta, N.; Cameán, I.; Ramos, A.; García, A.B. Graphitized biogas-derived carbon nanofibers as anodes for lithium-ion batteries. Electrochim. Acta 2016, 222, 264–270. [Google Scholar] [CrossRef]


| Electrode | Cdisc 1st Cycle (mAh g−1) | Cdisc 2nd Cycle (mAh g−1) | Cdisc 10th Cycle (mAh g−1) | Cdisc 20th Cycle (mAh g−1) | Cdisc 30th Cycle (mAh g−1) | Cirr a 1st Cycle (%) | R b 2nd–30th Cycles (%) |
|---|---|---|---|---|---|---|---|
| 80nSi/10BCNF27*G | 1871 | 1343 | 607 | -- | -- | -- | -- |
| 60nSi/30BCNF27*G | 1803 | 1473 | 512 | -- | -- | -- | -- |
| 45nSi/45BCNF27*G | 1900 | 1549 | 237 | 146 | 136 | 21 | 9 |
| 30nSi/60BCNF27*G | 1187 | 867 | 218 | 108 | 62 | 30 | 7 |
| 10nSi/80BCNF27*G | 718 | 468 | 287 | 155 | 187 | 39 | 40 |
| 80nSi/10BCNF27*A | 2643 | 1890 | 464 | 346 | 229 | 30 | 12 |
| 60nSi/30BCNF27*A | 2525 | 1957 | 753 | 464 | 314 | 37 | 16 |
| 45nSi/45BCNF27*A | 3191 | 2852 | 1558 | 904 | 534 | 28 | 19 |
| 30nSi/60BCNF27*A | 1181 | 1043 | 748 | 407 | 183 | 14 | 15 |
| 10nSi/80BCNF27*A | 738 | 465 | 343 | 183 | 126 | 49 | 27 |
| 80nSi/10BCNF16A | 3964 | 3167 | 1272 | 946 | 748 | 28 | 24 |
| 60nSi/30BCNF16A | 2439 | 2213 | 874 | 569 | 391 | 18 | 18 |
| 45nSi/45BCNF16A | 1916 | 1640 | 834 | 696 | 545 | 15 | 33 |
| 30nSi/60BCNF16A | 1516 | 1325 | 458 | 174 | 99 | 27 | 7 |
| 10nSi/80BCNF16A | 800 | 574 | 405 | 242 | 235 | 38 | 41 |
| nSi | 3554 | 1747 | 302 | 155 | 118 | -- | 8 |
| Carbon Matrix | SBET (m2 g−1) | Vt (cm3 g−1) | VMESO (cm3 g−1) | VMICRO (cm3 g−1) |
|---|---|---|---|---|
| BCNF16A | 96 | 0.240 | 0.171 | 0.011 |
| BCNF27*A | 113 | 0.392 | 0.333 | 0.010 |
| BCNF27*G | 73 | 0.279 | 0.279 | 0.010 |
| rGO | 460 | -- | -- | -- |
| PR24G | 29 | -- | -- | -- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cameán, I.; Cuesta, N.; Ramos, A.; García, A.B. Silicon/Biogas-Derived Carbon Nanofibers Composites for Anodes of Lithium-Ion Batteries. C 2020, 6, 25. https://doi.org/10.3390/c6020025
Cameán I, Cuesta N, Ramos A, García AB. Silicon/Biogas-Derived Carbon Nanofibers Composites for Anodes of Lithium-Ion Batteries. C. 2020; 6(2):25. https://doi.org/10.3390/c6020025
Chicago/Turabian StyleCameán, Ignacio, Nuria Cuesta, Alberto Ramos, and Ana B. García. 2020. "Silicon/Biogas-Derived Carbon Nanofibers Composites for Anodes of Lithium-Ion Batteries" C 6, no. 2: 25. https://doi.org/10.3390/c6020025
APA StyleCameán, I., Cuesta, N., Ramos, A., & García, A. B. (2020). Silicon/Biogas-Derived Carbon Nanofibers Composites for Anodes of Lithium-Ion Batteries. C, 6(2), 25. https://doi.org/10.3390/c6020025







